Abstract
Objective
Management of intermediate degrees of mitral regurgitation (MR) during aortic valve replacement (AVR) for aortic stenosis remains controversial. We sought to evaluate the degree of reduction of MR in patients undergoing AVR, as well as the relationship between the pre-operative gradient across the aortic valve and the degree of reduction in MR.
Methods
We retrospectively analyzed demographic, intraoperative, and echocardiographic data on 802 patients that underwent AVR or aortic root replacement between January 2010 and March 2011. 578 patients underwent AVR or aortic root replacement without intervention on the mitral valve. We excluded 88 patients with severe aortic insufficiency, 3 patients that underwent ventricular assist device placement, 4 patients that underwent prior mitral valve replacement, and 21 patients with incomplete data yielding 462 patients for analysis. MR was graded for each patient and the degree of change in MR for each patient was determined by subtracting the grade of pre-operative MR from the degree of post-operative MR.
Results
Of the 462 patients, 289 patients had at least mild MR. On average, MR was downgraded by 0.24 degrees per patient for this cohort of 289 patients. Of the 56 patients with at least moderate MR, MR was downgraded 0.54 degrees per patient. Of 62 patients that underwent AVR only, had at least mild MR, and no evidence of structural mitral valve disease, downgrading of MR was 0.24 degrees per patient. Linear regression analysis revealed no relationship between reduction in MR and pre-operative gradient across the aortic valve.
Conclusions
Reduction in MR after relief of aortic outflow tract obstruction is modest at best. Further, the magnitude of the pre-operative gradient across the aortic valve has little influence on the degree of reduction in MR. These observations argue in favor of performing a prospective evaluation of the clinical benefits of addressing moderate MR at the time of aortic valve intervention.
INTRODUCTION
Co-existent mitral regurgitation is commonly encountered in the setting of aortic valve replacement for aortic stenosis.1 Severe mitral regurgitation mandates surgical intervention at the time of aortic valve replacement. Some evidence suggests that the presence of intermediate degrees of mitral regurgitation after AVR carries a worse prognosis.3–6 However, the mortality of double valve surgery is substantially higher than that of isolated aortic valve replacement.7,8 Further, it is commonly thought that functional mitral regurgitation, when present in the setting of severe aortic stenosis, improves significantly after AVR. However, there is substantial disagreement in the literature over the proportion of patients that will experience improvement in MR after AVR and the degree of improvement that can be expected is not well defined.1 For these reasons, the appropriate management of intermediate degrees of MR in the setting of AVR is undefined and remains controversial, with some authors advocating for a more aggressive surgical approach2,3,5,8 and others advocating a more conservative approach.9,10 No randomized controlled clinical trials that address this issue have been published to date. Accordingly, after collating existing data in systematic fashion, one group concluded that the evidence was inconclusive to make recommendations regarding surgical intervention on the mitral valve in the setting of moderate MR at the time of AVR.11 We sought to define the degree of improvement in MR that can be expected after AVR in a contemporary cohort of patients from a single center and to examine the relationship between the magnitude of the pre-operative gradient across the aortic valve and the change in MR after AVR.
METHODS
Demographic, intraoperative, and echocardiographic data on 802 patients that underwent intervention on the aortic valve (AVR or aortic root replacement) with or without intervention on the mitral valve (repair or replacement) between January 2010 and March 2011 was extracted from the Society of Thoracic Surgeons compliant database from our institution. 578 patients underwent AVR or aortic root replacement without intervention on the mitral valve. The decision to perform concomitant mitral valve surgical intervention at the time of AVR was made at the discretion of the operating surgeon. Typically, the presence of severe MR would prompt either repair or replacement of the mitral valve at the time of AVR. Intervention on the mitral valve would be considered in the setting of intermediate degrees of mitral regurgitation in the presence of structural mitral valve disease. An individual patient's age and medical comorbidities would be considered in the ultimate decision as to whether to proceed with intervention on the mitral valve. We excluded 88 patients with severe aortic insufficiency, 3 patients that underwent ventricular assist device placement at the time of AVR, 4 patients that underwent prior mitral valve replacement, and 21 patients with incomplete data yielding 462 patients for analysis.
Grading of Mitral Regurgitation
Data on MR for each patient was extracted from pre- and post-operative echocardiograms. Routine intraoperative transesophageal echocardiography (TEE) is employed at our institution and the intra-operative completion TEE was used as the post-op echo in this study. Measurement of vena contracta width is the most commonly employed method of classifying MR. MR was numerically graded as follows: none = 0, trace = 0.5, mild = 1, moderate = 2, moderate-severe = 3, severe = 4. Intermediate degrees were assigned half of a degree. Change in the degree of MR for each patient was quantitated by subtracting the grade of pre-operative MR from the degree of post-operative MR. Any patient with any abnormality of the either the mitral valve leaflets, the chordae tendonae, the papillary muscles, or the annulus (including MAC but excluding pure annular dilatation) was classified as having structural mitral valve disease. Patients with any degree of leaflet disease including tethering, thickening, calcification, or prolapse were considered to have a leaflet abnormality. In order to strictly identify patients with completely normal mitral valve architecture, even minor abnormalities (such as small amounts of leaflet calcification) were considered abnormalities.
Statistical analysis
Data were expressed as mean ± standard deviation for continuous variables and as percentage for categorical variables. Categorical variables were compared using Chi-square tests and continuous variables were compared with either t-tests or rank-sum tests. Statistical significance was defined as a P value < 0.05. Simple linear regression was used to evaluate the relationship between change in mitral regurgitation and pre-operative mean gradient across the aortic valve.
RESULTS
Baseline patient characteristics and operative data
Baseline characteristics and operative data of this cohort of 462 patients are presented in Tables 1A and 1B. The patients in this cohort are predominantly elderly (average age 72.9 ± 10.9 years), Caucasian (90.9%), and male (58.7%). A large fraction of the patients (81.4%) suffer from hypertension. A majority of these patients (84.0%) had symptoms of heart failure pre-operatively. NHYA class 3 symptoms were observed in 37.4% and 10.0% had class 4 symptoms. Diabetes (29.4%), previous MI (19.5%), cerebrovascular disease (19.9%), and peripheral arterial disease (16.4%) were also observed in substantial proportion of the patients. Five percent of the patients in this cohort require chronic hemodialysis.
TABLE 1.
| A. Baseline patient characteristics | |
|---|---|
| Age (years) | 72.9 ±10.9 |
| Sex | |
| Male | 58.7% (n=271) |
| Female | 41.3% (n= 191) |
| Race | |
| Caucasian | 90.9% (n=420) |
| African American | 5.0% (n=23) |
| Asian | 0.6% (n=3) |
| Hispanic | 0.4% (n=2) |
| Other/unspecified | 3.0% (n=14) |
| Diabetes | 29.4% (n=135) |
| Hypertension | 81.4% (n=376) |
| Previous MI | 19.5% (n=90) |
| Heart failure | 84.0% (n=388) |
| NYHA class | |
| 1 | 2.6% (n=12) |
| 2 | 34.0% (n=157) |
| 3 | 37.4% (n=173) |
| 4 | 10.0% (n=46) |
| Cerebrovascular disease | 19.9% (n=92) |
| Peripheral arterial disease | 16.4% (n=76) |
| Dialysis | 5.0% (n=23) |
| Creatinine | 1.11 ± 0.893 |
| Ejection fraction (n=454) | 56.4% ±12.8% |
| Mean aortic valve gradient (mmHg, n=434) | 44.6 ±15.9 |
| Mean pulmonary artery pressure(mmHg, n=290) | 26.5 ± 9.873 |
| B. Operative data | |
|---|---|
| Nature of operation | |
| AVR | 59.3% (n=274) |
| AVR + CABG | 27.5% (n=127) |
| AVR + CABG +other | 1.9% (n=9) |
| AVR + other | 8.0% (n=37) |
| Aortic root replacement | 1.7% (n=8) |
| Aortic root replacement + other | 1.5% (n=7) |
| Incidence | |
| First cardiac surgery operation | 82.9% (n=383) |
| First re-operation | 15.6% (n=72) |
| Second re-operation | 1.3% (n=6) |
| Third re-operation | 0.2% (n=1) |
| Urgency | |
| Elective | 71.9% (n=332) |
| Urgent | 26.4% (n=122) |
| Emergent | 1.7% (n=8) |
| Cardiopulmonary bypass time (min) | 119 ± 49.6 |
| Cross clamp time (min) | 85.8 ± 39.1 |
| Valve type | |
| Bioprosthesis | 93.3% (n=432) |
| Mechanical | 6.1% (n=28) |
| Homograft | 0.4% (n=2) |
| Implant Size (mm) | 23.7 ± 2.2 |
Abbreviations: MI, myocardial infarction; NYHA, New York Heart Association; mmHg, millimeters of mercury.
Abbreviations: AVR, aortic valve replacement; CABG, coronary artery bypass grafting; min, minutes; mm, millimeters.
Of the 462 patients in this cohort, a majority of patients (59.3%) underwent AVR only. However, 27.5% underwent AVR and CABG, 1.9% underwent AVR/CABG combined with another procedure (including left atrial appendage excision, maze procedure, patent foramen ovale closure, septal myomectomy, and excision of cardiac tumor), and 8.0% underwent AVR combined with another procedure (including ascending aortic aneurysm repair, septal myomectomy, aortic valve tumor resection, atrial septal defect repair, excision of left atrial myxoma, maze procedure, PFO closure, pacemaker removal, left atrial appendage excision, and pulmonary embolectomy). Aortic root replacement was performed in 1.7% and aortic root replacement combined with another procedure (including septal myomectomy, pulmonary artery patch, CABG) was performed in 1.5%. Since patients with severe aortic insufficiency were excluded from this analysis, the predominant indication for aortic valve replacement in these cases was aortic stenosis. This was the first cardiac surgery operation for most of the patients (82.9%) in the cohort, but first (15.6%), second (1.3%), and third (0.2%) re-operations were also performed in this series. Most of the cases in this series (71.9%) were elective, but some (26.4%) were considered urgent and few (1.7%) conducted on an emergent basis. Bioprosthetic aortic valves were used in the majority of cases (93.3%). Mechanical valves were used less frequently (6.1%) and homografts were rarely employed (0.4%). The average size of the valve implanted was 23.7 ± 2.2 mm.
Fate of MR
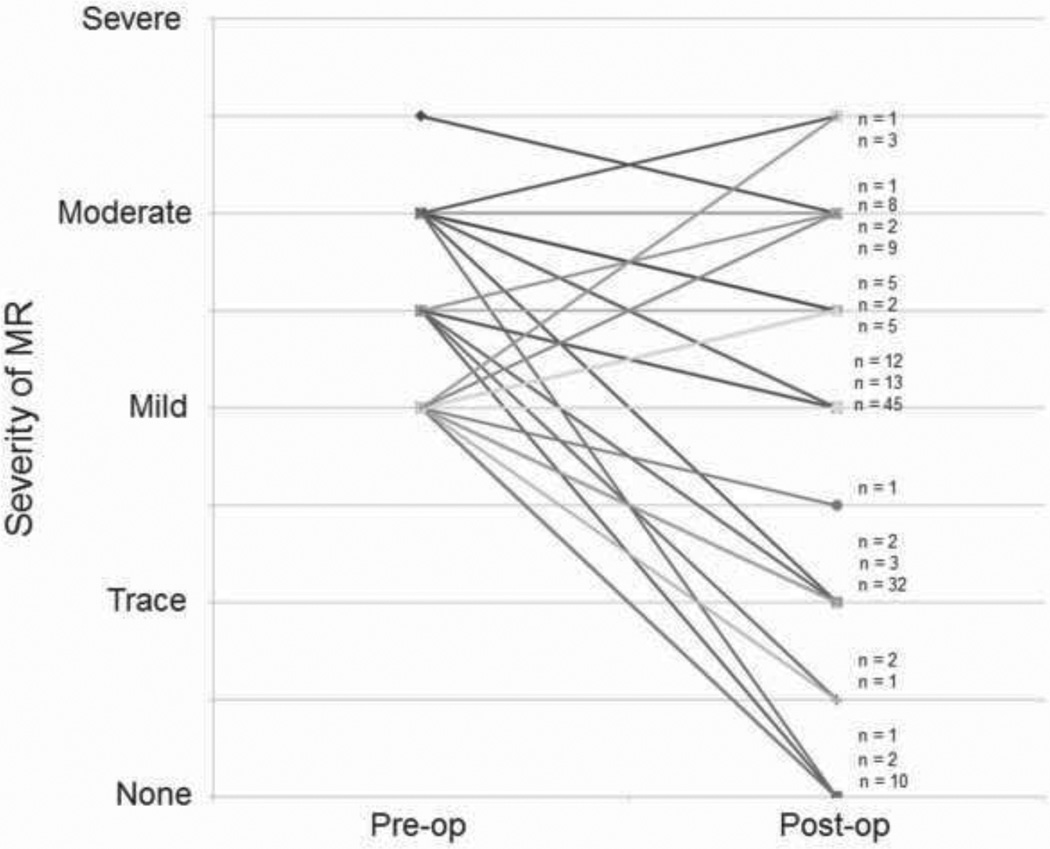
We next sought to determine the fate of co-existent mitral regurgitation after AVR. Overall trends in change in MR were examined by plotting the grade of pre-operative and post-operative MR for individual patients. As an example, a plot demonstrating changes in MR after AVR for patients that underwent AVR only (no other concomitant procedure) who had at least mild MR is illustrated in Figure 1. As can be seen in the figure, there is substantial variability in the change in MR after AVR without a clear trend towards improvement. Plots of other subgroups of patients yielded similar results.
FIGURE 1. Trend plot of MR for patients undergoing AVR.
The pre-operative and post-operative grades of MR for individual patients undergoing AVR alone (without any concomitant procedure) that had at least mild pre-operative MR were plotted. Because there is substantial overlap of individual plots, n values are used to indicate the number of patients represented by each line.
Quantitative change in MR
In order to quantify the change in mitral regurgitation that occurred in sub-groups of patients in this cohort, we next employed our numeric scoring system to evaluate the net change in mitral regurgitation in each group and on average per patient (Tables 2A, 2B). The change in MR per patient was −0.11 degrees in patients with any pre-operative MR, −0.24 degrees per patient in patients with at least mild MR, and –0.54 degrees in patients with at least moderate MR. Patients that underwent isolated AVR and had at least mild pre-operative MR had downgrading of −0.28 degrees per patient. For patients that underwent isolated AVR, had at least mild pre-operative MR, and had no mitral leaflet or chordal disease, the average change in mitral regurgitation was −0.26 degrees per patient. Lastly, for patients that underwent isolated AVR, had at least mild pre-operative MR, and had no disease of the mitral apparatus (including MAC), the average change in mitral regurgitation was −0.24 degrees per patient.
TABLE 2.
| A. Quantitative change in mitral regurgitation in patients undergoing aortic valve intervention | |
|---|---|
| Change in MR per patient | |
| Patients with MR (n=399) | −0.11 per patient |
| Patients with at least mild MR (n=289) | −0.24 per patient |
| Patients with at least moderate MR (n=56) | −0.54 per patient |
| B. Quantitative change in mitral regurgitation in patients undergoing isolated aortic valve replacement | |
|---|---|
| Change in MR per patient | |
| AVR only with at least mild MR (n=169) | −0.28 per patient |
| AVR only, mild MR, no leaflet disease (n=135) | −0.26 per patient |
| AVR only, mild MR, no mitral disease (n=62) | −0.24 per patient |
Abbreviations: MR, mitral regurgitation.
Abbreviations: AVR, aortic valve replacement; MR, mitral regurgitation.
Change in MR and pre-operative AV gradient
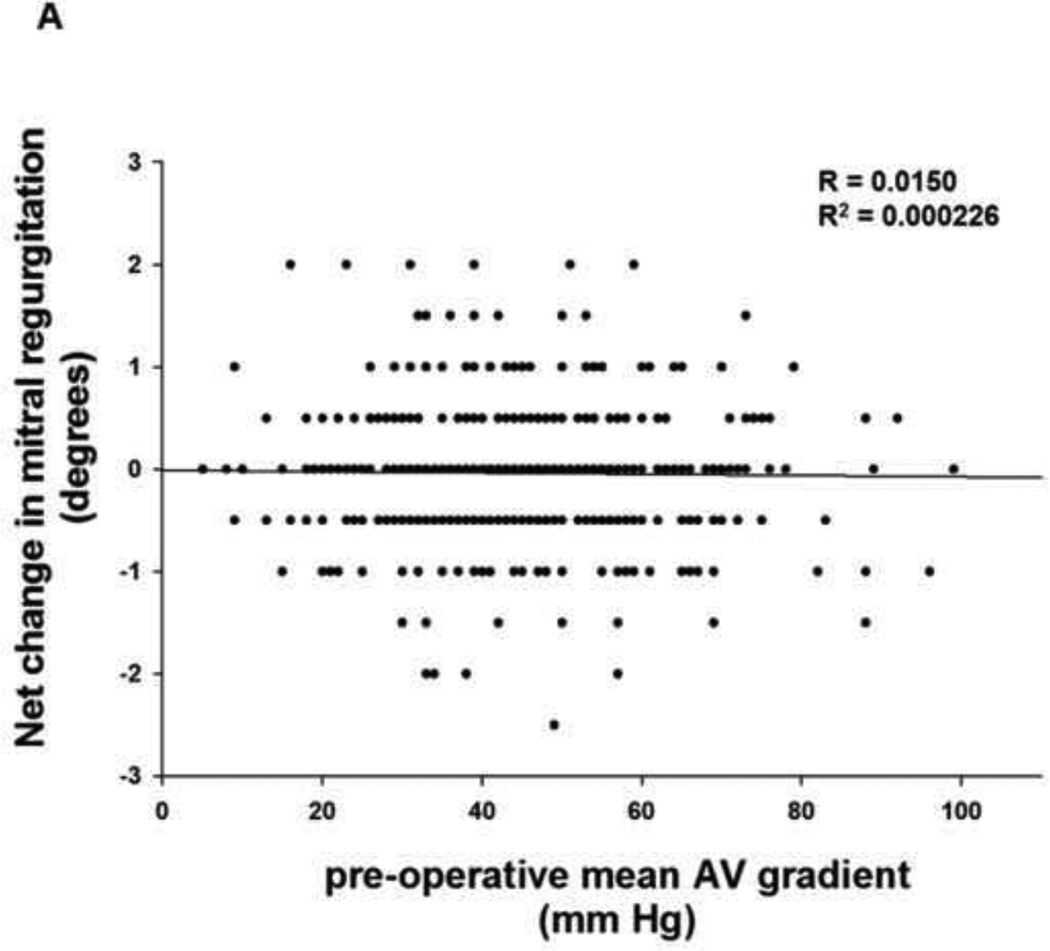
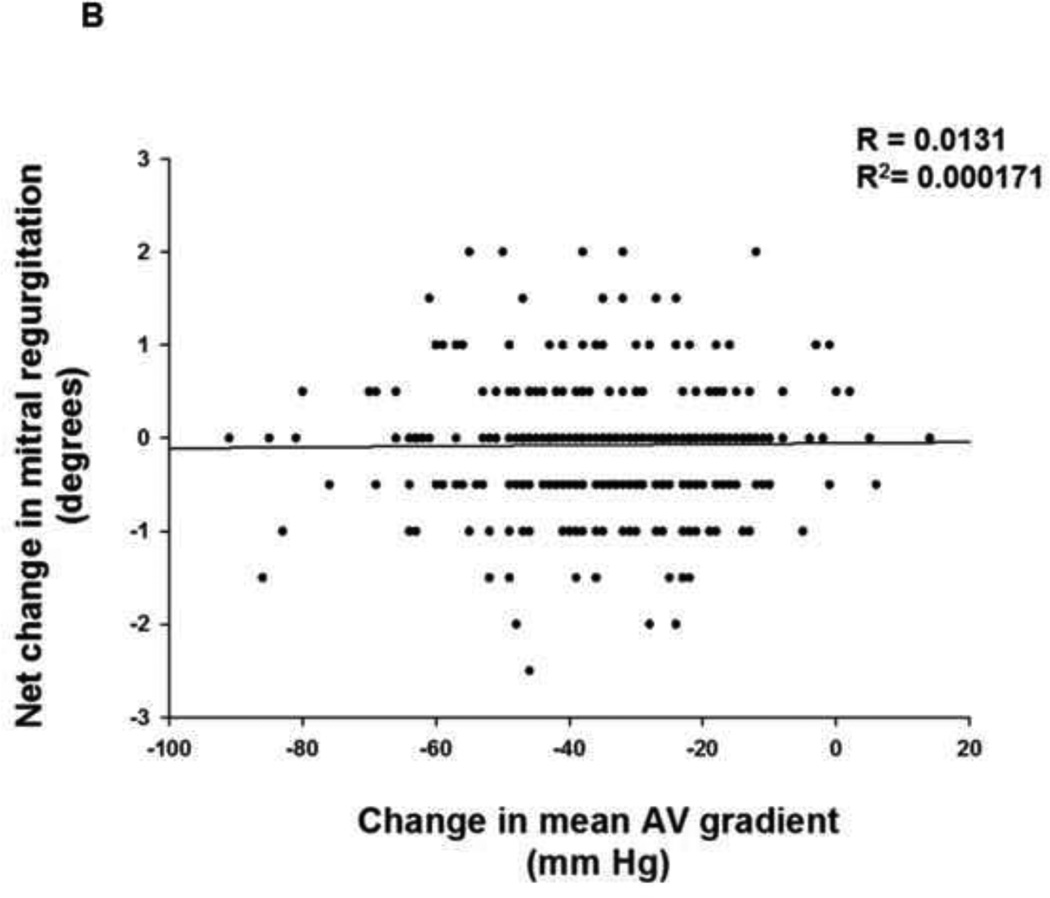
To evaluate whether the change in MR observed after relief of aortic outflow tract obstruction is dependent upon the pre-operative gradient across the aortic valve and presumably the extent of pressure overload reduction with AVR, we next performed simple linear regression analysis. The change in MR for each patient after aortic valve intervention was plotted as a function of pre-operative mean gradient across the aortic valve. When this analysis was performed for the entire cohort of 462 patients (Figure 2A), no clear relationship between change in MR and pre-operative mean gradient across the aortic valve was observed (R2 = 0.0002). In order to evaluate whether the net change in gradient across the aortic valve after AVR might influence the degree of improvement in mitral regurgitation, we also obtained data on the mean post-operative gradient across the aortic valve where available (n = 335). The change in gradient across the aortic valve was calculated by subtracting the mean pre-operative gradient from the mean post-operative gradient. The change in MR for each patient after aortic valve intervention was then plotted as a function of change in mean gradient across the aortic valve and linear regression analysis was performed (Figure 2B). No clear relationship between change in MR and change in mean gradient across the aortic valve was observed (R2 = 0.0002).
FIGURE 2. Change in MR as a function of thegradient across the aortic valve.
(A) Simple linear regression analysis of the change in MR for each patient according to the pre-operative mean gradient across the aortic valve was performed for the entire cohort of 462 patients. (B) Simple linear regression analysis of the change in MR for each patient according to the net change in mean gradient across the aortic valve was performed.
Predictors of improvement of MR
While the overall change in MR observed after relief of aortic outflow tract obstruction was modest, some patients did have some degree of improvement in MR, while others did not. In order to help identify factors that might predict improvement in MR, patients that underwent aortic valve intervention with mild or greater MR preoperatively were evaluated for improvement in MR. Patients were divided into groups depending on whether there was improvement in MR (n=137) or no improvement in MR (n=150). Pre-operative and operative characteristics were compared (Table 3A, B). In the group of patients that did not experience improvement in MR, a greater proportion of patients had mild MR, whereas a greater proportion of patients in the group that did experience improvement had greater than mild degrees of MR. There were no significant differences in other pre-operative or operative characteristics examined, including ejection fraction, mean aortic valve gradient, mean pulmonary artery pressure, left atrial diameter.
TABLE 3.
| A. Comparison of baseline characteristics of patients that experienced improvement in MR after aortic valve intervention versus those that did not improve | |||
|---|---|---|---|
| Improved (n=137) |
No Improvement (n=150) |
P value | |
| Age (years) | 74.7 ± 9.5 | 74.5 ± 9.9 | p = ns |
| Sex | |||
| Male | 54.7% (n=75) | 56.7% (n=85) | p = ns |
| Female | 45.3% (n= 62) | 43.3% (n=65) | |
| Race | |||
| Caucasian | 90.5% (n=124) | 91.3% (n=137) | p = ns |
| African American | 8.0% (n=11) | 4.7% (n=7) | |
| Asian | 0.0% (n=0) | 1.3% (n=2) | |
| Hispanic | 0.0% (n=0) | 1.3% (n=2) | |
| Other/unspecified | 1.5% (n=2) | 1.3% (n=2) | |
| Atrial fibrillation/flutter | 24.1% (n=33) | 28.7% (n=43) | p = ns |
| Diabetes | 30.7% (n=42) | 28.0% (n=42) | p = ns |
| Hypertension | 81.0% (n=111) | 81.3% (n=122) | p = ns |
| Previous MI | 19.7% (n=27) | 25.3% (n=38) | p = ns |
| Heart failure | 82.5% (n=113) | 90.0% (n=135) | p = ns |
| NYHA class | |||
| 1 | 1.5% (n=2) | 1.3% (n=2) | p = ns |
| 2 | 26.2% (n=36) | 32.7% (n=49) | |
| 3 | 43.0% (n=59) | 42.7% (n=64) | |
| 4 | 10.2% (n=14) | 13.3% (n=20) | |
| Cerebrovascular disease | 19.9% (n=26) | 20.7% (n=31) | p = ns |
| Peripheral arterial disease | 19.0% (n=22) | 19.3% (n=29) | p = ns |
| Dialysis | 2.2% (n=3) | 2.0% (n=3) | p = ns |
| Creatinine | 1.19 ± 0.972 | 1.12 ± 0.769 | p = ns |
| Ejection fraction (%) | 54.0% ±13.9% (n=135) | 56.0% +13.6% (n=147) | p = ns |
| Mean aortic valve gradient (mm Hg) | 45.4±16.0 (n=136) | 43.4±16.5 (n=149) | p = ns |
| Mean pulmonary artery pressure (mm Hg) | 27.2 ± 9.7 (n=90) | 27.9 ± 10.5 (n=94) | p = ns |
| Left atrial diameter (cm) | 4.46 ± 0.85 (n=102) | 4.50 ± 0.83 (n=107) | p = ns |
| Degree of pre-op MR | |||
| Mild | 49.6% (n=68) | 70.7% (n=106) | p = 0.003 |
| Mild-moderate | 25.5% (n=35) | 14.7% (n=22) | |
| Moderate | 21.9% (n=30) | 14.7% (n=22) | |
| Moderate-severe | 2.2% (n=3) | 0.0% (n=0) | |
| Severe | 0.7% (n=1) | 0.0% (n=0) | |
| Any structural MV disease | 69.3% (n=95) | 63.3% (n=95) | p = ns |
| Mitral annular calcification | 62.0% (n=85) | 53.3% (n=80) | p = ns |
| Moderate or worse mitral annular calcification | 24 1% (n=33) | 30.0% (n=45) | p = ns |
| Leaflet abnormality | 23.4% (n=32) | 21.3% (n=32) | p = ns |
| B. Comparison of operative characteristics of patients that experienced improvement in MR after aortic valve intervention versus those that did not improve | |||
|---|---|---|---|
| Improved (n=137) |
No improvement (n=150) |
||
| Nature of operation | |||
| AVR | 64.2% (n=88) | 54.0% (n=81) | p = ns |
| AVR + CABG | 21.9% (n=30) | 32.0% (n=48) | |
| AVR + CABG +other | 2.2% (n=3) | 2.7% (n=4) | |
| AVR + other | 10.2% (n=14) | 7.3% (n=11) | |
| Aortic root replacement | 0.7% (n=1) | 0.7% (n=1) | |
| Aortic root replacement + other | 0.7% (n=1) | 3.3% (n=5) | |
| Incidence | |||
| First cardiac surgery operation | 81.0% (n=111) | 81.3% (n=122) | p = ns |
| First re-operation | 17.9% (n=24) | 17.3% (n=26) | |
| Second re-operation | 1.5% (n=2) | 1.3% (n=2) | |
| Third re-operation | 0.0% (n=0) | 0.0% (n=0) | |
| Urgency | |||
| Elective | 70.1% (n=96) | 70.7% (n=106) | p = ns |
| Urgent | 27.0% (n=37) | 27.3% (n=41) | |
| Emergent | 2.9% (n=4) | 2.0% (n=3) | |
| Cardiopulmonary bypass time (min) | 113 ± 42.6 | 125 ± 53.9 | p = ns |
| Valve type | |||
| Bioprosthesis | 96.3% (n=132) | 95.3% (n=143) | p = ns |
| Mechanical | 3.6% (n=5) | 4.7% (n=7) | |
| Homograft | 0.0% (n=0) | 0.0% (n=0) | |
| Implant Size (mm) | 23.8 ± 2.1 | 23.5 ± 2.2 | p = ns |
Abbreviations: MI, myocardial infarction; NYHA, New York Heart Association; mmHg millimeters of mercury; cm, centimeters; MV, mitral valve, ns, not significant.
Abbreviations: AVR, aortic valve replacement; CABG, coronary artery bypass grafting; min, minutes; mm, millimeters; ns, not significant.
DISCUSSION
Previous studies have shown significant variability in the degree of improvement in MR after AVR.1 The most prominent finding of our study is that there is little improvement in MR after relief of aortic outflow tract obstruction by AVR. Looking at the entire cohort of 462 patients that were analyzed, we found that there was a decrease of only 0.08 degrees of MR per patient. As this group contains patients with no MR, it is not surprising that there was very little net change in MR for the group. However, when the same analysis was performed for patients with MR (n =399) and patients with at least mild MR (n=289), we found that the change in MR was less than a half of a degree per patient. Patients with at least moderate MR (n =56) were found to have the greatest improvement in MR, but again, the change in MR observed was only slightly more than half of a degree on average per patient. Since some of these patients underwent concomitant CABG and some had evidence of intrinsic mitral valve disease (most commonly MAC), one could argue that these groups may include patients with some degree of ischemic MR or organic mitral regurgitation. However, when patients that had at least mild MR that underwent AVR only and had no evidence of any structural mitral valve disease (including MAC) were considered, there was still less than half of a degree of improvement in MR per patient after AVR. Thus, regardless of the scenario, one can expect on average roughly a half of a degree reduction in MR at best with AVR for AS. This observation argues in favor of an aggressive approach of concomitant MV repair during AVR when pre-op MR is present.
Several studies have found that the degree of improvement in MR varies based on etiology.2,12 However, Unger and co-investigators found that etiology of MR was not predictive of improvement in MR.13 Our results support the finding by Unger that the etiology of MR does not predict reduction in MR. Specifically, there was no statistically significant difference in the proportion of patients that had leaflet abnormalities, mitral annular calcification, or any structural MV disease between groups of patients that experienced improvement in MR compared to those that did not.
Aside from the etiology of MR, a number of studies have identified pre-operative parameters that appear to predict improvement in MR after AVR. Parameters identified include presence of coronary artery disease, absence of diabetes, absence of pulmonary hypertension, left atrial diameter < 4.5 cm, presence of congestive heart failure, lesser degrees of tricuspid regurgitation, absence of cerebrovascular disease, lower LV EF .4,14,15 Substantial variability exists in these findings and is likely attributable to differences in patient populations included in these studies as well as variability in factors examined and quantitation of these variables. Our analysis was unable to confirm any of these variables as predictors of improvement in MR after AVR. One possibility is that the presence of functional MR after AVR is a symptom of advanced underlying left ventricular pathology. For this reason, echocardiographic parameters of LV dysfunction, particularly diastolic dysfunction, may serve as better predictors of change in MR after AVR.
Pre-operative MR severity was found to be a predictor of improvement in our study as well as others.14,15 While more severe degrees of MR predict improvement, over 40% of patients with moderate MR did not improve in our analysis. Furthermore, the degree of improvement in MR was modest. Specifically, only 0.56 degrees of improvement in MR was observed in the sub-group of patients that derived the greatest benefit. While some patients clearly derived greater benefit than others, we found that improvement in MR is difficult to predict based on simple pre-operative characteristics.
The use of transcatheter aortic valve replacement (TAVR) is becoming an important method of addressing aortic stenosis in high risk patients. Our observation that there is only modest improvement in MR after relief of aortic outflow tract obstruction through open aortic valve or aortic root replacement suggests similar results would be observed after TAVR. A recent study aimed at addressing changes in MR after TAVR concurs with our observations.16 These results, in conjunction with our findings, suggest that one can expect only a modest reduction in MR after TAVR.
Limitations
The major limitation of this study is the retrospective nature of the analysis, as it is subject to the inherent limits of this design. Additionally, since LV remodeling may occur over time after AVR, MR may subsequently change over a longer period of time. One limitation of the current study is the lack of long-term echocardiographic data on patients. Of note, however, no changes were noted in post-procedural MR, compared to early or late follow-up in one study in which these parameters were examined.12 Another limitation of the use of intra-operative echocardiography is that both cardioplegic arrest and loading conditions may alter the degree of mitral regurgitation. For these reasons, both short and long term post-operative assessment of the degree of residual mitral regurgitation should be included in future prospective studies. Additionally, quantitative volumetric measurements of the degree of mitral regurgitation should also be utilized in such studies.
Conclusions
Repairing severe MR in virtually every scenario yields significant benefit.17–22 Not treating intermediate levels of MR in certain settings such as ischemic heart disease portends a worse prognosis.23–25 MR in the setting of aortic stenosis has not been as extensively studied, in part because intermediate MR may decrease with relief of left ventricular pressure overload from AVR. Thus, the optimal management of intermediate degrees of MR at the time of AVR is undefined. Our data demonstrate that there is little improvement in MR after AVR for aortic stenosis. Furthermore, the degree of improvement in MR does not depend on the pre-operative gradient across the aortic valve, otherwise interpreted as: the extent of MR reduction does not correlate with extent of LV pressure gradient reduction. Importantly, our results indicate that it is very difficult to predict which patients will experience any improvement in MR after AVR. Thus, our findings argue in favor of performing a prospective evaluation of the clinical merits of addressing moderate MR at the time of aortic valve intervention.
REFERENCES
- 1.Unger P, Dedobbeleer C, Van Camp G, Plein D, Cosyns B, Lancellotti P. Mitral regurgitation in patients with aortic stenosis undergoing valve replacement. Heart. 2010 Jan;96(1):9–14. doi: 10.1136/hrt.2009.165548. [DOI] [PubMed] [Google Scholar]
- 2.Barreiro CJ, Patel ND, Fitton TP, Williams JA, Bonde PN, Chan V, Alejo DE, Gott VL, Baumgartner WA. Aortic valve replacement and concomitant mitral valve regurgitation in the elderly: impact on survival and functional outcome. Circulation. 2005 Aug 30;112(9 Suppl):I443–1447. doi: 10.1161/CIRCULATIONAHA.104.526046. [DOI] [PubMed] [Google Scholar]
- 3.Moazami N, Diodato MD, Moon MR, Lawton JS, Pasque MK, Herren RL, et al. Does functional mitral regurgitation improve with isolated aortic valve replacement? J Card Surg. 2004 Sep-Oct;19(5):444–448. doi: 10.1111/j.0886-0440.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 4.Caballero-Borrego J, Gómez-Doblas JJ, Cabrera-Bueno F, García-Pinilla JM, Melero JM, Porras C, Olalla E, De Teresa Galván E. Incidence, associated factors and evolution of non-severe functional mitral regurgitation in patients with severe aortic stenosis undergoing aortic valve replacement. Eur J Cardiothorac Surg. 2008 Jul;34(1):62–66. doi: 10.1016/j.ejcts.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Matsumiya G, Sakaguchi T, Miyagawa S, Yamauchi T, Shudo Y, Izutani H, Sawa Y. Impact of untreated mild-to-moderate mitral regurgitation at the time of isolated aortic valve replacement on late adverse outcomes. Eur J Cardiothorac Surg. 2010 May;37(5):1033–1038. doi: 10.1016/j.ejcts.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Rankin JS, Hammill BG, Ferguson TB, Jr, Glower DD, O'Brien SM, DeLong ER, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006 Mar;131(3):547–557. doi: 10.1016/j.jtcvs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Litmathe J, Boeken U, Kurt M, Feindt P, Gams E. Predictive risk factors in double-valve replacement (AVR and MVR) compared to isolated aortic valve replacement. Thorac Cardiovasc Surg. 2006 Oct;54(7):459–463. doi: 10.1055/s-2006-924247. [DOI] [PubMed] [Google Scholar]
- 8.Harling L, Saso S, Jarral OA, Kourliouros A, Kidher E, Athanasiou T. Aortic valve replacement for aortic stenosis in patients with concomitant mitral regurgitation: should the mitral valve be dealt with? Eur J Cardiothorac Surg. 2011 Nov;40(5):1087–1096. doi: 10.1016/j.ejcts.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Christenson JT, Jordan B, Bloch A, Schmuziger M. Should a regurgitant mitral valve be replaced simulataneously with a stenotic aortic valve? Tex Heart Inst J. 2000;27(4):350–355. [PMC free article] [PubMed] [Google Scholar]
- 10.Absil B, Dagenais F, Mathieu P, Métras J, Perron J, Baillot R, et al. Does moderate mitral regurgitation impact early or mid-term clinical outcome in patients undergoing isolated aortic valve replacement for aortic stenosis? Eur J Cardiothorac Surg. 2003 Aug;24(2):217–222. doi: 10.1016/s1010-7940(03)00251-3. [DOI] [PubMed] [Google Scholar]
- 11.Alghamdi AA, Elmistekawy EM, Singh SK, Latter DA. Is concomitant surgery for moderate functional mitral regurgitation indicated during aortic valve replacement for aortic stenosis? A systematic review and evidence-based recommendations. J Card Surg. 2010 Mar;25(2):182–187. doi: 10.1111/j.1540-8191.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Eynden F, Bouchard D, El-Hamamsy I, Butnaru A, Demers P, Carrier M, et al. Effect of aortic valve replacement for aortic stenosis on severity of mitral regurgitation. Ann Thorac Surg. 2007 Apr;83(4):1279–1284. doi: 10.1016/j.athoracsur.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 13.Unger P, Plein D, Van Camp G, Cosyns B, Pasquet A, Henrard V, et al. Effects of valve replacement for aortic stenosis on mitral regurgitation. Am J Cardiol. 2008 Nov 15;102(10):1378–1382. doi: 10.1016/j.amjcard.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Wan CK, Suri RM, Li Z, Orszulak TA, Daly RC, Schaff HV et al. Management of moderate functional mitral regurgitation at the time of aortic valve replacement: is concomitant mitral valve repair necessary? J Thorac Cardiovasc Surg. 2009 Mar;137(3):635–640. doi: 10.1016/j.jtcvs.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Waisbren EC, Stevens LM, Avery EG, Picard MH, Vlahakes GJ, Agnihotri AK. Changes in mitral regurgitation after replacement of the stenotic aortic valve. Ann Thorac Surg. 2008 Jul;86(1):56–62. doi: 10.1016/j.athoracsur.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Tzikas A, Piazza N, van Dalen BM, Schultz C, Geleijnse ML, van Geuns RJ, et al. Changes in mitral regurgitation after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010 Jan 1;75(1):43–49. doi: 10.1002/ccd.22197. [DOI] [PubMed] [Google Scholar]
- 17.Suri RM, Aviernos JF, Dearani JA, Mahoney DW, Michelena HI, Schaff HV, et al. Management of less-than-severe mitral regurgitation: should guidelines recommend earlier surgical intervention? Eur J Cardiothorac Surg. 2011 Aug;40(2):496–502. doi: 10.1016/j.ejcts.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 18.Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ, et al. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovasc Surg. 2009 May;137(5):1071–1076. doi: 10.1016/j.jtcvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Stulak JM, Suri RM, Dearani JA, Burkhart HM, Sundt TM, 3rd, Enriquez-Sarano M, et al. Does early surgical intervention improve left ventricular mass regression after mitral valve repair for leaflet prolapse? J Thorac Cardiovasc Surg. 2011 Jan;141(1):122–129. doi: 10.1016/j.jtcvs.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Vaishnava P, Fuster V, Goldman M, Bonow RO. Surgery for asymptomatic degenerative aortic and mitral valve disease. Nat Rev Cardiol. 2011 Mar;8(3):173–177. doi: 10.1038/nrcardio.2010.203. [DOI] [PubMed] [Google Scholar]
- 21.Carabello BA. The management of functional mitral regurgitation. Curr Cardiol Rep. 2007 Apr;9(2):112–117. doi: 10.1007/BF02938337. [DOI] [PubMed] [Google Scholar]
- 22.Schmitto JD, Lee LS, Mokashi SA, Bolman RM 3rd, Cohn LH, Chen FY. Functional mitral regurgitation. Cardiol Rev. 2010 Nov-Dec;18(6):285–291. doi: 10.1097/CRD.0b013e3181e8e648. [DOI] [PubMed] [Google Scholar]
- 23.Grossi EA, Bizekis CS, LaPietra A, Derivaux CC, Galloway AC, Ribakove GH, et al. Late results of isolated mitral annuloplasty for "functional" ischemic mitral insufficiency. J Card Surg. 2001;16(4):328–332. doi: 10.1111/j.1540-8191.2001.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim YH, Czer LS, Soukiasian HJ, De Robertis M, Magliato KE, Blanche C, et al. Ischemic mitral regurgitation: revascularization alone versus revascularization and mitral valve repair. Ann Thorac Surg. 2005 Jun;79(6):1895–1901. doi: 10.1016/j.athoracsur.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MI, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004 Sep 14;110(11 Suppl 1):II103–II108. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]