Abstract
Neuroblastoma remains responsible for a disproportionate amount of childhood cancer morbidity and mortality despite recent significant advances in understanding the genetic basis of tumor initiation and progression. About half of newly diagnosed patients can be reliably identified as having tumors of low malignant potential, and these children have cure rates of greater than 95% with little or no cytotoxic therapy. On the other hand, the other half of neuroblastomas typically present in an explosive fashion with widely metastatic disease, and reliable tumor-specific biomarkers have been defined for this phenotype as well. Empiric approaches to high-risk neuroblastoma therapy have relied on dramatic escalation of chemotherapy dose intensity, and recently the incorporation of targeted immunotherapy, but nearly 50% of children with high-risk disease will be refractory to therapy or suffer a relapse, both of which are invariably fatal. Future improvements in high-risk neuroblastoma outcomes will require the identification of disease and patient-specific oncogenic vulnerabilities that can be leveraged therapeutically. Rational development of novel approaches to neuroblastoma therapy requires forward-thinking strategies to unequivocally prove activity in the relapse setting, and ultimately efficacy in curing patients when integrated into frontline treatment plans.
BACKGROUND
Neuroblastoma is a pediatric cancer typically occurring in young children that arises from the developing sympathetic nervous system1. Tumors arise in adrenal medullary tissue or paraspinal ganglia and may be localized or widely metastatic at diagnosis. Children with localized neuroblastoma and favorable tumor genomic characteristics have an excellent overall survival probability with little or no cytotoxic therapy. However, approximately 50% of neuroblastoma patients have a clinically aggressive form of the disease with overall survival rates of less than 40% 1, 2. While high-risk neuroblastoma accounts for only 4% of all pediatric cancer diagnoses, it is responsible for 12% of pediatric cancer deaths 3 and new therapies are clearly needed.
For children with localized disease, the general trend has been on therapy reduction focused on maintaining outstanding cure rates while minimizing treatment related morbidity. On the other hand, there has been a several decades long trend of escalating chemotherapeutic dose intensity for patients with the high-risk form of the disease. For example, neuroblastoma is perhaps the only solid human malignancy where myeloablative chemotherapy followed by autologous stem cell rescue has been proven by several investigators to improve survival4–6. Thus, transplant after intensive chemotherapeutic induction chemotherapy has been standard practice in this disease. However, the improvements in outcome are modest and associated with significant immediate and late toxicity. For patients who show a good anti-tumor response to upfront chemotherapy, two major advances inform current practice. First, Matthay et al showed that patients randomized to treatment with the differentiation agent isotretinoin after myeloablative consolidation had a decreased rate of relapse compared to randomized controls4. More recently, after years of preclinical and pilot studies, Yu et al published results of a randomized phase 3 trial that was stopped early due to meeting early criteria for efficacy, demonstrating that maintenance immunotherapy of a chimeric monoclonal antibody ch14.18 directed against the GD2 ganglioside combined with cytokines and isotretinoin was superior to treatment with isotretinoin alone (66% +/− 5% vs 46% +/− 5% at 2 years, p = 0.01) 7. Despite these recent advances in treatment, 10–20% of patients with high-risk neuroblastoma will never achieve a remission and be refractory to current treatment and 50–60% of patients who complete treatment will experience a relapse. There are currently no known curative salvage regimens for refractory and recurrent high-risk neuroblastoma. Identifying oncogenic vulnerabilities in children with refractory neuroblastoma that can be leveraged therapeutically have the hope of changing this current reality and ultimately impacting the newly diagnosed patient as well.
ON THE HORIZON
Biomarker discovery
Neuroblastoma has been in the forefront of incorporation of genomics into clinical practice. Shortly after the identification of the MYCN oncogene in 1983, it was shown that children with amplification of this gene have a worse clinical outcome, thus establishing MYCN as the first DNA-based biomarker for therapy selection in cancer 8–10. Other tumor DNA aberrations are likewise prognostic and are currently used clinically such as overall tumor DNA index (ploidy) and copy number status at chromosome arms 1p and 11q2. Likewise, RNA signatures appear to provide robust prognostic information11–15, but these have not found their way into clinical practice. International cooperation will be absolutely essential to provide robust validation cohorts in order to establish genomics-based prognostic signatures with high enough sensitivity and specificity for clinical utility. Ultimately, many of these biomarkers will also predict activity of many of the targeted therapeutics discussed below.
Therapeutic target discovery
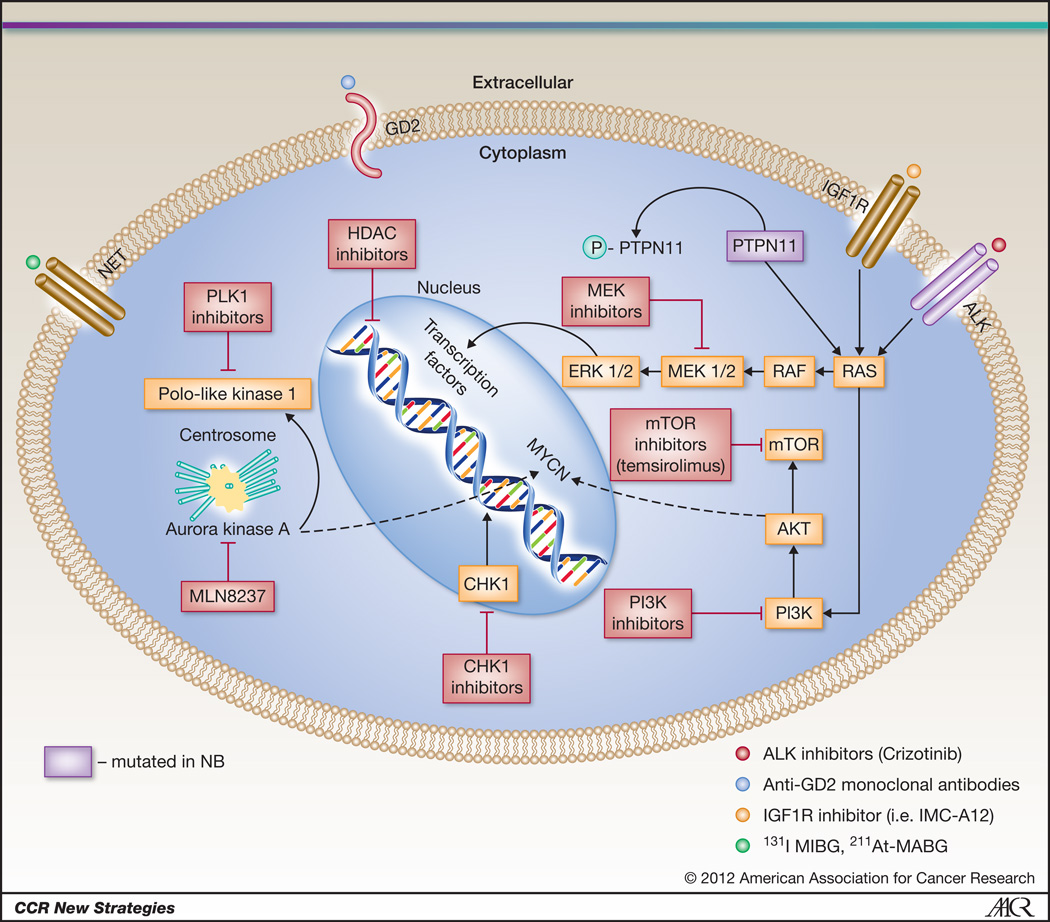
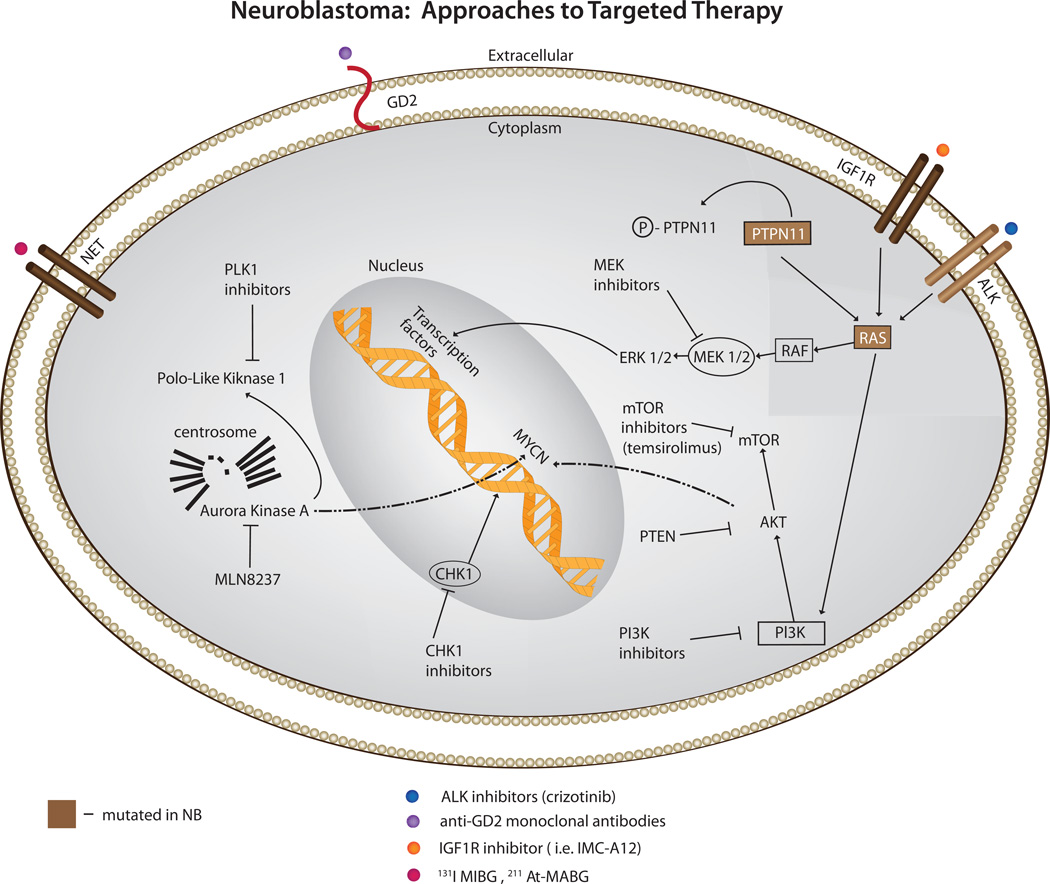
Several groups are exploring the hypothesis that oncogenic vulnerabilities in neuroblastoma cells can be discovered via highly parallel resequencing of tumor genomes. Data will be emerging shortly that will define the mutational landscape of high-risk neuroblastoma. An early success was the identification of gain of function mutations in the anaplastic lymphoma kinase gene (ALK), Figure 1. In addition to being the major familial neuroblastoma predisposition gene, mutations or amplification of ALK occur in about 10–15% of high-risk neuroblastoma cases16–20. Mutations result in constitutive activation of this receptor tyrosine kinase, providing an oncogenic driver analogous to activation via translocation events such as NPM-ALK in anaplastic large cell lymphoma and EML4-ALK in non-small cell lung cancer. The remarkable activity, with minimal toxicity, of the ALK inhibitor Crizotinib in ALK fusion positive small cell lung cancer21, provided additional proof-of-concept for an ongoing pediatric phase I/II clinical trial testing ALK inhibition strategies in neuroblastoma and other ALK activated pediatric neoplasms such as ALCL and inflammatory myofibroblastic tumor.
Figure 1.
Targeted therapy in neuroblastoma: Current approaches to targeted therapy for neuroblastoma involve several modalities including 1. small molecular inhibitors of activated signaling pathways (ALK, IGF1R, MEK, PLK1, AURKA, PI3K and mTOR inhibitors) 2. radiopharmaceuticals targeting the NET receptor (131I-MIBG, 211At-MABG) and 3. immunotherapy (anti-GD2 antibodies).
The aurora kinase A (AURKA) gene provides another promising therapeutic target in neuroblastoma. It has been shown that AURKA is highly expressed in high-risk tumors, and in addition to AURKA’s expected growth promoting roles, also stabilizes the MYCN protein by direct physical association, preventing MYCN’s degradation 22. The Pediatric Preclinical Testing Program (PPTP), which tests early phase agents in xenograft models of pediatric cancers, identified the AURKA inhibitor MLN8237 as a potent inhibitor of neuroblastoma – the only potent small molecular activity identified to date in this screening program in the neuroblastoma panel, resulting in fast-tracked development of an ongoing pediatric clinical trial23.
The insulin-like growth factor (IGF) signaling pathway plays an important role in the development and maintenance of pediatric tumors, including neuroblastoma24. IGF1R signaling has been shown to promote neuroblastoma tumorigenesis, inhibit apoptosis 24, 25 and disruption of IGF1R signaling by a small molecule inhibitor or antibody inhibits neuroblastoma growth in both in vitro and in vivo models of the disease 26, 27. Inhibitors against IGF1R and its downstream target mTOR, are currently being evaluated in early phase pediatric clinical trials27, 28.
While not yet translated to the clinic, there are several other promising neuroblastoma targets that also have small molecule inhibitors in adult trials. Through an unbiased RNAi screen of the protein kinome in neuroblastoma cell lines, the cell cycle checkpoint kinase CHK1 was identified as uniquely potent in inducing cytotoxicity following protein depletion29, 30. CHK1 is constitutively activated in neuroblastoma cell lines and primary tumor tissues. The unique single agent activity is likely through myc-induced replication stress, a finding that was recently supported in MYC driven lymphoma models 30. Other druggable targets include PLK1 which emerged from a small molecule screen in neuroblastoma tumor initiating cells 31, MEK inhibitors which may reverse hyperactivated ras mediated retinoid resistance in neuroblastoma32 and dual PI3K/mTOR inhibitors which destabilize MYCN and inhibit tumor vasculature 33. Molecularly targeted agents are currently being tested in combination with Irinotecan and Temozolamide because this combination was well tolerated and had modest activity in a phase II clinical trial for children with recurrent and refractory neuroblastoma34, providing a chemotherapeutic backbone for new agent integration into more traditional therapeutic regimens (ClinicalTrials.gov Identifier: NCT01141244)35.
Immunotherapy
A recent randomized phase 3 trial showed a significant improvement in high-risk neuroblastoma patient survival in those treated with the chimeric monoclonal antibody ch14.18 following conventional cytotoxic therapy7. This approach is both disease and patient specific as this antibody targets the disialoganglioside GD2 expressed on the surface of most neuroblastoma cells, and the effect is augmented by the addition of the cytokines interleukin-2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GMCSF) due to antibody-dependent cytotoxicity (ADCC) 7, 36, 37. This pivotal trial followed years of development of anti-GD2 approaches in early phase clinical trials that all documented some degree of activity, as well as considerable toxicity such as dose limiting pain, since GD2 is also expressed on pain fibers38–41. Current efforts are underway to potentially improve both the antibody and augmentation of the immune response so that future immunotherapy will be even more effective and less toxic. These strategies include a fully humanized anti-GD2 protein, and physically linking this molecule to IL-2, potentially allowing for ADCC in the proper microenvironment but limiting systemic toxicity such as capillary leak, hypotension and fever42, 43. The results of the phase II study of the hu-14.18-IL2 antibody showed that patients with a low burden of disease (stratum 2) had a 22% complete response rate 43. Another humanized antibody, Hu14.18K322A, has modifications that decrease antibody complement activation, which may result in fewer side effects, and alternative post translational modification that may allow higher ADCC 44, 45. Most investigators have considered immunotherapy as a post-chemotherapy remission-consolidation strategy targeting minimal residual disease, but it is also possible to use these anti-GD2 agents with chemotherapy to more effectively induce remission. Future randomized phase III trials of immunotherapeutic strategies will be required to definitively establish which strategy provides the right balance of anti-tumor efficacy with toxicity.
Targeted radiotherapy
Neuroblastoma is an exquisitely radiosensitive neoplasm, but its disseminated nature challenges successful implementation of conventional radiotherapeutic approaches. Since neuroblastoma arises from neural crest progenitors, nearly 85% of tumors have a functional sympathetic norepinephrine transporter (NET) protein on their cell surface. Investigators have taken advantage of this by targeting radiolabeled benzylguanidine (norepinephrine analogue) for diagnostic (low dose) and therapeutic (high dose) purposes. Indeed, a large phase II clinical trial of children with recurrent and refractory neuroblastoma treated with a single dose of 131I-metaiodobenzylguanidine (131I-MIBG) showed an impressive objective response rate (CR and PR) in 36% of patients, with an additional 34% having stable disease for a median time of 6 months, and palliation of symptoms such as pain46. There are plans to test this agent in a randomized controlled trial for newly diagnosed neuroblastoma patients in first response to see if this targeted radiotherapeutic can provide improved durable remission rates when integrated into consolidation therapy. Additional studies are aimed at providing enhanced activity of 131I- MIBG by combination with radiosensitization agents such as Irinotecan, histone deacetylase inhibitors (http://www.nant.org/), and perhaps targeted agents such as CHK1 inhibitors47, 48. It should be noted that a hypothetical limitation of the 131I-MIBG therapy is that I-131 emits largely beta-energy, which because of its relatively long path-length, the resident cell itself is not targeted. Therefore, efforts are underway to test the α-particle emitting norepinephrine analogue 211At-MABG, which may have superior efficacy against minimal residual disease in neuroblastoma than 131I-MIBG due to its higher energy transfer and much shorter path length49.
Epigenetic therapy
An emerging paradigm is that pediatric cancers have relatively few somatic mutations compared to common adult malignancies 50–52. In neuroblastoma, this is despite the fact that the genome is highly rearranged, with large chromosomal copy number alterations. Thus, it is likely that defects in DNA methylation and acetylation play a major role in regulating the high-risk neuroblastoma transcriptome. Ongoing efforts in epigenomic profiling are focused on defining the likelihood that any such aberrancies result in targetable vulnerabilities for drug development. Non-specific histone deacetylase inhibitors are currently being tested in the clinic, and investigators must take advantage of any observed anti-tumor activity to determine what defects in the tumor genome and/or epigenome could predict for clinical efficacy47, 53.
Rational Drug Development Strategies
A paradigm that is emerging in cancer therapeutics is that while clearly there is differential sensitivity to therapeutics between tumor types, the same is true within histologies, at diagnosis and at relapse. For any therapeutic, be it a traditional chemotherapy, radiation or a targeted small molecule, by identifying individuals most likely to respond, effective agents will continue in clinical development in the appropriate populations, and more importantly there will be improved efficacy. Our current understanding of the underlying molecular circuitry of neuroblastoma is just emerging, and is years from contributing to a comprehensive personalized approach to therapy, but this is on the horizon. To achieve this goal, investigators must identify biomarkers of therapy response that are useful to the clinician. The ultimate goal will be to have robust makers of specific oncogenic vulnerabilities for each individual patient, and this will require access to tumor material in real time. This is especially critical in the traditional phase 1 setting, as most molecular data is derived from diagnostic specimens and relapsed tumors are rarely biopsied. In order to achieve this goal, several milestones must be reached. First, ongoing genomic profiling and systems biology approaches must deliver on the accurate and complete characterization of all mutant pathways in this disease, at diagnosis and at relapse. Second, investigators must utilize large and well-annotated collections of neuroblastoma-derived cell lines and transgenic preclinical models to test the hypotheses that specific biomarkers indeed predict response to a therapy under development. Finally, pediatric oncologists will need to reconsider traditional drug development paradigms, and seek to design early phase clinical trials that select for patients most likely to show signs of anti-tumor efficacy but understanding the tumor at the time of trial entry in order to support rapid development in the refractory disease setting, allowing for early testing for impact on survival in newly diagnosed high-risk neuroblastoma patients.
Figure 2.
REFERENCES
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 5.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 6.Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R, et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–965. doi: 10.1200/JCO.1998.16.3.953. [DOI] [PubMed] [Google Scholar]
- 7.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab M, Alitalo K, Klempnauer KH, Varmus HE, Bishop JM, Gilbert F, et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305:245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 9.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 10.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 11.Abel F, Dalevi D, Nethander M, Jornsten R, De Preter K, Vermeulen J, et al. A 6-gene signature identifies four molecular subgroups of neuroblastoma. Cancer Cell Int. 2011;11:9. doi: 10.1186/1475-2867-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asgharzadeh S, Pique-Regi R, Sposto R, Wang H, Yang Y, Shimada H, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98:1193–1203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 13.De Preter K, Vermeulen J, Brors B, Delattre O, Eggert A, Fischer M, et al. Accurate outcome prediction in neuroblastoma across independent data sets using a multigene signature. Clin Cancer Res. 2010;16:1532–1541. doi: 10.1158/1078-0432.CCR-09-2607. [DOI] [PubMed] [Google Scholar]
- 14.Oberthuer A, Hero B, Berthold F, Juraeva D, Faldum A, Kahlert Y, et al. Prognostic impact of gene expression-based classification for neuroblastoma. J Clin Oncol. 2010;28:3506–3515. doi: 10.1200/JCO.2009.27.3367. [DOI] [PubMed] [Google Scholar]
- 15.Wei JS, Greer BT, Westermann F, Steinberg SM, Son CG, Chen QR, et al. Prediction of clinical outcome using gene expression profiling and artificial neural networks for patients with neuroblastoma. Cancer Res. 2004;64:6883–6891. doi: 10.1158/0008-5472.CAN-04-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 19.Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 20.Weiser D, Laudenslager M, Rappaport E, Carpenter E, Attiyeh E, Diskin S, et al. Stratification of patients with neuroblastoma for targeted ALK therapy. J Clin Oncol. 2011;29 (suppl; abstr 9514) [Google Scholar]
- 21.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Toretsky JA, Scher D, Helman LJ. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton JR, Randolph AE, Feldman EL. Insulin-like growth factor I receptor prevents apoptosis and enhances neuroblastoma tumorigenesis. Cancer Res. 1996;56:4522–4529. [PubMed] [Google Scholar]
- 26.Tanno B, Mancini C, Vitali R, Mancuso M, McDowell HP, Dominici C, et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–6780. doi: 10.1158/1078-0432.CCR-06-1479. [DOI] [PubMed] [Google Scholar]
- 27.Houghton PJ, Morton CL, Gorlick R, Kolb EA, Keir ST, Reynolds CP, et al. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54:921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29:2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, Bosse K, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci U S A. 2011;108:3336–3341. doi: 10.1073/pnas.1012351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrao PT, Bukczynska EP, Johnstone RW, McArthur GA. Efficacy of CHK inhibitors as single agents in MYC-driven lymphoma cells. Oncogene. 2011 doi: 10.1038/onc.2011.358. [DOI] [PubMed] [Google Scholar]
- 31.Grinshtein N, Datti A, Fujitani M, Uehling D, Prakesch M, Isaac M, et al. Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res. 2011;71:1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 32.Holzel M, Huang S, Koster J, Ora I, Lakeman A, Caron H, et al. NF1 is a tumor suppressor in neuroblastoma that determines retinoic acid response and disease outcome. Cell. 2010;142:218–229. doi: 10.1016/j.cell.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanthery YH, Gustafson WC, Itsara M, Persson A, Hackett CS, Grimmer M, et al. Paracrine Signaling Through MYCN Enhances Tumor-Vascular Interactions in Neuroblastoma. Sci Transl Med. 2012;4:115ra3. doi: 10.1126/scitranslmed.3002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipsitz E, Nguyen H, Zhao H, Ecsedy J, Maris J, Adamson PC, et al. J Clin Oncol. Vol. 28. Chicago, IL: 2010. Modeling MLN8237, an aurora kinase A inhibitor, with irinotecan (IRN) and temozolamide (TMZ) in neuroblastoma (NB) p. 15s. 2010 (suppl; abstr 10593) [Google Scholar]
- 36.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–1941. [PubMed] [Google Scholar]
- 37.Hank JA, Surfus J, Gan J, Chew TL, Hong R, Tans K, et al. Treatment of neuroblastoma patients with antiganglioside GD2 antibody plus interleukin-2 induces antibody-dependent cellular cytotoxicity against neuroblastoma detected in vitro. Journal of immunotherapy with emphasis on tumor immunology : official journal of the Society for Biological Therapy. 1994;15:29–37. doi: 10.1097/00002371-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 39.Cheung NK, Kushner BH, Yeh SD, Larson SM. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: a phase II study. Int J Oncol. 1998;12:1299–1306. doi: 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- 40.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 41.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16:2169–2180. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 42.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets. 2010;10:200–209. doi: 10.2174/156800910791054167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorkin LS, Otto M, Baldwin WM, 3rd, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149:135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 47.More SS, Itsara M, Yang X, Geier EG, Tadano MK, Seo Y, et al. Vorinostat increases expression of functional norepinephrine transporter in neuroblastoma in vitro and in vivo model systems. Clin Cancer Res. 2011;17:2339–2349. doi: 10.1158/1078-0432.CCR-10-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubois SG, Chesler L, SG G, Hawkins F, Goodarzian F, Yanik G, et al. J Clin Oncol. Vol. 29. Chicago, IL: 2011. Phase I study of vincristine, irinotecan, and 131I-MIBG for patients with relapsed or refractory neuroblastoma: A New Approach to Neuroblastoma Therapy (NANT) study. 2011 (suppl; abstr 9513) [Google Scholar]
- 49.Cunningham SH, Mairs RJ, Wheldon TE, Welsh PC, Vaidyanathan G, Zalutsky MR. Toxicity to neuroblastoma cells and spheroids of benzylguanidine conjugated to radionuclides with short-range emissions. British journal of cancer. 1998;77:2061–2068. doi: 10.1038/bjc.1998.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugh T, Lawrence M, Sougnez C, Getz G, Attiyeh E, Hogarty M, et al. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research. Orlando, FL: AACR; 2011. Exome sequencing of 81 neuroblastomas identifies a wide diversity of somatic mutation. [Google Scholar]
- 52.Morozova O, Birol I, Corbett R, Mungall K, Attiyeh E, Asgharzadeh S, et al. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research. Orlando, FL: AACR; 2011. Whole genome and transcriptome sequencing defines the spectrum of somatic changes in high-risk neuroblastoma. [Google Scholar]
- 53.Hahn CK, Ross KN, Warrington IM, Mazitschek R, Kanegai CM, Wright RD, et al. Expression-based screening identifies the combination of histone deacetylase inhibitors and retinoids for neuroblastoma differentiation. Proc Natl Acad Sci U S A. 2008;105:9751–9756. doi: 10.1073/pnas.0710413105. [DOI] [PMC free article] [PubMed] [Google Scholar]