Abstract
Classic cognitive theory conceptualizes executive functions as involving multiple specific domains, including initiation, inhibition, working memory, flexibility, planning, and vigilance. Lesion and neuroimaging experiments over the past two decades have suggested that both common and unique processes contribute to executive functions during higher cognition. It has been suggested that a superordinate fronto–cingulo–parietal network supporting cognitive control may also underlie a range of distinct executive functions. To test this hypothesis in the largest sample to date, we used quantitative meta-analytic methods to analyze 193 functional neuroimaging studies of 2,832 healthy individuals, ages 18–60, in which performance on executive function measures was contrasted with an active control condition. A common pattern of activation was observed in the prefrontal, dorsal anterior cingulate, and parietal cortices across executive function domains, supporting the idea that executive functions are supported by a superordinate cognitive control network. However, domain-specific analyses showed some variation in the recruitment of anterior prefrontal cortex, anterior and midcingulate regions, and unique subcortical regions such as the basal ganglia and cerebellum. These results are consistent with the existence of a superordinate cognitive control network in the brain, involving dorsolateral prefrontal, anterior cingulate, and parietal cortices, that supports a broad range of executive functions.
Keywords: Cognitive control, Prefrontal cortex, Executive function, Activation likelihood estimation, Meta-analysis
Early cognitive theories posited that cognitive functions are modular in nature and located within separable but interconnected parts of the brain (Luria, 1970; Shallice, 1988). Within this framework, executive functions have been described as a set of superordinate processes that guide thought and behavior and allow purposive action toward a goal (Miller, 2000). These functions are critical for normal day-to-day cognitive functioning and appear to be particularly susceptible to altered development, injury, and disease. From a traditional cognitive or neuropsychological perspective, executive functions have been thought to comprise a set of distinct cognitive domains that include vigilance, or sustained attention (Pennington & Ozonoff, 1996; Smith & Jonides, 1999); initiation of complex goal-directed behaviors (Lezak, 1995); inhibition of prepotent but incorrect responses (Luna, Padmanabhan, & O’Hearn, 2010; Smith & Jonides, 1999); flexibility to shift easily between goal states (Ravizza & Carter, 2008); planning the necessary steps to achieve a goal (Smith & Jonides, 1999); and working memory, the ability to hold information in mind and manipulate it to guide response selection (Goldman-Rakic, 1996).
These theoretically distinct domains are supported by discrete neural systems (Luria, 1970; Shallice, 1988), which typically include elements of the prefrontal cortex (PFC). Early animal lesion studies provided evidence for PFC involvement in the coordination of complex behaviors, by serving as a temporary store for incoming information, making this information immediately available to guide response selection (Fuster, 1990; Goldman-Rakic, 1987; Jacobsen, 1936). Prefrontal damage in humans also impairs various executive functions, including planning (Owen, Downes, Sahakian, Polkey, & Robbins, 1990; Shallice, 1982, 1988), flexibility (Milner, 1982), response inhibition (Leimkuhler & Mesulam, 1985), and working memory (Milner, 1982).
Early neuroimaging and human lesion studies revealed that the frontal cortex is just one element in a network of spatially distinct regions associated with executive functions (Baddeley & Wilson, 1988). For example, neuroimaging studies of a prototypical working memory task, the n-back paradigm, have consistently shown activated regions in the frontal and posterior parietal cortex and cerebellum (Owen, McMillan, Laird, & Bullmore, 2005). Within this task, Broca’s area and premotor cortex have been associated with subvocal rehearsal processes, while posterior parietal areas were associated with the storage of verbal information (Awh et al., 1996). On tasks that require flexibility, the ability to flexibly switch attention and behavioral responses between different rules is associated with activation of dorsolateral PFC (DLPFC), while switching attention responses between different perceptual features of a stimulus is associated with parietal activation (Ravizza & Carter, 2008).
While traditional theories of executive functions have posited a set of distinct domains supported by at least partially unique brain regions, increasing numbers of functional neuroimaging studies examining diverse executive functions have suggested that these tasks may engage very similar brain networks (e.g., Duncan & Owen, 2000). Recent views of the PFC highlight its role in higher cognitive functions by supporting coordinated activation of multiple brain areas within the “cognitive control network,” including the DLPFC, medial frontal cortex (including the anterior cingulate cortex [ACC]), parietal cortex, motor areas, and cerebellum (Bellebaum & Daum, 2007; Braver, Cohen, & Barch, 2002; D’Esposito, 2007; Fuster, 2002). Furthermore, analyses of functional connectivity in healthy adults revealed that coordinated temporal activation across the network of prefrontal and posterior brain regions is associated with better performance on cognitive control tasks (Fornito, Yoon, Zalesky, Bullmore, & Carter, 2011; Yoon et al., 2008). Miller and Cohen proposed that the PFC supports “cognitive control” by actively maintaining “rules” online in order to evaluate incoming information, as well as internal states to guide response selection toward a current goal (Miller, 2000; Miller & Cohen, 2001). According to this view, cognitive control mechanisms support the range of executive functions, including working memory, selective attention, stimulus–response mapping, and performance monitoring (Carter et al., 1998; Cohen, Dunbar, & McClelland, 1990; Miyake & Shah, 1999; Shallice, 1988), and are not restricted to a particular cognitive domain (Banich, 1997; Smith & Jonides, 1999).
The diverse array of executive functions has limited our ability to directly test the unitary or modular nature of the underlying brain systems within a single set of experiments. Capitalizing on the unique power of activation likelihood estimation (ALE) meta-analytic tools, this study is the first to synthesize almost 200 published reports, testing the hypothesis that traditional executive functions are supported by a common PFC-related cognitive control network. The ALE meta-analytic approach models three-dimensional coordinates (from reported activations in standard space) as the center of a three-dimensional Gaussian distribution (Laird, Fox, et al., 2005). By combining published data from a wide variety of studies, the ALE method provides the unique opportunity to examine this question in the largest sample of control subjects published to date. Activation likelihood estimation has been has been used to address similar research questions in both healthy and patient samples (Binder, Desai, Graves, & Conant, 2009; Caspers, Zilles, Laird, & Eickhoff, 2010; Chouinard & Goodale, 2010; Dickstein, Bannon, Castellanos, & Milham, 2006; Fusar-Poli et al., 2009; Glahn et al., 2005; Goghari, 2010; Mana, Paillere Martinot, & Martinot, 2010; Minzenberg, Laird, Thelen, Carter, & Glahn, 2009; Molenberghs, Cunnington, & Mattingley, 2009; Owen et al., 2005; Ragland et al., 2009; Richlan, Kronbichler, & Wimmer, 2009; Samson, Mottron, Soulieres, & Zeffiro, 2011; Schwindt & Black, 2009; Spaniol et al., 2009; Turkeltaub & Coslett, 2010; Yu et al., 2010). We hypothesized that healthy adults would show a common pattern of activation across prefrontal (DLPFC, ACC) and parietal regions when performing executive function tasks across multiple domains (see Table 1). Furthermore, we hypothesized that additional areas of domain-specific activation may be observed, but these would occur in addition to the common pattern of activation within the cognitive control network.
Table 1.
Definitions of the cognitive domains examined within this study, tasks included within each of the domains, the total numbers of available studies examined, and the total numbers of studies and subjects included in the present analysis, by domain and task
| Cognitive Domain | Definition | Task Included in Domain |
Number of Available Studies |
Number of Studies Included in Current Analysis |
Total Number of Subjects Included |
|---|---|---|---|---|---|
| Flexibility1,2 | Switch from one task OR rule to another | Task switching | 26 | 12 | 201 |
| Wisconsin Card Sorting Test | 16 | 9 | 129 | ||
| Inhibition1,3 | Inhibit prepotent response in order to make correct, but less common, response | Antisaccades | 13 | 11 | 149 |
| Flanker task | 10 | 9 | 108 | ||
| Go/no-go task | 40 | 23 | 417 | ||
| Simon task | 12 | 10 | 192 | ||
| Stroop task | 55 | 26 | 346 | ||
| Working memory4 | Maintain information/context/ temporal or spatial relationships online and manipulate or use that information to guide response selection | Complex calculation/ PASAT | 39 | 11 | 152 |
| Delayed match to sample | 24 | 12 | 150 | ||
| N-back/AXCPT | 73 | 37 | 502 | ||
| Spatial span/sequence recall | 20 | 3 | 24 | ||
| Sternberg task | 21 | 15 | 232 | ||
| Initiation5 | Initiate sequence of complex behaviors | Word generation | 85 | 9 | 115 |
| Planning1 | Identify and organize steps and elements needed to carry out an intention or achieve a goal | Tower Maze test | 13 | 4 | 51 |
| Vigilance1,6 | Maintaining set in the face of interference | Oddball discrimination | 10 | 2 | 64 |
| TOTAL = | 457 | 193 | 2,832 |
Method
Study selection
A search of the BrainMap database (Fox & Lancaster, 2002; Laird, Fox, et al., 2005) was performed to identify all English-language, peer-reviewed studies that investigated executive function tasks in multiple healthy individuals, ages 18–60 years, using functional magnetic resonance imaging (fMRI) or positron emission tomography (PET). Executive functions were defined as processes that are required in order to regulate or guide other cognitive processes in order to support goal-directed behavior (Minzenberg et al., 2009). For the purpose of this investigation, we examined studies that used task paradigms that are typically considered measures of executive function or cognitive control. As outlined in Table 1, these included measures of vigilance, inhibition, flexibility, planning, working memory, and initiation. Within each study, we included data from healthy individuals on specific contrasts that examined within-group whole-brain activation in response to a task of interest that was compared to an active control task, rather than to rest or fixation. Studies were excluded if the subject pool overlapped with other published studies on smaller subsets of the same sample or included subjects outside of the age range (18–60 years), if the task of interest did not require an appropriate behavioral response (e.g., a buttonpress), or if contrasts with the available coordinate data did not examine a specific executive function or rather examined differences between patients and controls. Table 1 provides the numbers of studies that were available and that met the criteria for inclusion within each domain. The BrainMap database archives the peak coordinates of activations as well as their corresponding metadata, such as the number and diagnosis of the subjects, the analysis technique, the paradigm, and the cognitive domain. Coordinates originally published in MNI space were converted to Talairach space using the Lancaster (icbm2tal) transformation (Laird et al., 2010; Lancaster et al., 2007). Further filtering and meta-analysis of the experiments was carried out using BrainMap’s software applications (Laird et al., 2009), as described below.
Activation likelihood estimation
We performed a series of coordinate-based meta-analyses of executive functioning using the ALE method (Laird, McMillan, et al., 2005; Turkeltaub, Eden, Jones, & Zeffiro, 2002), in which the voxel-wise correspondence of neuroimaging results is assessed across a large number of studies. The ALE algorithm aims to identify areas showing a higher convergence of findings across experiments than would be expected under a spatially random spatial association. The identified literature coordinates were modeled with a three-dimensional Gaussian probability distribution reflecting the spatial uncertainty of each focus on the basis of an estimation of the intersubject and interlaboratory variability typically observed in neuroimaging experiments. This algorithm limits the meta-analysis to an anatomically constrained space specified by a gray-matter mask and includes a method that calculates the above-chance clustering between experiments (i.e., random-effects analysis), rather than between foci (i.e., fixed-effects analysis), and it also accounts for differences in sample sizes across the included studies (Eickhoff et al., 2009). The probabilities of all foci reported in a given experiment were combined, resulting in a modeled activation map for each experiment, and the union of these probabilities was computed in order to derive voxel-wise ALE values that described the convergence of results across the whole brain. To determine which ALE values were statistically significant, ALE scores were compared with an empirical null distribution reflecting a random spatial association between experiments, thereby estimating convergence between studies rather than the clustering of foci within a particular study.
ALE was performed in Talairach space using GingerALE 2.0 (http://brainmap.org/ale/index.html) to analyze the global set of activation foci for concordance, as well as subsets of foci that corresponded to the cognitive components of interest within executive function. From the set of included studies (Table 2), the results for a global set of within-group activations across all six domains were meta-analyzed to address the primary hypothesis. To examine the foci of greatest concordance across studies, we also performed a conjunction analysis across the three domains in which the data from more than nine studies were available (flexibility, inhibition, and working memory). To examine potential domain-specific patterns of activation, we completed within-group meta-analyses for the domains in which data from more than nine studies were available. The resultant ALE maps were thresholded at a false-discovery rate (FDR)-corrected threshold of p < .05. Images were viewed in Mango (“multi-image analysis GUI”), developed at the Research Imaging Institute in San Antonio (http://ric.uthscsa.edu/mango/).
Table 2.
Published studies included in the ALE meta-analysis of executive functions, by domain
| Author | Task | PET vs. MRI |
Sample Size |
Age (Range or Mean) |
Included Contrasts | Materials Used | Perceptual Domain |
|---|---|---|---|---|---|---|---|
| FLEXIBILITY | |||||||
| K. F. Berman et al., 1995 | WCST | PET | 40 | 18–39 | 1. WCST > Control | Pictures (Cross Shape Array) | Visual |
| Brass & von Cramon, 2004 | Task Switching | fMRI | 14 | Mean = 24 | 1. Meaning Switch vs. Cue Switch | Shapes | Visual |
| Braver, Reynolds, & Donaldson, 2003 | Task Switching | fMRI | 13 | 19–26 | 1. Switch × Time | Words | Visual |
| Cools, Clark, & Robbins, 2004 | Task Switching | fMRI | 16 | 18–45 | 1. Object-Rule Switch vs. Nonswitch | Pictures (Abstract Patterns) | Visual |
| Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000 | Task Switching | fMRI | 16 | 21–29 | 1. Task Switch –Task Repetition | Shapes | Visual |
| Dreher & Grafman, 2003 | Task Switching | fMRI | 8 | 20–31 | 1. Task Switching vs. Baseline | Letters | Visual |
| Goldberg et al., 1998 | WCST | PET | 12 | 24–39 | 1. WCST – Control, Activations | Pictures (Five Card Stimulus) | Visual |
| Kimberg, Aguirre, & D’Esposito, 2000 | Task Switching | fMRI | 9 | College age | 1. Switch – Repeat | Letters | Visual |
| Konishi, Nakajima, Uchida, Kameyama, et al., 1998 | WCST | fMRI | 7 | 20–31 | 1. Three-Dimensional – (Two- + One-Dimensional) | Pictures (Five Card Stimulus), Letters | Visual |
| Luks, Simpson, Feiwell, & Miller, 2002 | Task Switching | fMRI | 11 | 24–45 | 1. Switch > Repeat | Numbers, Shapes | Visual |
| Monchi, Petrides, Petre, Worsley, & Dagher, 2001 | WCST | fMRI | 11 | 18–34 | 1. Matching After Negative Feedback – Control Matching (Increases) | Pictures (Five Card Stimulus) | Visual |
| Nagahama et al., 1996 | WCST | PET | 18 | 21–35 | 1. Modified Card Sorting Test (MCST) vs. Matching | Pictures (Five Card Stimulus) | Visual |
| Nagahama et al., 1997 | WCST | PET | 12 | 21–24 | 1. WCST > Matching, Young | Pictures (Five Card Stimulus) | Visual |
| Nagahama et al., 2001 | WCST | fMRI | 6 | Mean = 27 | 1. Set Shifting Task | Pictures (Three Card Stimulus) | Visual |
| Rao et al., 1997 | WCST | fMRI | 11 | 19–45 | 1. Conceptual Reasoning – Control | Words | Visual |
| Rogers, Andrews, Grasby, Brooks, & Robbins, 2000 | WCST | PET | 12 | Mean = 43 | 1. Extradimensional (ED) – Intradimensional (ID) Shift | Shapes | Visual |
| Rubia et al., 2006 | Task Switching | fMRI | 52 | 20–43 | 1. Switch Task, Adults | Shapes | Visual |
| Ruff, Woodward, Laurens, & Liddle, 2001 | Task Switching | fMRI | 12 | Mean = 23 | 1. Switching Color Naming, Incongruent vs. Neutral | 1. Letters, Words, | Visual |
| 2. Switching Word Reading, Incongruent vs. Neutral | 2. Words | ||||||
| Rushworth, Hadland, Paus, & Sipila, 2002 | Task Switching | fMRI | 18 | 19–31 | 1. Switch – Stay, RS, Increases | Shapes | Visual |
| Smith, Taylor, Brammer, & Rubia, 2004 | Task Switching | fMRI | 20 | 20–43 | 1. Switch vs. Repeat | Shapes | Visual |
| Sohn, Ursu, Anderson, Stenger, & Carter, 2000 | Task Switching | fMRI | 12 | 18–36 | 1. Foreknowledge Effects | Numbers, Digits | Visual |
| 2. Transition Effects | |||||||
| INHIBITION | |||||||
| Altshuler et al., 2005 | Go–No Go | fMRI | 13 | Mean = 31 | 1. No Go > Go, Normals | Letters | Visual |
| Asahi, Okamoto, Okada, Yamawaki, & Yokota, 2004 | Go–No-Go | fMRI | 17 | 23–30 | 1. Response Inhibition | Letters | Visual |
| Banich et al., 2000 | Stroop | fMRI | 10 | College age | 1. Incongruent > Congruent, Color | Words | Visual |
| Banich et al., 2001 | Stroop | fMRI | 14 | 21–35 | 1. Incongruent, Color vs. Neutral | Words | Visual |
| 2. Incongruent, Object vs. Neutral | |||||||
| Bellgrove, Hester, & Garavan, 2004 | Go–No-Go | fMRI | 42 | 18–46 | 1. Response Inhibition | Letters | Visual |
| Bench et al., 1993 | Stroop | PET | 12 | 21–34 | 1. Stroop I vs. Crosses I | 1. Words, Shapes | Visual |
| 2. Stroop I vs. Neutral | 2. Words | ||||||
| 3. Stroop II vs. Crosses II | 3. Words, Shapes | ||||||
| G. G. Brown et al., 1999 | Stroop | fMRI | 8 | Under age 55 | 1. Incongruent – Nonlexical | 1. Words, Shapes | Visual |
| 2. Incongruent – Neutral | 2. Words | ||||||
| M. R. G. Brown, Goltz, Vilis, Ford, & Everling, 2006 | Antisaccade | fMRI | 10 | 22–33 | 1. Antisaccade Response > Prosaccade Response | Shapes | Visual |
| M. R. G. Brown, Vilis, & Everling, 2007 | Antisaccade | fMRI | 11 | 20–28 | 1. Preparation, Antisaccade > Preparation, Prosaccade | Shapes | Visual |
| 2. Response, Antisaccade – Preparation, Antisaccade | |||||||
| Bunge et al., 2002 | Flanker | fMRI | 10 | 18–44 | 1. Incongruent vs. Neutral | Letters | Visual |
| Bush et al., 1998 | Stroop | fMRI | 9 | Mean = 24 | 1. Interference – Neutral | Words | Visual |
| Carter, Mintun, & Cohen, 1995 | Stroop | PET | 15 | 22–49 | 1. Incongruent – Neutral | Words | Visual |
| 2. Incongruent – Congruent | |||||||
| Chikazoe, Konishi, Asari, Jimura, & Miyashita, 2007 | Antisaccade | fMRI | 25 | 20–29 | 1. Antisaccade – Control Saccade | Shapes | Visual |
| Coderre, Filippi, Newhouse, & Dumas,2008 | Stroop | fMRI | 9 | 18–36 | 1. Kana Incongruent > Kana Congruent | Words/Symbols | Visual |
| 2. Kanji Incongruent > Kanji Congruent | |||||||
| 3. Kana Incongruent > Kana Words | |||||||
| 4. Kanji Incongruent > Kanji Words | |||||||
| de Zubicaray, Andrew, Zelaya, Williams, & Dumanoir, 2000 | Go–No-Go | fMRI | 8 | Mean = 27 | 1. Effect of Decreased # of No-Go Trials | Shapes | Visual |
| 2. Linear Decreases With Number of Trials Equated per Block | |||||||
| de Zubicaray, Wilson, McMahon, & Muthiah, 2001 | Stroop | fMRI | 8 | Mean = 29 | 1. Semantically Related Distractor vs. Control | Words, Letters | Visual |
| Dichter & Belger, 2007 | Flanker | fMRI | 17 | Mean = 25 | 1. Incongruent Arrows > Congruent Arrows, Controls | 1. Shapes | Visual |
| 2. Incongruent Gaze > Congruent Gaze, Controls | 2. Faces | ||||||
| Doricchi et al., 1997 | Antisaccade | PET | 10 | 20–26 | 1. Antisaccades vs. Fast–Regular | Shapes | Visual |
| Durston et al., 2003 | Flanker | fMRI | 9 | Mean = 26 | 1. Compatible Increased, Incompatible Decreased | Shapes | Visual |
| Ettinger et al., 2008 | Antisaccade | fMRI | 17 | 20–40 | 1. Saccade-by-Delay Interaction | Shapes | Visual |
| Fan, Flombaum, McCandliss, Thomas, & Posner, 2003 | Stroop & Flanker | fMRI | 12 | 18–34 | 1. Stroop Incongruent – Congruent, | 1. Words | Visual |
| 2. Flanker Incongruent – Congruent | 2. Shapes | ||||||
| Fassbender et al., 2004 | Go–No-Go | fMRI | 21 | 19–37 | 1. Activations for Correct Inhibitions | Numbers | Visual |
| K. D. Fitzgerald et al., 2005 | Flanker | fMRI | 7 | Mean = 30 | 1. High > No Interference, Normals | Letters | Visual |
| 2. High > Low Interference, Normals | |||||||
| Ford, Goltz, Brown, & Everling, 2005 | Antisaccade | fMRI | 10 | Mean = 28 | 1. Late Preparatory Period Comparison: Anti vs. Pro | Shapes | Visual |
| Forstmann, van den Wildenberg, & Ridderinkhof, 2008 | Simon | fMRI | 24 | Mean = 24 | 1. Incongruent vs. Neutral | Shapes | Visual |
| Garavan, Ross, & Stein, 1999 | Go–No-Go | fMRI | 14 | 19–44 | 1. Response Inhibition | Letters | Visual |
| Garavan, Ross, Murphy, Roche, & Stein, 2002 | Go–No-Go | fMRI | 14 | 19–45 | 1. Successful No-Gos | Letters | Visual |
| Garavan, Ross, Kaufman, & Stein, 2003 | Go–No-Go | fMRI | 16 | 18–46 | 1. Event-Related STOPS | Letters | Visual |
| George et al., 1993 | Stroop | PET | 21 | Mean = 38 | 1. Standard Stroop – Control | Words, Shapes | Visual |
| Hazeltine, Bunge, Scanlon, & Gabrieli, 2003 | Flanker | fMRI | 10 | 18–44 | 1. Incongruent – Neutral (Conjunction of Color and Letter) | Letters, Shapes (Circle) | Visual |
| Heckers et al., 2004 | Stroop | fMRI | 15 | Mean = 47 | 1. Interference vs. Control, Normals | Numbers, Letters/Digits | Visual |
| Hester et al., 2004 | Go–No-Go | fMRI | 15 | 23–40 | 1. Cued and Uncued Successful Response Inhibition | Letters | Visual |
| Horn, Dolan, Elliott, Deakin, & Woodruff, 2003 | Go–No-Go | fMRI | 21 | 18–50 | 1. Go/No-Go > Go | Letters | Visual |
| Kelly et al., 2004 | Go–No-Go | fMRI | 15 | 23–40 | 1. Fast and Slow Successful Response Inhibitions | Letters | Visual |
| Kerns et al., 2005 | Stroop | fMRI | 13 | Mean = 36 | 1. Conflict-Related Activity in Normal Subjects | Words | Visual |
| Kerns, 2006 | Simon | fMRI | 26 | 18–36 | 1. Incongruent, Activations | Shapes | Visual |
| Kimmig et al., 2001 | Antisaccade | fMRI | 15 | 20–37 | 1. Prosaccade and Antisaccade | Shapes | Visual |
| Konishi, Nakajima, Uchida, Sekihara, & Miyashita, 1998 | Go–No-Go | fMRI | 5 | 20–31 | 1. No-Go Dominant Foci | Shapes | Visual |
| Konishi et al., 1999 | Go–No-Go | fMRI | 6 | 20–31 | 1. No-Go Dominant Area | Shapes | Visual |
| Kronhaus et al., 2006 | Stroop | fMRI | 11 | Mean = 36 | 1. Incongruent Stroop > Letter String, Healthy Controls | Words, Letters | Visual |
| Lee, Dolan, & Critchley, 2008 | Simon | fMRI | 14 | Mean = 24 | 1. Activation Associated with Interference Effect of the Simon Task | Film, Words | Visual, Auditory |
| Leung, Skudlarski, Gatenby, Peterson, & Gore, 2000 | Stroop | fMRI | 19 | 20–45 | 1. Stroop Positive | Words | Visual |
| Liddle, Kiehl, & Smith, 2001 | Go–No-Go | fMRI | 16 | Mean = 30 | 1. Correct No-Go – Go | Letters | Visual |
| Liu, Banich, Jacobson, & Tanabe, 2004 | Simon & Stroop | fMRI | 11 | 24–40 | 1. Simon Incongruent > Simon Congruent | Shapes | Visual |
| 2. Stroop Incongruent > Stroop Congruent | |||||||
| MacDonald et al., 2000 | Stroop | fMRI | 12 | 18–30 | 1. Color, Incongruent > Color, Congruent | Words | Visual |
| Maclin, Gratton, & Fabiani, 2001 | Simon | fMRI | 8 | 18–47 | 1. Incongruent > Congruent | Shapes | Visual |
| Maguire et al., 2003 | Go–No-Go | fMRI | 6 | 22–30 | 1. Go/No-Go vs. Go | Shapes | Visual |
| Maltby, Tolin, Worhunsky, O’Keefe, & Kiehl, 2005 | Go–No-Go | fMRI | 14 | Mean = 37 | 1. Correct Inhibition, Normals | Letters | Visual |
| Matsuda et al., 2004 | Antisaccade | fMRI | 21 | Mean = 39 | 1. Antisaccades > Saccades | Shapes | Visual |
| Mead et al., 2002 | Stroop | fMRI | 18 | 18–46 | 1. Incongruent > Congruent | Words | Visual |
| 2. Incongruent > Neutral | |||||||
| Menon, Adelman, White, Glover, & Reiss, 2001 | Go–No-Go | fMRI | 14 | 17–41 | 1. Go/No-Go – Go | Letters | Visual |
| Milham et al., 2001 | Stroop | fMRI | 16 | 18–30 | 1. Incongruent > Neutral | Words | Visual |
| Milham et al., 2002 | Stroop | fMRI | 22 | 21–27 | 1. Incongruent > Congruent or Neutral, Young Subjects | Words | Visual |
| Milham & Banich, 2005 | Stroop | fMRI | 18 | 18–40 | 1. Incongruent vs. Congruent | Words | Visual |
| Mostofsky et al., 2003 | Go–No-Go | fMRI | 48 | Mean = 27 | 1. Primary No-Go Effects | Pictures (Spaceships) | Visual |
| 2. Primary Counting No-Go Effects | |||||||
| Norris, Zysset, Mildner, & Wiggins, 2002 | Stroop | fMRI | 7 | 23–31 | 1. Incongruent vs. Neutral, GE-EPI, Activations | Words, Letters | Visual |
| O’Driscoll et al., 1995 | Antisaccade | PET | 10 | 22–39 | 1. Antisaccade – Saccade | Shapes | Visual |
| Paus, Petrides, Evans, & Meyer, 1993 | Antisaccade | PET | 9 | 19–30 | 1. Oculomotor, Antistimulus – Prostimulus | Shapes | Visual |
| Peterson et al., 2002 | Simon & Stroop | fMRI | 10 | 24–29 | 1. Simon Incongruent vs. Congruent | 1. Shapes | Visual |
| 2. Stroop Incongruent vs. Congruent | 2. Words | ||||||
| Roth et al., 2006 | Stroop | fMRI | 11 | 18–55 | 1. Incongruent > Congruent, Normals | Words | Visual |
| Roth et al., 2007 | Go–No Go | fMRI | 14 | Mean = 38 | 1. Response Inhibition, Normals | Shapes | Visual |
| Rubia et al., 2001 | Go–No-Go | fMRI | 15 | 26–58 | 1. Generic Go/No-Go Activation | Pictures (Planes, Airplanes Bombs) | Visual |
| 2. Generic Stop Activation | |||||||
| Rubia et al., 2006 | Go–No-Go & Simon | fMRI | 52 | 10–43 | 1. Go/No-Go Task, Adults | Shapes | Visual |
| 2. Simon Task, Adults | |||||||
| Sommer, Hajak, Döhnel, Meinhardt, & Müller, 2008 | Simon | fMRI | 12 | 22–37 | 1. Incompatible > Compatible | Letters | Visual |
| Sweeney et al., 1996 | Antisaccade | PET | 11 | Mean = 27 | 1. Antisaccades – Visually Guided Saccades, Increases | Shapes | Visual |
| 2. Conditional Antisaccades – Visually Guided Saccades, Increases | |||||||
| Tang, Critchley, Glaser, Dolan, & Butterworth, 2006 | Stroop | fMRI | 18 | 21–38 | 1. Numerical Task Conflict Trials > Numerical Task Nonconflict Trials | Numbers | Visual |
| 2. Physical Task Conflict Trials > Physical Task Nonconflict Trials | |||||||
| Taylor, Kornblum, Lauber, Minoshima, & Koeppe, 1997 | Stroop | PET | 18 | Under age 30 | 1. Stroop – Neutral Words | Words | Visual |
| Ullsperger & von Cramon, 2001 | Flanker | fMRI | 12 | 21–29 | 1. Response Competition (Incompatible Correct vs. Compatible Correct) | Shapes | Visual |
| van Veen, Cohen, Botvinick, Stenger, & Carter, 2001 | Flanker | fMRI | 12 | Mean = 27 | 1. (Congruent = Stimulus Incongruent) < Response Incongruent | Letters | Visual |
| 2. Congruent < Stimulus Incongruent < Response Incongruent | |||||||
| Vink et al., 2005 | Go–No-Go | fMRI | 20 | Mean = 20 | 1. Go/Stop > Go Only | Shapes | Visual |
| 2. Parametric Analysis | |||||||
| Watanabe et al., 2002 | Go–No-Go | fMRI | 11 | 19–40 | 1. Specific Activation Areas During NO-GO Phase | Shapes | Visual |
| Wittfoth, Buck, Fahle, & Herrmann, 2006 | Simon | fMRI | 20 | 21–31 | 1. Motion-Based: Incompatible > Compatible | Shapes | Visual |
| 2. Location-Based: Incompatible > Compatible | |||||||
| Wittfoth, Kustermann, Fahle, & Herrmann, 2008 | Simon | fMRI | 15 | 21–31 | 1. Incompatible > Compatible | Shapes | Visual |
| Yücel et al., 2007 | Flanker | fMRI | 19 | Mean = 31 | 1. Incongruent > Congruent, Normals | Numbers | Visual |
| Zysset, Muller, Lohmann, & von Cramon, 2001 | Stroop | fMRI | 9 | 21–34 | 1. Incongruent vs. Neutral | 1. Words, Letters | Visual |
| 2. Incongruent vs. Congruent | 2. Words | ||||||
| WORKING MEMORY | |||||||
| Audoin et al., 2005 | Complex Calculation | fMRI | 18 | 19–40 | 1. PASAT – REPEAT, Controls | Numbers | Auditory |
| Awh et al., 1996 | Sternberg & N-back | PET | 20 | 18–27 | 1. Sternberg Item Recognition Memory – Control | Letters | Visual |
| 2. 2-Back – Search Control | |||||||
| Barch et al., 2001 | N-back | fMRI | 12 | Mean = 25 | 1. Main Effect of Delay | Letters | Visual |
| Bedwell et al., 2005 | Sternberg | fMRI | 14 | 22–40 | 1. Brain regions significantly active during encoding period | Letters | Visual |
| 2. Brain regions significantly active during retrieval period | |||||||
| Braver et al., 1997 | N-back | fMRI | 8 | 18–25 | 1. Brain Areas Showing Monotonic Increases in Activity as a Function of Memory Load | Letters | Visual |
| Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001 | Sternberg | fMRI | 16 | 18–40 | 1. Load 6 > Load 4 | Letters | Visual |
| Cairo, Liddle, Woodward, & Ngan, 2004 | Sternberg | fMRI | 18 | 18–35 | 1. Encoding, Linear Regression with Load | Letters | Visual |
| 2. Retrieval, Linear Regression with Load | |||||||
| Callicott et al., 1999 | N-back | fMRI | 9 | 18–39 | 1. Significant Increases in Activation as a Function of Working Memory Load | Numbers | Visual |
| Carlson et al., 1998 | N-back | fMRI | 7 | 17–23 | 1. Two-Back vs. Zero-Back | Shapes | Visual |
| 2. One-Back vs. Zero-Back | |||||||
| 3. Two-Back vs. One-Back | |||||||
| Casey et al., 1998 | N-back | fMRI | 32 | 19–43 | 1. Memory – Motor, Pooled Data | Shapes | Visual |
| Chen et al., 2004 | Delayed Match to Sample | fMRI | 8 | Mean = 28 | 1. Verbal Working Memory | 1. Words | 1. Visual |
| 2. Visual Abstract Working Memory | 2. Pictures (Abstract Patterns) | 2. Shapes | |||||
| Clark et al., 2000 | N-back | PET | 10 | Mean = 47 | 1. Variable Target > Fixed Target | Words | Visual |
| Cohen et al., 1994 | N-back | fMRI | 12 | 20–29 | 1. Memory – Control | Letters | Visual |
| Cohen et al., 1997 | N-back | fMRI | 10 | 18–34 | 1. Load | Letters | Visual |
| 2. Load × Time | |||||||
| Crespo-Facorro et al., 2001 | Delayed Match to Sample | PET | 34 | Mean = 26 | 1. Novel – Well-learned, Normals | Shapes | Visual |
| Dade, Zatorre, Evans, & Jones-Gorman, 2001 | N-back | PET | 12 | 20–30 | 1. Odor Working Memory – Baseline | 1. Scent | 1. Ofactory, |
| 2. Face Working Memory – Baseline | 2. Faces | 2. Visual | |||||
| Delazer et al., 2003 | Complex Calculation | fMRI | 13 | Mean = 31 | 1. Untrained vs. Trained Multiplication Set | Numbers | Visual |
| Dolcos & McCarthy, 2006 | Delayed Match to Sample | fMRI | 15 | 18–31 | 1. Neutral – Scrambled Pictures | Faces | Visual |
| Druzgal & D’Esposito, 2001a | N-back | fMRI | 9 | 21–27 | 1. Match > No Match | Faces | Visual |
| Druzgal & D’Esposito, 2001b | Sternberg | fMRI | 9 | 22–27 | 1. Working Memory Load | Faces | Visual |
| Fehr, Code, & Hermann, 2007 | Complex Calculation | fMRI | 11 | 22–40 | 1. Addition, Complex > Simple | Numbers | Visual |
| 2. Subtraction, Complex > Simple | |||||||
| 3. Multiplication, Complex > Simple | |||||||
| 4. Division, Complex > Simple | |||||||
| 5. Conjunction (All Conditions) | |||||||
| Garavan, Kelley, Rosen, Rao, & Stein, 2000 | Delayed Match to Sample | fMRI | 17 | Mean = 26.6 | 1. Visual working memory vs. Control | Shapes | Visual |
| Garavan, Ross, Li, & Stein, 2000 | Complex Calculation | fMRI | 11 | 19–41 | 1. Function of Switching Frequency | Shapes | Visual |
| Ghatan, Hsieh, Petersson, Stone-Elander, & Ingvar, 1998 | Complex Calculation | PET | 6 | 20–24 | 1. Irrelevant Speech + Arithmetical Task vs. Arithmetical Task (Increase in rCBF) | Words & Digits | Auditory & Visual |
| Harvey et al., 2005 | N-back | fMRI | 10 | 18–45 | 1. n-Back vs. 0-Back, Healthy Subjects | Letters | Visual |
| Honey et al., 2003 | N-back | fMRI | 27 | Mean = 35 | 1. Working Memory, Normals | Letters | Visual |
| Hugdahl et al., 2004 | Complex Calculation | fMRI | 12 | Mean = 31 | 1. Mental Arithmetic – Vigilance, Healthy Subjects | Numbers | Visual |
| Ischebeck et al., 2006 | Complex Calculation | fMRI | 12 | Mean = 27 | 1. Multiplication Untrained vs. Number Matching | Numbers | Visual |
| 2. Subtraction Untrained vs. Number Matching | |||||||
| Johnson et al., 2006 | Sternberg | fMRI | 18 | Mean = 37 | 1. Controls, Activation Modulated by Load, Encoding | Letters | Visual |
| 2. Controls, Activation Modulated by Load, Retrieval | |||||||
| 3. Controls, Difficult 6 > Medium 6, Encoding | |||||||
| 4. Controls, Difficult 6 > Medium 6, Retrieval | |||||||
| Jonides et al., 1997 | N-back | PET | 19 | College age | 1. 3-back minus Control, Activations | Letters | Visual |
| 2. 2-back minus Control, Activations | |||||||
| 3. 1-back minus Control, Activations | |||||||
| 4. 0-back minus Control, Activations | |||||||
| Kim et al., 2002 | N-back | PET | 14 | Mean = 25 | 1. Simple Pictures – Control | 1. Shapes | Visual |
| 2. Korean Words – Control | 2. Words | ||||||
| Kim et al., 2003 | N-back | fMRI | 12 | 19–35 | 1. 2-Back – Control, Normals | Shapes | Visual |
| Kirschen, Chen, Schraedley-Desmond, & Desmond, 2005 | Sternberg | fMRI | 16 | Mean = 25 | 1. Linear Activations (effect of increasing load) | Letters | Visual |
| Kumari et al., 2006 | N-back | fMRI | 13 | 18–55 | 1. 1 Back > 0 Back, Normals | Shapes | Visual |
| 2. 2 Back > 0 Back, Normals | |||||||
| LaBar, Gitelman, Parrish, & Mesulam, 1999 | N-back | fMRI | 11 | Mean = 33 | 1. Working Memory | Letters | Visual |
| Lagopoulos, Ivanovski, & Malhi, 2007 | Sternberg | fMRI | 10 | 20–54 | 1. Encoding, Healthy Controls | Words | Visual |
| 2. Response, Healthy Controls | |||||||
| Landau, Schumacher, Garavan, Druzgal, & D’Esposito, 2004 | Delayed Match to Sample | fMRI | 10 | 22–27 | 1. Encoding: Main Effect Only | Faces | Visual |
| 2. Retrieval: Main Effect Only | |||||||
| Lange et al., 2005 | Complex Calculation | fMRI | 44 | 21–45 | 1. mPASAT vs. Auditory Monitoring using a random effects model, Controls | Numbers | Auditory |
| Lazeron, Rombouts, de Sonneville, Barkhof, & Scheltens, 2003 | Complex Calculation | fMRI | 9 | 19–30 | 1. High (rapid math) vs. Low (slow simple math) | Numbers | Visual |
| Leung, Gore, & Goldman-Rakic, 2002 | Delayed Match to Sample | fMRI | 6 | Mean = 28 | 1. Late Delay | Shapes | Visual |
| 2. Main Task Effects | |||||||
| 3. Time Effects | |||||||
| 4. Interaction Between Task and Time | |||||||
| Linden et al., 2003 | Sternberg | fMRI | 12 | 24–31 | 1. Encoding | Shapes | Visual |
| MacDonald & Carter, 2003 | N-back | fMRI | 17 | Mean = 34 | 1. Cue by Scan Interaction, Normals | Letters | Visual |
| MacDonald et al., 2005 | N-back | fMRI | 28 | Mean = 25 | 1. Nontarget vs. Target, Normals | Letters | Visual |
| 2. Long vs. Short Delay, Normals | |||||||
| Manoach et al., 2000 | Delayed Match to Sample | fMRI | 9 | 28–49 | 1. 5 Targets WM vs. Arrows, Normals | Numbers, Shapes | Visual |
| Martinkauppi, Rama, Aronen, Korvenoja, & Carlson, 2000 | N-back | fMRI | 10 | 20–30 | 1. 3-Back vs. 1-Back | Tones | Auditory |
| 2. 2-Back vs. 1-Back | |||||||
| Matsuo et al., 2007 | N-back | fMRI | 15 | Mean = 38 | 1. 1-back > 0-back, Normals | Numbers | Visual |
| 2. 2-back > 0-back, Normals | |||||||
| Mayer et al., 2007 | Delayed Match to Sample | fMRI | 18 | 20–44 | 1. Working Memory Selective, WM Load | Shapes | Visual |
| Mendrek et al., 2005 | N-back | fMRI | 12 | Mean = 28 | 1. Activations, 2-Back vs. 0-Back, Normals | Letters | Visual |
| Menon, Anagnoson, Mathalon, Glover, & Pfefferbaum, 2001 | N-back | fMRI | 13 | 37–49 | 1. Average Group Activation For Controls | Numbers | Auditory |
| Monks et al., 2004 | N-back & Sternberg | fMRI | 12 | Mean = 46 | 1. 2-Back vs. Baseline, Controls | 1. Letters | Visual |
| 2. Sternberg, Controls | 2. Numbers | ||||||
| Owen et al., 1998 | N-back | fMRI | 6 | College age | 1. Spatial Working Memory vs. Control | 1. Shapes | Visual |
| 2. Nonspatial Working Memory vs. Control | 2. Pictures (Abstract Patterns) | ||||||
| Owen et al., 1999 | N-back & Sequencing | PET | 5 | 44–55 | 1. Spatial Manipulation – Visuomotor Control | Shapes | Visual |
| 2. Spatial Span – Visuomotor Control | |||||||
| Perlstein, Dixit, Carter, Noll, & Cohen, 2003 | N-back | fMRI | 15 | 26–47 | 1. N-Back Load Main Effect | Letters | Visual |
| 2. AX-CPT Cue Type Main Effect | |||||||
| Petit, Courtney, Ungerleider, & Haxby, 1998 | Delayed Match to Sample | fMRI | 12 | Mean = 28 | 1. Spatial Working Memory | Faces | Visual |
| 2. Face Working Memory | |||||||
| Petrides, Alivisatos, Meyer, & Evans, 1993 | Complex Calculation | PET | 10 | 19–39 | 1. Self-Ordered – Counting | Numbers | Auditory |
| 2. Externally Ordered – Counting | |||||||
| Pochon et al., 2001 | N-back & Sequencing | fMRI | 8 | 20–25 | 1. Visuospatial matching (MAT) vs. Control (MAT CONT) | Shapes | Visual |
| 2. Visuospatial reproduction (REP) vs. Control (REP CONT) | |||||||
| Ragland et al., 2002 | N-back | fMRI | 11 | 21–53 | 1. Letter 1-Back – 0-Back | 1. Letters | Visual |
| 2. Fractal 1-Back – 0-Back | 2. Shapes (Fractals) | ||||||
| 3. Letter 2-Back – 0-Back | 3. Letters | ||||||
| 4. Fractal 2-Back – 0-Back | 4. Shapes | ||||||
| 5. Letter 2-Back – 1-Back | 5. Letters | ||||||
| 6. Fractal 2-Back – 1-Back | 6. Shapes (Fractals) | ||||||
| Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000 | Delayed Match to Sample | fMRI | 6 | 24–34 | 1. Working Memory Maintenance | Shapes | Visual |
| 2. Selection from Memory | |||||||
| Rypma, Prabhakaran, Desmond, Glover, & Gabrieli, 1999 | Sternberg | fMRI | 6 | Mean = 25 | 1. 3–1 Contrast | Letters | Visual |
| 2. 6–1 Contrast | |||||||
| Rypma, Prabhakaran, Desmond, & Gabrieli, 2001 | Sternberg | fMRI | 12 | 22–29 | 1. Sternberg, Load 6 vs. Load 1, Young Subjects | Letters | Visual |
| Sanchez-Carrion et al., 2008 | N-back | fMRI | 14 | Mean = 24 | 1. 2-back vs. 0-back, Normals | Numbers | Visual |
| 2. 3-back vs. 0-back, Normals | |||||||
| Schumacher et al., 1996 | N-back | PET | 8 | Under age 60 | 1. Visual Memory – Control, Increases | 1. Letters | 1. Visual |
| 2. Auditory Memory – Control, Increases | 2. Letters | 2. Auditory | |||||
| Sheridan, Hinshaw, & D’Esposito, 2007 | Delayed Match to Sample | fMRI | 10 | 12–17 | 1. Encoding, High Load > Low Load, Normals | Letters | Visual |
| Smith, Jonides, & Koeppe, 1996 | N-back & Sternberg | PET | 30 | College age | 1. Verbal 3-Back – Control | 1. Letters | Visual |
| 2. Spatial 3-Back – Control | 2. Letters | ||||||
| 3. Verbal 2-Back – Control | 3. Letters | ||||||
| 4. Verbal Memory – Control | 4. Letters, Shapes | ||||||
| 5. Spatial Memory – Control | 5. Letters, Shapes | ||||||
| Stern et al., 2000 | Delayed Match to | fMRI | 5 | Missing | 1. Working Memory I vs. Control | Pictures (Abstract Patterns) | Visual |
| Sample | 2. Working Memory II vs. Control | ||||||
| 3. Working Memory I vs. Working Memory II | |||||||
| van der Wee et al., 2003 | N-back | fMRI | 11 | Mean = 35 | 1. 1+2 + 3-Back vs. 0-Back, Normals | Shapes | Visual |
| Veltman, Rombouts, & Dolan, 2003 | N-back & Sternberg | fMRI | 22 | Mean = 23 | 1. n-Back vs. Control | Letters | Visual |
| 2. Sternberg vs. Control | |||||||
| Volle et al., 2005 | Sequencing | fMRI | 11 | 22–34 | 1. MemG Delay One, 3 Square vs. 1 Square | Shapes | Visual |
| 2. MemG Delay One, 5 Square vs. 3 Square | |||||||
| 3. MemG Delay Two vs. VisG Delay Two | |||||||
| Walter, Wolf, Spitzer, & Vasic, 2007 | Sternberg | fMRI | 17 | Mean = 31 | 1. Load 1 > Simple Reaction, Normals | Letters | Visual |
| 2. Load 2 > Simple Reaction, Normals | |||||||
| 3. Load 3 > Simple Reaction, Normals | |||||||
| Yoo et al., 2005 | N-back | fMRI | 10 | 20–30 | 1. Working Memory, Normals | Faces, Abstract Patterns | Visual |
| Zago et al., 2001 | Complex Calculation | PET | 6 | Mean = 21 | 1. Compute (complex math) vs. Read | Numbers | Visual |
| Zurowski et al., 2002 | N-back | PET | 8 | Mean = 27 | 1. Main Effect of Working Memory | Syllables | Visual |
| INITIATION | |||||||
| Audenaert et al., 2000 | Word Generation | fMRI | 20 | 19–28 | 1. Letter Fluency vs. Control | 1. Letters, Words | Auditory |
| 2. Category Fluency vs. Control | 2. Words | ||||||
| Basho, Palmer, Rubio, Wulfeck, & Muller, 2007 | Word Generation | fMRI | 12 | 21–37 | 1. Factor-Specific Effects for Overt | Words | Auditory, Visual |
| Crosson et al., 1999 | Word Generation | fMRI | 17 | 28–32 | 1. Emotionally Neutral words – Repetition | Words | Auditory |
| Frith, Friston, Liddle, & Frackowiak, 1991 | Word Generation | PET | 6 | 25–45 | 1. Fixed Words, Generate – Random Words, Repeat | Words | Auditory |
| Fu et al., 2002 | Word Generation | fMRI | 11 | Mean = 30 | 1. Easy Letter Fluency vs. Repetition | Letters | Visual |
| 2. Difficult Letter Fluency vs. Repetition | |||||||
| Klein, Milner, Zatorre, Meyer, & Evans, 1995 | Word Generation | PET | 12 | Mean = 22 | 1. L1 Synonym Generation – L1 Word Repeating | Words | Auditory |
| Klein, Milner, Zatorre, Zhao, & Nikelski, 1999 | Word Generation | PET | 13 | 18–28 | 1. Verb Generation minus Word Repetition (Chinese words) | Words | Auditory |
| Petersen, Fox, Posner, Mintun, & Raichle, 1988 | Word Generation | PET | 17 | Under age 40 | 1. Generate Words – Repeat Words, Visual | Words | |
| 2. Generate Words – Repeat Words, Auditory | |||||||
| Petersen, Fox, Posner, Mintun, & Raichle, 1989 | Word Generation | PET | 7 | 18–49 | 1. Generate Verbs, Visual vs. Repeat Words, Visual | Words | 1. Visual |
| 2. Generate Verbs, Auditory vs. Repeat Words, Auditory | 2. Auditory | ||||||
| PLANNING | |||||||
| Fincham, Carter, van Veen, Stenger, & Anderson, 2002 | Tower Test | fMRI | 8 | 18–32 | 1. Planning (Tower vs. control) | Numbers | Visual |
| P. B. Fitzgerald et al., 2008 | Tower Test | fMRI | 13 | Mean = 35 | 1. Regions Correlated with Reaction Time During TOL Task in Controls | Shapes | Visual |
| Ghatan et al., 1995 | Maze Task | PET | 8 | 41–59 | 1. Perceptual Maze vs. Motor Control, Increase in rCBF | Shapes | Visual |
| van den Heuvel et al., 2005 | Tower Test | fMRI | 22 | Mean = 30 | 1. Planning (Tower) vs. Counting, Normals | Shapes | Visual |
| 2. Increases Correlating With Increased Task Load, Normals | |||||||
| VIGILANCE | |||||||
| Gur et al., 2007 | Oddball | fMRI | 36 | 18–48 | 1. Target Green Circles > Standard Red Circles | Shapes | Visual |
| Laurens, Kiehl, Ngan, & Liddle, 2005 | Oddball | fMRI | 28 | Mean = 28 | 1. Novel Stimuli vs. Nontarget Stimuli, Controls | Tones | Auditory |
WCST, Wisconsin Card Sort task.
Results
Global analysis across all domains
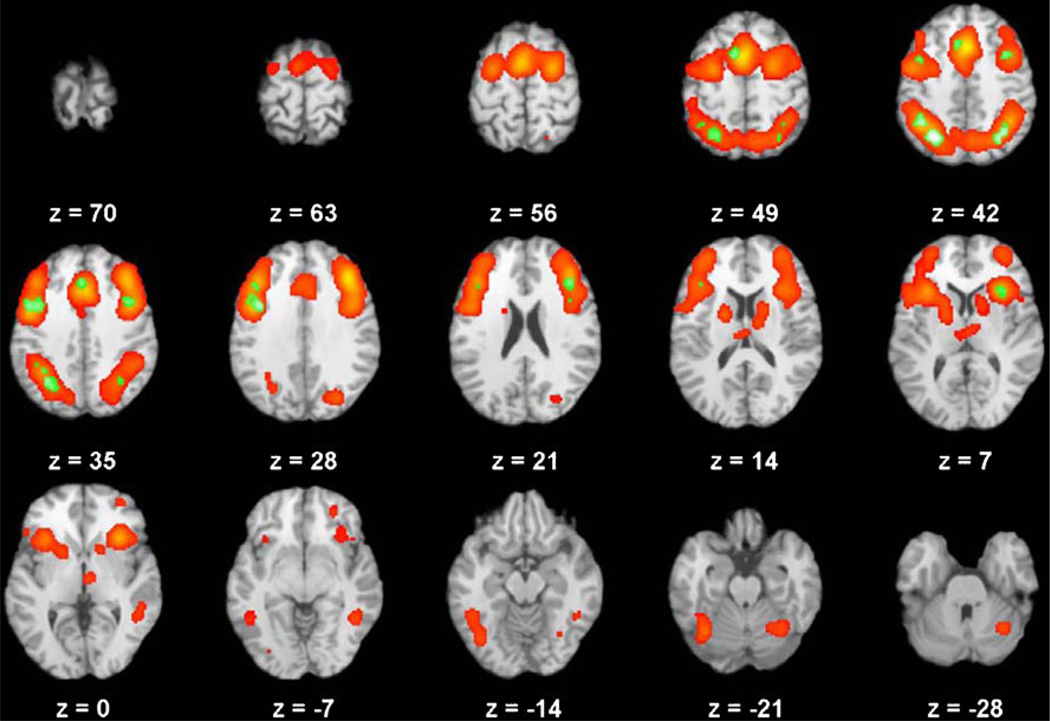
Across all domains (shown in red in Fig. 1; see also Table 3a), large clusters of significant activation were observed within lateral and medial PFC bilaterally, encompassing superior, middle, and inferior frontal gyri including the DLPFC (Brodmann areas [BAs] 9, 46), as well as the ACC (BA 32) on the medial wall. In addition to prefrontal activation, the overall contrast revealed large parietal clusters, including the inferior (BA 40) and superior (BA 7) parietal lobe. This combined frontal–parietal activation is consistent with previous findings related to the cognitive control circuit (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Carter, Botvinick, & Cohen, 1999; Cohen, Botvinick, & Carter, 2000; Yarkoni et al., 2005). Additional activation in frontal regions included the premotor cortex (BA 6), frontopolar cortex (BA 10), and orbitofrontal cortex (BA 11). Activation was also observed in occipital (BA 19) and temporal (BAs 13, 22, 37) regions, which are consistent with processing of the verbal and auditory stimuli, respectively, that are presented as part of the included tasks. Finally, significant activation was found in subcortical structures, including the thalamus, caudate, and putamen, as well as areas of the cerebellum, including the posterior declive and anterior culmen. These findings are consistent with the hypothesis that executive functions are supported by a common set of cortical and subcortical regions within the cognitive control network.
Fig. 1.
Global analysis of executive function in 193 studies of healthy adults, showing brain regions with significant activation across all executive function domains (red) and the areas of conjunction (green) across the three domains for which data from more than nine studies were available (flexibility, inhibition, and working memory).
Table 3.
Brain regions (Brodmann areas in parentheses) with significant activation within healthy adults from (a) a combined meta-analysis across all six executive function domains and (b) a conjunction meta-analysis for domains with more than nine included studies (flexibility, inhibition, and working memory)
| Maxima |
||||
|---|---|---|---|---|
| Brain Region (BA) | Volume (mm3) | x | y | z |
| (a) Combined Across All Six Domains | ||||
| Right Middle Frontal Gyrus (9) | 20,048 | 40 | 30 | 28 |
| Right Insula (13) | 32 | 18 | 4 | |
| Right Middle Frontal Gyrus (10) | 32 | 48 | 14 | |
| Right Inferior Parietal Lobule (40) | 12,328 | 38 | −50 | 42 |
| Right Superior Parietal Lobule (7) | 32 | −60 | 42 | |
| Right Cuneus (19) | 28 | −76 | 28 | |
| Left Superior Parietal Lobule (7) | 11,200 | −28 | −60 | 44 |
| Right Precuneus (7) | 8 | −68 | 46 | |
| Left Precuneus (7) | −6 | −62 | 44 | |
| Left Superior Frontal Gyrus (6) | 9,112 | −2 | 6 | 50 |
| Left Insula (13) | 6,744 | −32 | 18 | 6 |
| Left Cerebellar Declive | 4,592 | −34 | −62 | −20 |
| Left Fusiform Gyrus (37) | −46 | −50 | −12 | |
| Left Middle Frontal Gyrus (10) | 3,608 | −36 | 44 | 18 |
| Right Frontal Lobe Subgyral (6) | 3,032 | 26 | −2 | 54 |
| Right Caudate Body | 2,984 | 16 | 2 | 12 |
| Right Thalamus | 12 | −8 | 14 | |
| Right Thalamus | 6 | −16 | 2 | |
| Left Inferior Frontal Gyrus (9) | 2,776 | −42 | 4 | 30 |
| Left Middle Frontal Gyrus (9) | 2,480 | −40 | 26 | 28 |
| Right Cerebellar Culmen | 2,352 | 32 | −60 | −24 |
| Left Middle Frontal Gyrus (6) | 1,936 | −28 | −4 | 50 |
| Right Temporal Lobe Subgyral (37) | 1,912 | 46 | −52 | −6 |
| Right Middle Temporal Gyrus (22) | 50 | −42 | 2 | |
| Right Inferior Frontal Gyrus (9) | 1,080 | 44 | 6 | 32 |
| Left Lentiform Nucleus Putamen | 1,016 | −20 | 8 | 4 |
| Left Inferior Parietal Lobule (40) | 864 | −38 | −52 | 40 |
| Right Caudate Head | 808 | 14 | 10 | 4 |
| Right Cingulate Gyrus (32) | 704 | 2 | 16 | 40 |
| Left Thalamus | 296 | −2 | −20 | 10 |
| Right Middle Frontal Gyrus (11) | 224 | 26 | 42 | −10 |
| Left Fusiform Gyrus (19) | 128 | −28 | −80 | −12 |
| Left Lentiform Nucleus Putamen | 128 | −18 | −2 | 12 |
| (b) Conjunction Analysis | ||||
| (Flexibility, Inhibition, and Working Memory) | ||||
| Left Superior Parietal Lobule (7) | 1,896 | −26 | −64 | 40 |
| Left Inferior Frontal Gyrus (9) | 1,880 | −38 | 6 | 28 |
| Left Middle Frontal Gyrus (9) | −48 | 6 | 36 | |
| Left Middle Frontal Gyrus (9) | −46 | 14 | 28 | |
| Left Middle Frontal Gyrus (9) | −42 | 22 | 28 | |
| Right Inferior Parietal Lobule (39) | 856 | 34 | −62 | 40 |
| Right Precuneus (19) | 30 | −66 | 44 | |
| Right Middle Frontal Gyrus (6) | 576 | 34 | 8 | 42 |
| Right Precentral Gyrus (9) | 40 | 8 | 36 | |
| Left Inferior Parietal Lobule (40) | 568 | −38 | −52 | 44 |
| Left Superior Frontal Gyrus (6) | 528 | −8 | 10 | 48 |
| Left Cingulate Gyrus (32) | −6 | 18 | 42 | |
| Right Middle Frontal Gyrus (46) | 432 | 40 | 26 | 22 |
Results of the conjunction analysis (shown in green in Fig. 1; see also Table 3b) across the three domains for which the data from more than nine studies were available (flexibility, inhibition, and working memory) revealed similar patterns of common activation in cognitive-control-related frontal and parietal regions, including the DLPFC (BAs 9, 46), anterior cingulate (BA 32), inferior (BAs 39, 40) and superior (BA 7) parietal lobe, and precuneus (BA 19). The results of these analyses can be examined through an interactive viewer at http://carterlab.ucdavis.edu/research/ale_analysis.php.
Domain-specific within-group analysis
Flexibility
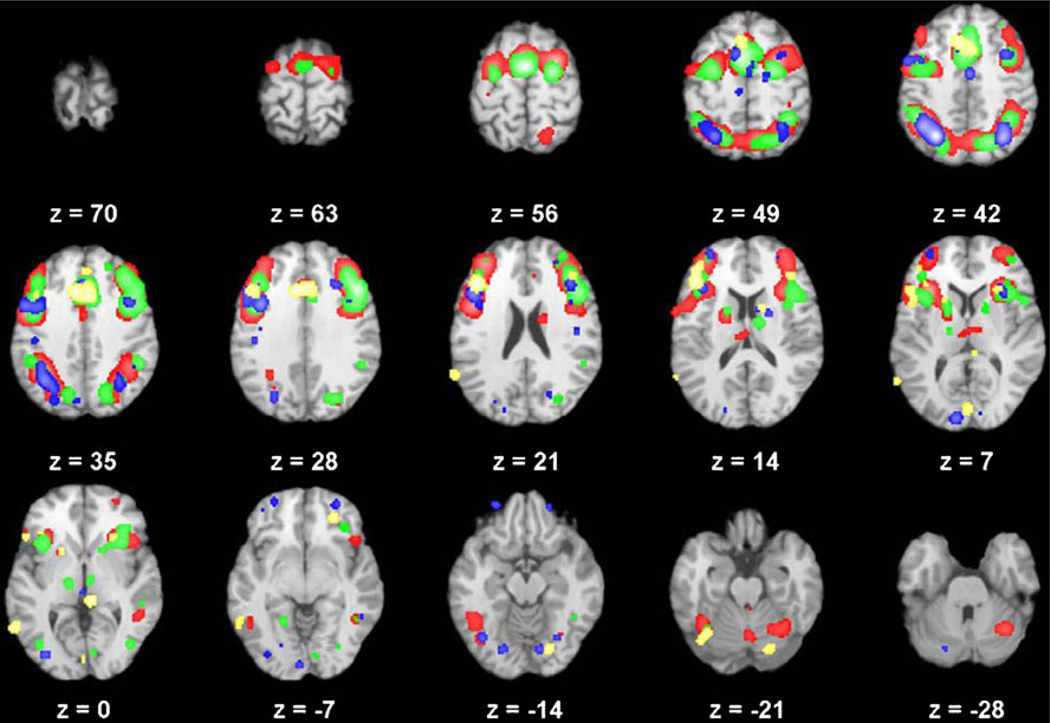
For tasks that examined flexibility, similar patterns of activation were observed in frontal and parietal regions supporting the cognitive control network (see Fig. 2 and Table 4), including the DLPFC (BAs 9, 46), cingulate (BAs 32, 24), as well as superior (BA 7) and inferior (BA 40) parietal lobe. Activation was also observed in additional prefrontal (BAs 6, 10, 11), occipital (BA 19), and temporal (BAs 13, 37) regions.
Fig. 2.
Domain-specific analysis showing patterns of common and distinct activation across the working memory (red; 78 studies), inhibition (green; 79 studies), flexibility (blue; 21 studies), and initiation (yellow; 9 studies) domains
Table 4.
Brain regions (Brodmann areas in parentheses) with significant activation within healthy adults for tasks within the flexibility domain
| Maxima |
||||
|---|---|---|---|---|
| Brain Region (BA) | Volume (mm3) | x | y | z |
| Left Inferior Frontal Gyrus (9) | 6,472 | −38 | 6 | 28 |
| Left Middle Frontal Gyrus (46) | −46 | 18 | 24 | |
| Left Middle Frontal Gyrus (9) | −50 | 6 | 36 | |
| Left Middle Frontal Gyrus (46) | −42 | 26 | 16 | |
| Left Superior Parietal Lobule (7) | 6,328 | −26 | −62 | 44 |
| Left Inferior Parietal Lobule (40) | −36 | −54 | 42 | |
| Left Precuneus (19) | −26 | −78 | 32 | |
| Right Precuneus (19) | 2,648 | 32 | −64 | 42 |
| Right Middle Frontal Gyrus (6) | 1,176 | 34 | 8 | 44 |
| Right Precentral Gyrus (9) | 40 | 8 | 36 | |
| Right Inferior Frontal Gyrus (9) | 40 | 10 | 24 | |
| Right Middle Frontal Gyrus (46) | 856 | 40 | 26 | 22 |
| Right Middle Frontal Gyrus (9) | 28 | 24 | 30 | |
| Left Superior Frontal Gyrus (6) | 688 | −8 | 10 | 48 |
| Left Cingulate Gyrus (32) | −6 | 18 | 42 | |
| Right Cingulate Gyrus (24) | 584 | 4 | −8 | 44 |
| Right Medial Frontal Gyrus (6) | 6 | 0 | 48 | |
| Left Fusiform Gyrus (19) | 544 | −38 | −68 | −14 |
| Left Cerebellar Declive | −38 | −68 | −18 | |
| Left Cuneus (17) | 544 | −10 | −92 | 8 |
| Left Middle Frontal Gyrus (10) | 464 | −32 | 52 | 10 |
| Right Middle Frontal Gyrus (11) | 408 | 22 | 46 | −12 |
| Right Middle Frontal Gyrus (10) | 28 | 50 | −8 | |
| Left Middle Frontal Gyrus (11) | 408 | −26 | 48 | −12 |
| Left Inferior Occipital Gyrus (18) | 368 | −32 | −82 | −2 |
| Right Insula (13) | 344 | 32 | 18 | 8 |
| Left Cerebellar Declive | 328 | −20 | −78 | −16 |
| Left Cingulate Gyrus (32) | 296 | 0 | 26 | 36 |
| Right Lentiform Nucleus Putamen | 272 | 20 | 0 | 14 |
| Right Caudate Body | 10 | 4 | 16 | |
| Left Postcentral Gyrus (2) | 240 | −42 | −24 | 30 |
| Right Cerebellar Declive | 208 | 12 | −78 | −14 |
| Right Medial Frontal Gyrus (6) | 208 | 18 | −10 | 52 |
| Right Cuneus (18) | 152 | 24 | −76 | 16 |
| Right Precuneus (31) | 22 | −70 | 24 | |
| Left Middle Occipital Gyrus (18) | 144 | −18 | −86 | 16 |
| Left Lingual Gyrus (18) | 136 | −4 | −90 | −8 |
| Right Temporal Lobe Sub-Gyral (37) | 104 | 50 | −48 | −10 |
| Left Paracentral Lobule (6) | 104 | −6 | −24 | 52 |
Inhibition
As is shown in Fig. 2 (see Table 5), tasks that require inhibition were associated with activation in frontal and parietal cognitive-control-related regions, including DLPFC (BAs 9, 46), ACC (BA 32), and superior (BA 7) and inferior (BA 40) parietal lobe. Such tasks also elicited activation in other prefrontal (BAs 6, 10), occipital (BA 19), and temporal (BA 13) regions. Activation of subcortical regions included the caudate, thalamus, putamen, and cerebellar declive.
Table 5.
Brain regions (Brodmann areas in parentheses) with significant activation within healthy adults for tasks within the inhibition domain
| Maxima |
||||
|---|---|---|---|---|
| Brain Region (BA) | Volume (mm3) | x | y | z |
| Right Middle Frontal Gyrus (9) | 20,464 | 46 | 20 | 28 |
| Right Middle Frontal Gyrus (46) | 40 | 32 | 24 | |
| Right Middle Frontal Gyrus (9) | 38 | 28 | 32 | |
| Right Inferior Frontal Gyrus (9) | 46 | 6 | 32 | |
| Right Precentral Gyrus (9) | 38 | 6 | 38 | |
| Right Claustrum | 32 | 16 | 2 | |
| Right Inferior Frontal Gyrus (47) | 34 | 26 | 0 | |
| Right Precentral Gyrus (44) | 50 | 10 | 8 | |
| Right Lentiform Nucleus Putamen | 16 | 2 | 10 | |
| Right Lentiform Nucleus Putamen | 16 | 8 | 2 | |
| Left Medial Frontal Gyrus (6) | 15,872 | 0 | −2 | 56 |
| Left Medial Frontal Gyrus (32) | 0 | 10 | 46 | |
| Left Precentral Gyrus (9) | 13,368 | −42 | 4 | 32 |
| Left Middle Frontal Gyrus (6) | −28 | −4 | 50 | |
| Left Middle Frontal Gyrus (9) | −40 | 28 | 32 | |
| Left Insula (13) | −36 | 12 | 4 | |
| Left Middle Frontal Gyrus (46) | −38 | 30 | 12 | |
| Right Inferior Parietal Lobule (40) | 8,720 | 38 | −48 | 46 |
| Right Precuneus (7) | 18 | −68 | 42 | |
| Right Supramarginal Gyrus (40) | 48 | −44 | 34 | |
| Right Cuneus (7) | 20 | −74 | 32 | |
| Right Cuneus (19) | 28 | –76 | 26 | |
| Right Angular Gyrus (39) | 34 | −60 | 38 | |
| Right Superior Temporal Gyrus (13) | 52 | −44 | 20 | |
| Left Precuneus (19) | 4,160 | –28 | −62 | 38 |
| Left Precuneus (7) | −20 | −70 | 42 | |
| Left Precuneus (7) | –18 | −64 | 48 | |
| Left Precuneus (7) | –12 | –72 | 34 | |
| Right Middle Frontal Gyrus (6) | 3,056 | 24 | −6 | 52 |
| Left Inferior Parietal Lobule (40) | 2,408 | −44 | −44 | 40 |
| Left Lentiform Nucleus Putamen | 912 | −18 | 8 | 4 |
| Left Caudate Body | –16 | 2 | 14 | |
| Left Thalamus Mammillary Body | 520 | −12 | −20 | 0 |
| Right Thalamus | 456 | 12 | −10 | 14 |
| Right Superior Frontal Gyrus (10) | 376 | 34 | 50 | 20 |
| Right Middle Frontal Gyrus (10) | 30 | 42 | 18 | |
| Right Inferior Frontal Gyrus (10) | 304 | 38 | 46 | 4 |
| Right Inferior Occipital Gyrus (19) | 232 | 40 | −72 | 0 |
| Left Inferior Occipital Gyrus (19) | 216 | −38 | −74 | 0 |
| Right Lingual Gyrus (18) | 200 | 10 | −82 | −4 |
| Left Parahippocampal Gyrus (19) | 192 | −18 | −52 | −6 |
| Right Thalamus | 176 | 6 | −18 | 0 |
| Left Cerebellar Declive | 160 | −34 | −62 | −20 |
| Left Middle Frontal Gyrus (10) | 160 | −34 | 46 | 18 |
| Right Superior Frontal Gyrus (9) | 160 | 22 | 40 | 34 |
Working memory
Working memory tasks elicited the common pattern of frontal–parietal activation associated with the cognitive control network (see Fig. 2 and Table 6), including the DLPFC (BAs 9, 46), cingulate (BAs 32, 24), and parietal lobe (BAs 7, 40). A consistent pattern of activation was also observed in prefrontal (BAs 6, 10), occipital (BA 19), temporal (BAs 13, 37), and subcortical (thalamus, caudate, putamen, cerebellar declive) regions.
Table 6.
Brain regions (Brodmann areas in parentheses) with significant activation within healthy adults within the working memory
| Maxima |
||||
|---|---|---|---|---|
| Brain Region (BA) | Volume (mm3) | x | y | z |
| Right Middle Frontal Gyrus (9) | 103,712 | 38 | 30 | 28 |
| Left Superior Frontal Gyrus (6) | −2 | 6 | 52 | |
| Right Frontal Lobe Sub-Gyral (6) | 26 | 2 | 54 | |
| Left Cingulate Gyrus (32) | 0 | 16 | 40 | |
| Right Insula (13) | 32 | 20 | 6 | |
| Left Inferior Frontal Gyrus (9) | −44 | 6 | 26 | |
| Left Precentral Gyrus (6) | −46 | 0 | 36 | |
| Left Insula (13) | −32 | 18 | 6 | |
| Right Inferior Frontal Gyrus (9) | 44 | 6 | 32 | |
| Left Middle Frontal Gyrus (6) | −26 | −4 | 50 | |
| Left Middle Frontal Gyrus (9) | −40 | 28 | 28 | |
| Left Middle Frontal Gyrus (10) | −36 | 42 | 20 | |
| Left Middle Frontal Gyrus (46) | −42 | 16 | 24 | |
| Left Lentiform Nucleus Putamen | −18 | −4 | 12 | |
| Right Inferior Frontal Gyrus (47) | 44 | 18 | −2 | |
| Left Precentral Gyrus (44) | −48 | 14 | 8 | |
| Left Lentiform Nucleus Putamen | −22 | 8 | 4 | |
| Left Middle Frontal Gyrus (10) | −28 | 54 | 4 | |
| Left Cingulate Gyrus (24) | 0 | −2 | 36 | |
| Left Precentral Gyrus (6) | −60 | 2 | 14 | |
| Right Inferior Parietal Lobule (40) | 8,896 | 40 | −50 | 40 |
| Right Cuneus (19) | 28 | −76 | 30 | |
| Right Precuneus (7) | 8,152 | 10 | −66 | 46 |
| Left Cerebellar Declive | 3,320 | −36 | −58 | −20 |
| Left Fusiform Gyrus (37) | −44 | −52 | −14 | |
| Left Cerebellar Declive | −38 | −70 | −14 | |
| Right Cerebellar Tuber | 3,056 | 34 | −60 | −30 |
| Left Thalamus | 864 | −4 | −20 | 12 |
| Right Thalamus | 8 | −14 | 4 | |
| Left Inferior Parietal Lobule (40) | 728 | −38 | −50 | 40 |
| Right Cerebellar Declive | 448 | 2 | −64 | −22 |
| Right Caudate Caudate Body | 264 | 16 | −6 | 20 |
Other domains
Domain-specific analyses for the planning and vigilance domains were not possible, due to the small number of studies available for inclusion within the ALE analysis (four and two studies, respectively). Although the number of studies for the initiation domain was also small (n = 9), the results are presented here as a preliminary analysis of site-specific activation within this domain. In contrast to the pattern of frontal–parietal activation observed in the other three domains, initiation tasks were associated with a pattern of activation primarily in frontal regions, including the DLPFC (BA 46), middle (BA 10) and inferior (BA 47) frontal, anterior cingulate (BA 32), and motor (BA 6) regions, with no observed activation in parietal regions (see Fig. 2, Table 7). Activation was also observed in the superior (BA 21) and middle (BA 22) temporal, occipital (BA 17), and subcortical (putamen, caudate, cerebellar declive and culmen) regions, in a manner similar to other executive domains.
Table 7.
Preliminary data for brain regions (Brodmann areas in parentheses) with significant activation within healthy adults for tasks within the initiation domain
| Maxima |
||||
|---|---|---|---|---|
| Brain Region (BA) | Volume (mm3) | x | y | z |
| Left Cingulate Gyrus (32) | 6,064 | −6 | 18 | 32 |
| Right Cingulate Gyrus (32) | 4 | 14 | 38 | |
| Right Cingulate Gyrus (32) | 2 | 22 | 30 | |
| Left Middle Frontal Gyrus (46) | 4,160 | −42 | 26 | 20 |
| Left Inferior Frontal Gyrus (46) | −42 | 38 | 12 | |
| Left Inferior Frontal Gyrus (45) | −50 | 20 | 4 | |
| Left Precentral Gyrus (44) | −46 | 12 | 8 | |
| Left Middle Temporal Gyrus (21) | 904 | −60 | −58 | 0 |
| Right Cerebellar Declive | 632 | 22 | −76 | −16 |
| Right Cerebellar Culmen | 584 | 6 | −36 | 2 |
| Left Cerebellar Declive | 504 | −34 | −64 | −22 |
| Right Occipital Lobe Lingual Gyrus (17) | 472 | 2 | −84 | 6 |
| Right Middle Frontal Gyrus (46) | 384 | 42 | 32 | 18 |
| Left Superior Temporal Gyrus (22) | 352 | −60 | −56 | 18 |
| Right Claustrum | 344 | 26 | 20 | 2 |
| Right Middle Frontal Gyrus (11) | 312 | 26 | 38 | −6 |
| Left Lentiform Nucleus Putamen | 216 | −20 | 10 | 4 |
| Right Caudate Body | 160 | 14 | 4 | 14 |
| Right Medial Frontal Gyrus (6) | 128 | 2 | 36 | 34 |
| Left Inferior Frontal Gyrus (45) | 120 | −36 | 24 | 4 |
Discussion
Using a meta-analytic approach, we examined 193 neuroimaging studies of tasks divided according to classic executive function domains, creating the largest sample of healthy adults to date. We sought to provide evidence that discrete executive functions (initiation, inhibition, working memory, flexibility, planning, and vigilance) are supported by a shared, superordinate network that has been previously associated with cognitive control. Results of the combined analysis across domains showed that executive functions are indeed associated with increased activity in this common cognitive control network (Bellebaum & Daum, 2007; Botvinick et al., 2001; Carter et al., 1999; Cohen et al., 2000; D’Esposito & Postle, 2002; Yarkoni et al., 2005), which includes the DLPFC (BAs 9, 46), frontopolar cortex (BA 10), orbitofrontal cortex (BA 11), and anterior cingulate (BA 32). Additional concurrent regions of activation included the superior and inferior parietal (BAs 7, 40), occipital (BA 19), and temporal (BAs 13, 22, 37) cortex, as well as subcortical areas including the caudate, putamen, thalamus, and cerebellum. These conclusions were further supported by a conjunction analysis across the three domains in which data from more than nine studies were available (flexibility, inhibition, and working memory), which revealed a similar pattern of common activation in cognitive-control-related frontal and parietal regions. Although the present analysis did not directly examine the functional connectivity of these brain regions during each task, previous studies of cognitive control (Fornito et al., 2011; Yoon et al., 2008) have consistently shown task-related increases in functional connectivity between the DLPFC and the network of brain regions shown here.
These results provide additional evidence that a superordinate cognitive control network supports executive functions across a range of “domains” previously considered to be distinct, including flexibility, working memory, initiation, and inhibition. As proposed by Miller and Cohen (2001), it has been common to stress the distributed nature of the network that supports cognitive control functions, as well as the unique functional contributions by specific regions within the network. Within this framework, elements of the network may be differentially engaged, depending on the task demands. For example, previous studies (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008) have shown that the frontoparietal control network is engaged across multiple goal-directed activities, flexibly engaging the default-mode network to support autobiographical planning, or engaging the dorsal attention network to support visual spatial planning. Similarly, demands for specific goal- or task-context-related activity may be associated with stronger engagement of the PFC, and demands for maintaining information over longer periods of time may lead to more sustained network activity (Dosenbach et al., 2006; Yarkoni, Barch, Gray, Conturo, & Braver, 2009). Its connectivity with sensory and motor regions, including the cerebellum, allows the DLPFC to play a central role in the maintenance of the rules for action, as well as response selection and inhibition (Asaad, Rainer, & Miller, 2000; Bellebaum & Daum, 2007; Watanabe, 1990, 1992). The ACC and related medial frontal regions are considered to support cognitive control by detecting conditions, such as processing conflicts, that indicate the demand for control, which then leads to the engagement of the DLPFC (Egner & Hirsch, 2005; Kerns et al., 2005; MacDonald, Cohen, Stenger, & Carter, 2000). Furthermore, parietal activation is considered to provide the DLPFC with information on stimulus salience and learned stimulus–response pairings, while the DLPFC is thought to support its ability to shift attentional focus according to the demands of the task at hand (Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002; Bunge, Kahn, Wallis, Miller, & Wagner, 2003; Miller & Cohen, 2001; Posner & Petersen, 1990).
Within the cognitive control network, it is likely that network-level subdivisions also exist and may be differentially engaged in the same manner. For example, Dosenbach, Fair, Cohen, Schlaggar, and Petersen (2008) proposed discrete circuits within this broader network that support task-sustained versus transient aspects of control, and that these networks may be differentially engaged across different forms of executive functions. Similarly, Braver, Paxton, Locke, and Barch (2009) emphasized that cognitive control has proactive and reactive elements. Proactive control may also depend more on sustained activity in the cognitive control network and, to the degree that these systems may be segregated, they may be differentially engaged during executive functions. A study of the degree to which systematic differences exist in the engagement of discrete elements (regions or subnetworks) of the cognitive control networks across different executive function domains is beyond the resolution of this meta-analysis, and our understanding of this issue will be informed by future experimental studies, particularly those that include direct measures of intraregion connectivity or network dynamics across task demands.
While the use of quantitative meta-analytic methods allowed us to examine executive functions across a variety of tasks and domains within the largest sample of healthy adults to date, it is important to recognize that these findings are limited by the quality of the data available in the extant literature. Activation likelihood estimation requires the reporting of imaging data in three-dimensional coordinates in a standard brain space. Therefore, this analysis did not include studies in which such data were not reported for relevant contrasts (e.g., a within-subjects contrast related to the primary effect of interest in healthy controls), analyses that focused on particular regions of interest, or studies that reported negative findings, as the ALE method does not allow for the modeling of null results (Li, Chan, McAlonan, & Gong, 2010). Furthermore, the lack of appropriate contrasts, such as contrasting an active task with rest or fixation, reduced the number of studies available for inclusion within each domain. However, the use of an active control condition is essential in order to isolate the cognitive process of interest in subtraction contrasts (Stark & Squire, 2001). Our approach to this analysis integrated findings from both fMRI and O15 PET studies, and the ALE method does not account for the potential influence of the different physiological signals associated with these two methods. Additionally, this method does not account for differences in behavioral performance across tasks or the influence of demographic factors, although the sample was restricted to studies that examined a specific age range (18–60 years). While all available studies within the BrainMap database were considered for this analysis, studies that were not included in the database at the time of the analysis have been omitted. Furthermore, this meta-analytic method does not allow for the weighting of results on the basis of levels of statistical significance or the numbers of activation foci that may have been reported by some studies within this investigation (Li, Chan, McAlonan, & Gong, 2010). Although Gaussian blurring of the coordinates will have tended to remove per-study bias of the peak activation localizations, noise within the data might have influenced the study results (M. G. Berman et al., 2010). Finally, our definition of executive functions was based on a traditional view that is often used in cognitive or neuropsychological research (Lezak, 1995; Luria, 1970; Shallice, 1988), and the use of other definitions might have altered the domains examined.
In conclusion, the present study used the meta-analysis of a very large number of published fMRI data sets to examine whether traditional taxonomies of executive functions purporting discrete modular cognitive domains are supported by a superordinate cognitive control system that is engaged during the performance of a range of executive function tasks. Our results suggest that a frontal–cingulate–parietal–subcortical cognitive control network is consistently recruited across a range of traditional executive function tasks. Further research investigating the contributions of modular (e.g., prefrontal) versus shared elements (e.g., frontal–parietal connectivity) of the cognitive control network will inform our understanding of common and unique patterns of impairment in traditional executive functions that are often associated with various brain disorders. Novel approaches to investigating the function of different component systems using single methodologies (e.g., resting state; Deshpande, Santhanam, & Hu, 2011) or combined methodologies (e.g., EEG and fMRI; Debener et al., 2005) have the potential to elucidated the complex brain dynamics underlying cognitive control. Further studies will be needed to make explicit the precise functional contributions of each individual element of the cognitive control network, as well as to understand the complex interactions between network nodes to support coordinated, goal-directed behavior. Through increased understanding of the function of modular components within this network, along with their anatomical connections and functional interactions, we will be able to more effectively investigate the mechanisms by which aberrant behavior or clinical symptoms may result from dysfunction in individual regions or in their connectivity within the broader network (Menon, 2011). Additional research on the relationship between various imaging modalities (e.g., resting state, task-related fMRI, or diffusion tensor imaging) will also help us to uncover ways in which discrete brain systems interact to support complex cognition and behavior.
Acknowledgments
The authors acknowledge the National Institute of Mental Health for its support via Grants K23MH087708 to T.A.N., R01MH074457 to A.R.L., R01MH078143 and R01MH083824 to D. C.G., and 2R01MH059883 and 1R24MH081807 to C.S.C. The authors also thank the various researchers who responded to inquires about their sample demographics during the course of this analysis.
Footnotes
The authors do not have any conflicts of interest to report in relation to this publication.
Contributor Information
Tara A. Niendam, Email: tniendam@ucdavis.edu, Imaging Research Center, University of California, Davis, 4701 X Street, Suite E, Sacramento, CA 95817, USA.
Angela R. Laird, Research Imaging Institute, University of Texas Health Science Center, San Antonio, TX, USA
Kimberly L. Ray, Research Imaging Institute, University of Texas Health Science Center, San Antonio, TX, USA
Y. Monica Dean, Imaging Research Center, University of California, Davis, 4701 X Street, Suite E, Sacramento, CA 95817, USA.
David C. Glahn, Olin Neuropsychiatric Research Center, Institute of Living, Yale University School of Medicine, New Haven, CT, USA Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Cameron S. Carter, Imaging Research Center, University of California, Davis, 4701 X Street, Suite E, Sacramento, CA 95817, USA
References
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Cohen MS. Blunted activation in orbitofrontal cortex during mania: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. Journal of Neurophysiology. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Audenaert K, Brans B, Van Laere K, Lahorte P, Versijpt J, van Heeringen K, Dierckx R. Verbal fluency as a prefrontal activation probe: A validation study using 99 m Tc-ECD brain SPET. European Journal of Nuclear Medicine. 2000;27:1800–1808. doi: 10.1007/s002590000351. [DOI] [PubMed] [Google Scholar]
- Audoin B, Au Duong MV, Ranjeva JP, Ibarrola D, Malikova I, Confort-Gouny S, Cozzone PJ. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Human Brain Mapping. 2005;24:216–228. doi: 10.1002/hbm.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychological Science. 1996;7:25–31. [Google Scholar]
- Baddeley A, Wilson B. Frontal amnesia and the dysexecutive syndrome. Brain and Cognition. 1988;7:212–230. doi: 10.1016/0278-2626(88)90031-0. [DOI] [PubMed] [Google Scholar]
- Banich M. Neuropsychology: The neural bases of mental function. Boston, MA: Houghton Mifflin; 1997. [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Brown C. Prefrontal regions play a predominant role in imposing an attentional “set”: Evidence from fMRI. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ, Kramer AF. Attentional selection and the processing of task-irrelevant information: Insights from fMRI examinations of the Stroop task. Progress in Brain Research. 2001;134:459–470. doi: 10.1016/s0079-6123(01)34030-x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, III, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, Muller RA. Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and overt speech. Neuropsychologia. 2007;45:1697–1706. doi: 10.1016/j.neuropsychologia.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell JS, Horner MD, Yamanaka K, Li X, Myrick H, Nahas Z, George MS. Functional neuroanatomy of subcomponent cognitive processes involved in verbal working memory. International Journal of Neuroscience. 2005;115:1017–1032. doi: 10.1080/00207450590901530. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hester RL, Garavan H. The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: A positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Berman MG, Park J, Gonzalez R, Polk TA, Gehrke A, Knaffla S, Jonides J. Evaluating functional localizers: The case of the FFA. NeuroImage. 2010;50:56–71. doi: 10.1016/j.neuroimage.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Barch DM. The role of prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brown MRG, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: Rapid event-related fMRI of prosaccades, antisaccades, and no-go trials. NeuroImage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown GG, Kindermann SS, Siegle GJ, Granholm E, Wong EC, Buxton RB. Brain activation and pupil response during covert performance of the Stroop color word task. Journal of the International Neuropsychological Society. 1999;5:308–319. doi: 10.1017/s1355617799544020. [DOI] [PubMed] [Google Scholar]
- Brown MRG, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: An interference task specialized for functional neuroimaging—Validation study with functional MRI. Human Brain Mapping. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ETC. The influence of working memory load on phase specific patterns of cortical activity. Cognitive Brain Research. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cerebral Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: An H2 15O PET study of Stroop task performance. NeuroImage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, O’Craven K, Davidson RJ, Irwin W, Nelson CA, Turski PA. Reproducibility of fMRI results across four institutions using a spatial working memory task. NeuroImage. 1998;8:249–261. doi: 10.1006/nimg.1998.0360. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley KJ, Ptito A. Functional abnormalities in symptomatic concussed athletes: An fMRI study. NeuroImage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of Cognitive Neuroscience. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Goodale MA. Category-specific neural processing for naming pictures of animals and naming pictures of tools: An ALE meta-analysis. Neuropsychologia. 2010;48:409–418. doi: 10.1016/j.neuropsychologia.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Clark CR, Egan GF, McFarlane AC, Morris P, Weber D, Sonkkilla C, Danguy HJ. Updating working memory for words: A PET activation study. Human Brain Mapping. 2000;9:42–54. doi: 10.1002/(SICI)1097-0193(2000)9:1<42::AID-HBM5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre EL, Filippi CG, Newhouse PA, Dumas JA. The Stroop effect in kana and kanji scripts in native Japanese speakers: An fMRI study. Brain and Language. 2008;107:124–132. doi: 10.1016/j.bandl.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: Who’s in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Human Brain Mapping. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. Journal of Neuroscience. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Neural basis of novel and well-learned recognition memory in schizophrenia: A positron emission tomography study. Human Brain Mapping. 2001;12:219–231. doi: 10.1002/1097-0193(200104)12:4<219::AID-HBM1017>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Radonovich K, Sadek JR, Gökçay D, Bauer RM, Fischler IS, Briggs RW. Left-hemisphere processing of emotional connotation during word generation. NeuroReport. 1999;10:2449–2455. doi: 10.1097/00001756-199908200-00003. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The neural basis of working memory storage, rehearsal, and control processes: Evidence from patient and functional magnetic resonance imaging studies. New York, NY: Guilford Press; 2002. [Google Scholar]
- Dade LA, Zatorre RJ, Evans AC, Jones-Gotman M. Working memory in another dimension: Functional imaging of human olfactory working memory. NeuroImage. 2001;14:650–660. doi: 10.1006/nimg.2001.0868. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Andrew CM, Zelaya FO, Williams SCR, Dumanoir C. Motor response suppression and the prepotent tendency to respond: A parametric fMRI study. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Wilson SJ, McMahon KL, Muthiah S. The semantic interference effect in the picture-word paradigm: An event-related fMRI study employing overt responses. Human Brain Mapping. 2001;14:218–227. doi: 10.1002/hbm.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, Benke T. Learning complex arithmetic—An fMRI study. Cognitive Brain Research. 2003;18:76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Santhanam P, Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. NeuroImage. 2011;54:1043–1052. doi: 10.1016/j.neuroimage.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. NeuroImage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Incoccia C, Grassi F, Cappa SF, Bettinardi V, Fazio F. Neural control of fast-regular saccades and antisaccades: An investigation using positron emission tomography. Experimental Brain Research. 1997;116:50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: An event-related fMRI study. Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cerebral Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Activity in fusiform face area modulated as a function of working memory load. Cognitive Brain Research. 2001a;10:355–364. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. A neural network reflecting decisions about human faces. Neuron. 2001b;32:947–955. doi: 10.1016/s0896-6273(01)00519-0. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Casey BJ. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC. Decomposing the neural correlates of antisaccade eye movements using event-related fMRI. Cerebral Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cognitive Brain Research. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fehr T, Code C, Hermann M. Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Research. 2007;1172:93–102. doi: 10.1016/j.brainres.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, van Veen V, Stenger VA, Anderson JR. Neural mechanisms of planning: A computational analysis using event-related fMRI. Proceedings of the National Academy of Sciences. 2002;99:3346–3351. doi: 10.1073/pnas.052703399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, Egan GF. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Human Brain Mapping. 2008;29:490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MRG, Everling S. Neural processes associated with antisaccade task performance investigated with event-related fMRI. Journal of Neurophysiology. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biological Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WOM, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. Journal of Cognitive Neuroscience. 2008;20:1854–1865. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Mapping context and content: The BrainMap model. Nature Reviews Neuroscience. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ. Willed action and the prefrontal cortex in man: A study with PET. Proceedings of the Royal Society B. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Morgan K, Suckling J, Williams SCR, Andrew C, Vythelingum GN, McGuire PK. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: Greater anterior cingulate activation with increased task demand. NeuroImage. 2002;17:871–879. [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, Politi PL. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters. 2009;452:262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Behavioral electrophysiology of the prefrontal cortex of the primate. Progress in Brain Research. 1990;85:313–324. [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of Neurocytology. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen AC, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microscopy Research and Technique. 2000a;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman JN, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Li SJ, Stein EA. A parametric manipulation of central executive functioning. Cerebral Cortex. 2000b;10:585–592. doi: 10.1093/cercor/10.6.585. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Post RM. Regional brain activity when selecting a response despite interference: An H2 15O PET study of the Stroop and an emotional Stroop. Human Brain Mapping. 1993;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Hsieh JC, Petersson KM, Stone-Elander S, Ingvar M. Coexistence of attention-based facilitation and inhibition in the human cortex. NeuroImage. 1998;7:23–29. doi: 10.1006/nimg.1997.0307. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Hsieh JC, Wirsén-Meurling A, Wredling R, Eriksson L, Stone-Elander S, Ingvar M. Brain activation induced by the perceptual maze test: A PET study of cognitive performance. NeuroImage. 1995;2:112–124. doi: 10.1006/nimg.1995.1014. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM. Executive functioning-related brain abnormalities associated with the genetic liability for schizophrenia: An activation likelihood estimation meta-analysis. Psychological Medicine. 2010:1–14. doi: 10.1017/S0033291710001972. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, Weinberger DR. Uncoupling cognitive workload and prefrontal cortical physiology: A PET rCBF study. NeuroImage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Development. 1987;58:601–622. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, Gur RE. Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event-related fMRI study. Human Brain Mapping. 2007;28:263–274. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P-O, Fossati P, Pochon J-B, Levy R, LeBastard G, Lehéricy S, Dubois B. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. NeuroImage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JDE. Material-dependent and material-independent selection processes in the frontal and parietal lobes: An event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. The American Journal of Psychiatry. 2004;161:707–715. doi: 10.1176/appi.ajp.161.4.707. [DOI] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: Patterns of cortical activation and deactivation prior to response inhibition. Journal of Cognitive Neuroscience. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Honey GD, Sharma TS, Suckling J, Giampietro V, Williams SCR, Bullmore ET. The functional neuroanatomy of schizophrenic subsyndromes. Psychological Medicine. 2003;33:1007–1018. doi: 10.1017/s0033291703007864. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: An fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Rund BR, Lund A, Asbjornsen A, Egeland J, Ersland L, Thomsen T. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. American Journal of Psychiatry. 2004;161:286–293. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Siedentopf C, Koppelstätter F, Benke T, Felber S, Delazer M. How specifically do we learn? Imaging the learning of multiplication and subtraction. NeuroImage. 2006;30:1365–1375. doi: 10.1016/j.neuroimage.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates: I. The functions of the frontal association areas in monkeys. Comparative Psychology Monographs. 1936;13:1–60. [Google Scholar]
- Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biological Psychiatry. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester RL, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. NeuroImage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Johnson MK, III, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. The American Journal of Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kim J-J, Kim MS, Lee JS, Lee DS, Lee MC, Kwon JS. Dissociation of working memory processing associated with native and second languages: PET investigation. Neuro-Image. 2002;15:879–891. doi: 10.1006/nimg.2001.1025. [DOI] [PubMed] [Google Scholar]
- Kim J-J, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, Lee MC. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: A [15O]H2O PET study. American Journal of Psychiatry. 2003;160:919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D’Esposito M. Modulation of task-related neural activity in task-switching: An fMRI study. Cognitive Brain Research. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Greenlee MW, Gondan M, Schira M, Kassubek J, Mergner T. Relationship between saccadic eye movements and cortical activity as measured by fMRI: Quantitative and qualitative aspects. Experimental Brain Research. 2001;141:184–194. doi: 10.1007/s002210100844. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SHA, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebrocerebellar activation in verbal working memory: An fMRI study. NeuroImage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: A bilingual functional-imaging study. Proceedings of the National Academy of Sciences. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Zhao V, Nikelski J. Cerebral organization in bilinguals: A PET study of Chinese-English verb generation. NeuroReport. 1999;10:2841–2846. doi: 10.1097/00001756-199909090-00026. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nature Neuroscience. 1998a;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior pre-frontal cortex revealed by functional magnetic resonance imaging. European Journal of Neuroscience. 1998b;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, Phillips ML. Stroop performance in bipolar disorder: Further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disorders. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Taylor P, Ffytche DH, Das M, Barkataki I, Sharma T. Neural dysfunction and violence in schizophrenia: An fMRI investigation. Schizophrenia Research. 2006;84:144–164. doi: 10.1016/j.schres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam MM. Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. NeuroImage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Ivanovski B, Malhi GS. An event-related functional MRI study of working memory in euthymic bipolar disorder. Journal of Psychiatry & Neuroscience. 2007;32:174–184. [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Fox PT. ALE meta-analysis workflows via the BrainMap database: Progress towards a probabilistic functional brain atlas. Frontiers in Neuroinformatics. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Fox PT. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Human Brain Mapping. 2005b;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutiérrez D, Moran ST, Gonzales SM, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D’Esposito M. A functional MRI study of the influence of practice on component processes of working memory. Neuro-Image. 2004;22:211–221. doi: 10.1016/j.neuroimage.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lange G, Steffener J, Cook DB, Bly BM, Christodoulou C, Liu WC, Natelson BH. Objective evidence of cognitive complaints in Chronic Fatigue Syndrome: A BOLD fMRI study of verbal working memory. NeuroImage. 2005;26:513–524. doi: 10.1016/j.neuroimage.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ETC, Liddle PF. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophrenia Research. 2005;75:159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lazeron RHC, Rombouts SARB, de Sonneville L, Barkhof F, Scheltens P. A paced visual serial addition test for fMRI. Journal of the Neurological Sciences. 2003;213:29–34. doi: 10.1016/s0022-510x(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: Behavioral and neural correlates of nonimitative emotional responses. Cerebral Cortex. 2008;18:104–113. doi: 10.1093/cercor/bhm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimkuhler ME, Mesulam MM. Reversible go–no go deficits in a case of frontal lobe tumor. Annals of Neurology. 1985;18:617–619. doi: 10.1002/ana.410180518. [DOI] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during online storage of spatial memoranda. Journal of Cognitive Neuroscience. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the Stroop color word interference task. Cerebral Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. 3rd ed. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: A meta-analysis of functional neuroimaging data. Schizophrenia Bulletin. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DEJ, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Munk MHJ. Cortical capacity constraints for visual working memory: Dissociation of fMRI load effects in a fronto-parietal network. NeuroImage. 2003;20:1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. NeuroImage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL. Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. NeuroImage. 2002;17:792–802. [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. The functional organization of the brain. Scientific American. 1970;222:66–72. doi: 10.1038/scientificamerican0370-66. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology. 2003;112:689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maclin EL, Gratton G, Fabiani M. Visual spatial localization conflict: An fMRI study. NeuroReport. 2001;12:3633–3636. doi: 10.1097/00001756-200111160-00051. [DOI] [PubMed] [Google Scholar]
- Maguire RP, Broerse A, de Jong BM, Cornelissen FW, Meiners LC, Leenders KL, den Boer JA. Evidence of enhancement of spatial attention during inhibition of a visuo-motor response. NeuroImage. 2003;20:1339–1345. doi: 10.1016/S1053-8119(03)00402-6. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: An event-related fMRI study. NeuroImage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mana S, Paillere Martinot ML, Martinot JL. Brain imaging findings in children and adolescents with mental disorders: A cross-sectional review. European Psychiatry. 2010;25:345–354. doi: 10.1016/j.eurpsy.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Martinkauppi S, Rama P, Aronen HJ, Korvenoja A, Carlson S. Working memory of auditory localization. Cerebral Cortex. 2000;10:889–898. doi: 10.1093/cercor/10.9.889. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: Cortical and subcortical networks. Psychiatry Research. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P, Soares JC. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry. 2007;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Bittner RA, Nikolic D, Bledowski C, Goebel R, Linden DEJ. Common neural substrates for visual working memory and attention. NeuroImage. 2007;36:441–453. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Mead LA, Mayer AR, Bobholz JA, Woodley SJ, Cunningham JM, Hammeke TA, Rao SM. Neural basis of the Stroop interference task: Response competition or selective attention? Journal of the International Neuropsychological Society. 2002;8:735–742. doi: 10.1017/s1355617702860015. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Kiehl KA, Smith AM, Irwin D, Forster BB, Liddle PF. Dysfunction of a distributed neural circuitry in schizophrenia patients during a working-memory performance. Psychological Medicine. 2005;35:187–196. doi: 10.1017/s0033291704003228. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Adelman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001a;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Mathalon DH, Glover GH, Pfefferbaum A. Functional neuroanatomy of auditory working memory in schizophrenia: Relation to positive and negative symptoms. NeuroImage. 2001b;13:433–446. doi: 10.1006/nimg.2000.0699. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek TM, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain and Cognition. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Some cognitive effects of frontal-lobe lesions in man. Philosophical Transactions of the Royal Society B. 1982;298:211–226. doi: 10.1098/rstb.1982.0083. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 1999. [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2009;33:975–980. doi: 10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley KJ, Dagher A. Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neu-roscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SCR, Curtis VA. A functional MRI study of working memory task in euthymic bipolar disorder: Evidence for task-specific dysfunction. Bipolar Disorders. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, Pekar JJ. fMRI evidence that the neural basis of response inhibition is task-dependent. Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Katsumi Y, Magata Y, Shibasaki H, Kimura J. Age-related changes in cerebral blood flow activation during a Card Sorting Test. Experimental Brain Research. 1997;114:571–577. doi: 10.1007/pl00005665. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Matsuzaki S, Konishi J, Shibasaki H, Kimura J. Cerebral activation during performance of a card sorting test. Brain. 1996;119:1667–1675. doi: 10.1093/brain/119.5.1667. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Shibasaki H. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cerebral Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- Norris DG, Zysset S, Mildner T, Wiggins CJ. An investigation of the value of spin-echo-based fMRI using a Stroop color-word matching task and EPI at 3T. NeuroImage. 2002;15:719–726. doi: 10.1006/nimg.2001.1005. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proceedings of the National Academy of Sciences. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SPMJ, Carpenter TA, Pickard JD. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. European Journal of Neuroscience. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and non-spatial working memory processing within the human lateral frontal cortex. Proceedings of the National Academy of Sciences. 1998;95:7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. Journal of Neurophysiology. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the processing of single words. Journal of Cognitive Neuroscience. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung H-C, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby JV. Sustained activity in the medial wall during working memory delays. Journal of Neuroscience. 1998;18:9429–9437. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proceedings of the National Academy of Sciences. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J-B, Levy R, Poline J-B, Crozier S, Lehéricy S, Pillon B, Dubois B. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: An fMRI study. Cerebral Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. The American Journal of Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Gur RE. Working memory for complexfigures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Bobholz JA, Hammeke TA, Rosen AC, Woodley SJ, Cunningham JM, Binder JR. Functional MRI evidence for subcortical participation in conceptual reasoning skills. NeuroReport. 1997;8:1987–1993. doi: 10.1097/00001756-199705260-00038. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS. Shifting set about task switching: Behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia. 2008;46:2924–2935. doi: 10.1016/j.neuropsychologia.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Roth RM, Koven NS, Randolph JJ, Flashman LA, Pixley HS, Ricketts SM, Saykin AJ. Functional magnetic resonance imaging of executive control in bipolar disorder. Neuro-Report. 2006;17:1085–1089. doi: 10.1097/01.wnr.0000227979.06013.57. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: Response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Taylor E. Mapping motor inhibition: Conjunctive brain activations across different versions of go/ no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Woodward TS, Laurens KR, Liddle PF. The role of the anterior cingulate cortex in conflict processing: Evidence from reverse Stroop interference. NeuroImage. 2001;14:1150–1158. doi: 10.1006/nimg.2001.0893. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. Journal of Neurophysiology. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JDE. Age differences in prefrontal cortical activity in working memory. Psychology and Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JDE. Load-dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulieres I, Zeffiro TA. Enhanced visual functioning in autism: An ALE meta-analysis. Human Brain Mapping. 2011 doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Cárrion R, Gómez PV, Junqué C, Fernández-Espejo D, Falcon C, Bargalló N, Bernabeu M. Frontal hypoactivation on functional magnetic resonance imaging in working memory after severe diffuse traumatic brain injury. Journal of Neurotrauma. 2008;25:479–494. doi: 10.1089/neu.2007.0417. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber EJ, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. NeuroImage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Schwindt GC, Black SE. Functional imaging studies of episodic memory in Alzheimer’s disease: A quantitative meta-analysis. NeuroImage. 2009;45:181–190. doi: 10.1016/j.neuroimage.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society B. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T. From neuropsychology to mental structure. New York, NY: Cambridge University Press; 1988. [Google Scholar]
- Sheridan MA, Hinshaw S, D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/ hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer MJ, Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Human Brain Mapping. 2004;21:247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Hajak G, Döhnel K, Meinhardt J, Müller JL. Emotion-dependent modulation of interference processes: An fMRI study. Acta Neurobiologiae Experimentalis. 2008;68:193–203. doi: 10.55782/ane-2008-1688. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: Evidence from functional magnetic resonance imaging. NeuroImage. 2000;11:392–399. doi: 10.1006/nimg.2000.0569. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Tang J, Critchley HD, Glaser DE, Dolan RJ, Butterworth B. Imaging informational conflict: A functional magnetic resonance imaging study of numerical Stroop. Journal of Cognitive Neuroscience. 2006;18:2049–2062. doi: 10.1162/jocn.2006.18.12.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB. Localization of sublexical speech perception components. Brain and Language. 2010;114:1–15. doi: 10.1016/j.bandl.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJLM, van Hartskamp J, van Dyck R. Frontal-striatal dysfunction during planning in obsessive–compulsive disorder. Archives of General Psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van der Wee NJA, Ramsey NF, Jansma JM, Denys DA, van Megen HJGM, Westenberg HMG, Kahn RS. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. NeuroImage. 2003;20:2271–2280. doi: 10.1016/j.neuroimage.2003.05.001. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SARB, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: An fMRI study. NeuroImage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Human Brain Mapping. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle E, Pochon JB, Lehericy S, Pillon B, Dubois B, Levy R. Specific cerebral networks for maintenance and response organization within working memory as evidenced by the “double delay/double response” paradigm. Cerebral Cortex. 2005;15:1064–1074. doi: 10.1093/cercor/bhh207. [DOI] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: An event-related, parametric, performance-controlled fMRI study. Journal of Affective Disorders. 2007;101:175–185. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Prefrontal unit activity during associative learning in the monkey. Experimental Brain Research. 1990;80:296–309. doi: 10.1007/BF00228157. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Experimental Brain Research. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: An event-related fMRI study. NeuroImage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Wittfoth M, Buck D, Fahle M, Herrmann M. Comparison of two Simon tasks: Neuronal correlates of conflict resolution based on coherent motion perception. Neuro-Image. 2006;32:921–929. doi: 10.1016/j.neuroimage.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Wittfoth M, Kustermann E, Fahle M, Herrmann M. The influence of response conflict on error processing: Evidence from event-related fMRI. Brain Research. 2008;1194:118–129. doi: 10.1016/j.brainres.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS One. 2009;4:e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Gray JR, Chrastil ER, Barch DM, Green L, Braver TS. Sustained neural activity associated with cognitive control during temporally extended decision making. Cognitive Brain Research. 2005;23:71–84. doi: 10.1016/j.cogbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Choi BG, Juh RH, Park JM, Pae CU, Kim JJ, Lee CU. Working memory processing of facial images in schizophrenia: fMRI investigation. International Journal of Neuroscience. 2005;115:351–366. doi: 10.1080/00207450590520957. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Walter RBS, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. The American Journal of Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Frontiers in Human Neuroscience. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Archives of General Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Zago L, Pesenti M, Mellet E, Crivello F, Mazoyer B, Tzourio-Mazoyer N. Neural correlates of simple and complex mental calculation. NeuroImage. 2001;13:314–327. doi: 10.1006/nimg.2000.0697. [DOI] [PubMed] [Google Scholar]
- Zurowski B, Gostomzyk J, Grön G, Weller R, Schirrmeister H, Neumeier B, Walter H. Dissociating a common working memory network from different neural substrates of phonological and spatial stimulus processing. NeuroImage. 2002;15:45–57. doi: 10.1006/nimg.2001.0968. [DOI] [PubMed] [Google Scholar]
- Zysset S, Muller K, Lohmann G, von Cramon DY. Color-word matching Stroop task: Separating interference and response conflict. NeuroImage. 2001;13:29–36. doi: 10.1006/nimg.2000.0665. [DOI] [PubMed] [Google Scholar]