Abstract
Lipid phosphate phosphatases (LPPs) are a class of enzymes that can dephosphorylate a number of lysophopholipids in vitro. Analysis of knockouts of LPP family members has demonstrated striking but diverse developmental roles for these enzymes. LPP3 is required for mouse vascular development while the Drosophila LPPs Wunen (Wun) and Wunen2 (Wun2) are required during embryogenesis for germ cell migration and survival. In a recent publication we examined if these fly LPPs have further developmental roles and found that Wun is required for proper tracheal formation. In particular we highlight a role for Wun in septate junction mediated barrier function in the tracheal system. In this paper we discuss further the possible mechanisms by which LPPs may influence barrier activity.
Keywords: Wunen, Wunen2, septate junction, trachea, Drosophila, Serpentine, Vermiform, barrier
Introduction
The ability of cells to organize themselves into tubes is critical to generate many animal tissues including the kidney, lungs and vasculature. The Drosophila trachea is a genetically tractable system that can be used to study not just tube formation but also the regulation of tube size and tube maintenance. The trachea arises from isolated placodes of cells that invaginate from the ectoderm of the embryo, undergo cell rearrangements and eventually fuse and form a continuous lumen. The lumen expands in both diameter and length due to tracheal cell surface expansion and secretion and modification of a chitin cable (reviewed in ref.1).
In our recent paper,2 we identify a novel role for Wun, a LPP, in the formation of the Drosophila trachea. LPPs are a class of enzymes that can dephosphorylate lysophosphatidic acid (LPA), phosphatidic acid (PA), sphingosine 1-phosphate (S1P) and ceramide 1-phosphate (C1P) in vitro.3-6 These enzymes have multiple transmembrane domains and if they are present on the cell surface, the catalytic domain would face outside of the cell.
Wun and its closely related homolog Wun2 act redundantly in germ cell migration where they are required both in the germ cells themselves and also in surrounding somatic tissues.7 Somatic Wun and Wun2 are hypothesized to produce an extracellular lipid gradient in the mesoderm that directs migration of germ cells, which require Wun or Wun2 for survival.
Wunen is Required Tissue Autonomously for Proper Trachea Formation
Trachea lacking Wun exhibit discontinuities in the lumen and bulbous chitin accumulations, and they do not gas fill at the end of embryogenesis.2 wun RNA is maternally provided and expressed in a dynamic pattern during embryogenesis but is not specifically enriched in tracheal cells.8 To determine whether Wun is required autonomously in the trachea itself or whether, like in germ cell migration, it acts non-cell autonomously, we expressed Wun or Wun2 in the trachea or surrounding tissues in an otherwise wun null background. Expression of these LPPs in the trachea fully rescued the tracheal defects while expression in the mesoderm or hindgut had no effect. Therefore, Wun is required tissue autonomously.
Where in the cell is Wun working? We expressed tagged versions of LPPs in tracheal cells and found that Wun-GFP localizes to the apical surface and a lateral membrane region that colocalizes with septate junction (SJ) markers. Interestingly, although other Drosophila and mammalian LPPs can rescue the wun phenotype, these LPPs generally localize over the entire plasma membrane. Thus, although the specific membrane domain localization of Wun is intriguing, the restriction of Wun to this domain is not critical for its function.
Wunen Affects Septate Junctions and Tracheal Permeability
SJs are intercellular junctions made up of at least 13 proteins9 that form a tight barrier between cells which prevents exchange of molecules across an epithelia. These junctions are functionally analogous to mammalian tight junctions. In the trachea, SJ components are restricted to a subapical region on the lateral membrane and prevent molecules from diffusing between the tracheal lumen and the surrounding hemolymph. Mutations in single SJ components are sufficient to cripple barrier function and the remaining SJ components lose their restricted localization and become present along the entire lateral membrane. Loss of SJs also results in an excessively long and convoluted dorsal trunk (the widest tracheal tube which runs along the anterior posterior axis on either side of the embryo). However this phenotype is reported to be independent of the failure in barrier function.10,11
Because Wun-GFP co-localizes with SJ markers, we examined SJ integrity in wun mutants. We found that all SJ components examined were mislocalized along the entire lateral membrane of tracheal cells in wun mutants. This is not due to overall polarity defects; the polarity marker Crumbs localizes normally in wun mutants. The wun trachea also display two other phenotypes associated with loss of SJ integrity: a loss of barrier function, as measured by permeability to injected fluorescently labeled dextran, and an absence of the putative chitin modifying enzymes Serpentine (Serp) and Vermiform (Verm) from the tracheal lumen.
The latter phenotype in SJ mutants is reported to result from a failure of the tracheal cells to secrete these particular proteins, leading to the concept of a SJ-dependent secretion pathway.12 This hypothesis is supported by the presence of intracellular Verm staining in the tracheal cells of certain SJ mutants including Atpα, lachesin/bulbous (lac), sinuous (sinu)12 and varicose (vari).13 Such intracellular Verm staining, however, is not detectable in the wun mutant2 or in other SJ mutants including kune kune (kune),14 coracle (cora)11 and nervana 2 (nrv2).15 In contrast, Serp is not reported to be intracellular in any mutant backgrounds including lac,12,13 vari13 and kune.14
These differing observations may reflect differences in the affinity of the antibodies used and on the precise embryonic stage examined, particularly if intracellularly accumulating Serp or Verm becomes a target for degradation. It should be noted, however, that Serp protein can be detected intracellularly when COP mediated vesicle trafficking is disrupted,16 so low antibody affinity or rapid degradation cannot entirely explain the lack of Serp protein in SJ mutants. Interestingly another luminal component, the 2A12 antigen, accumulates in the lumen normally in SJ mutants.12 This implies that luminal proteins are packaged into different secretory vesicles and these vesicles are differentially affected by the loss of SJs. This is supported by the observation that mutants in diaphanous (which encodes a Formin actin-nucleating factor) have no luminal 2A12 staining but normal luminal levels of Verm.17
It is also theoretically possible that the 2A12 antigen is expressed and secreted slightly earlier than Serp or Verm, before the defects in SJs would start to affect secretion. However by live and fixed tissue analysis the timing of secretion of the various luminal proteins seems to be identical.18
In contrast with these scenarios, we find evidence that Serp and Verm are simply leaking out of the tracheal lumen rather than not being secreted in wun mutants. First we do not detect intracellular Serp and Verm but instead we observe abnormal accumulation of Serp and Verm in the hemolymph in wun mutant embryos. This hemolymph staining is not seen in serp and verm deficiency flies, indicating that this is not non-specific antibody binding. Second we do not see intracellular accumulation of Verm-RFP when expressed in wun mutant tracheal cells. Such tagged protein is stable in tracheal cells because it accumulates intracellularly when COP mediated vesicle trafficking is disrupted.19,20 Third, when ANF-GFP, a heterologous secretion marker, is expressed in trachea cells, instead of accumulating in the lumen as in wild type, it becomes abundant in the hemolymph in wun mutant embryos.
In conclusion, the lack of luminal Serp and Verm in different mutant backgrounds can result from a failure or reduction in secretion and/or a failure of the secreted proteins to remain in the lumen.
Trachea of Wun and a Septate Junction Mutant are Permeable to 70 kDa Molecules
Our hypothesis that Serp and Verm leak out of the wun trachea is based on the assumption that the SJ defects lead to large enough pores that Serp and Verm can leak out between tracheal cells. The dextran exclusion assay used to test SJ integrity is normally performed with fluorescently labeled 10 kDa dextran.2,10,14,21,22 However Serp and Verm are 65 and 70 kDa respectively, so this assay does not predict whether Serp and Verm can leak through defective SJs. Therefore, we tested whether a 70 kDa dextran was capable of leaking into the trachea of wun wun2 or cora (which encodes a SJ component) mutant embryos. We found that within 10 min after injection, the 70 kDa dextran was visible in the tracheal lumen of cora and wun wun2 mutants, but not a wild-type control (Fig. 1). The fluorescence intensity of dextran in the tracheal lumen was less than that in the hemolymph, and this difference was seen even 1 h after injection. This contrasts to the 10 kDa dextran in which the fluorescence intensity was equivalent in both the hemolymph and tracheal lumen 10 min after injection.2 Though the leakage of the 70 kDa marker into the tracheal lumen is less than with the 10 kDa marker, the presence of 70 kDa dextran in the tracheal lumen of cora and wun wun2 mutants leads us to conclude that loss of SJ function causes permeability to 70 kDa molecules.
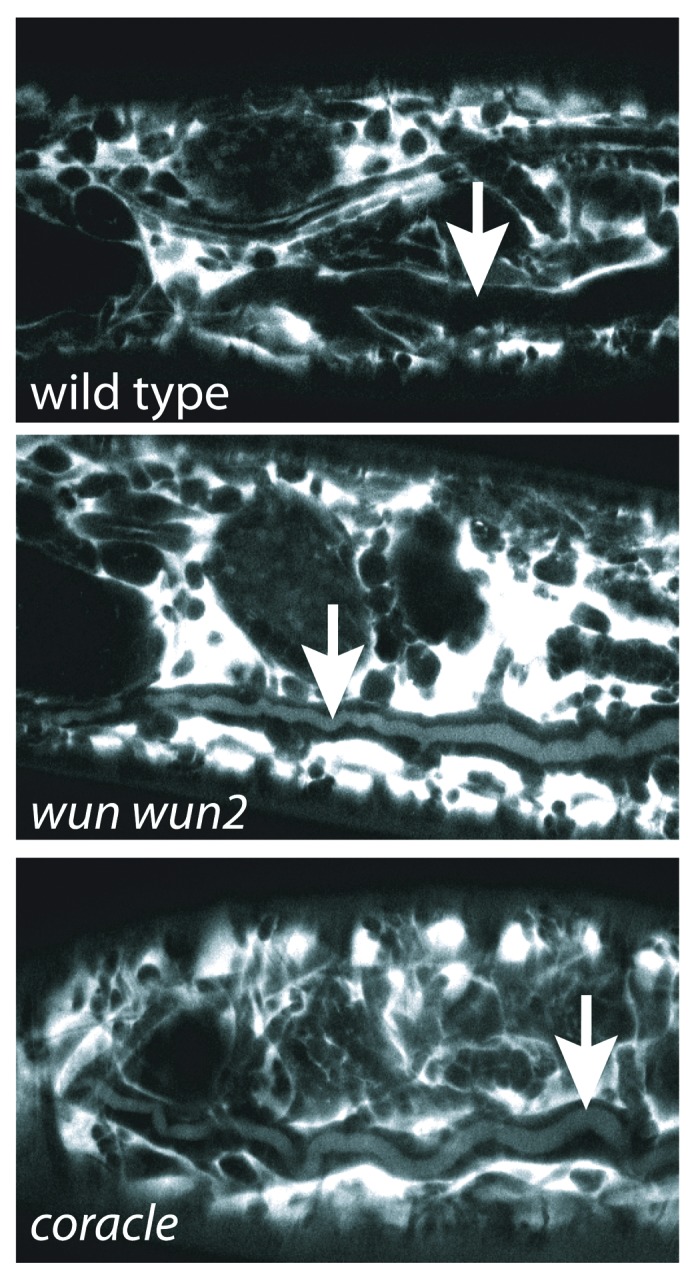
Figure 1. 70 kDa dextran can leak into the trachea of cora and wun mutants. 70 kDa dextran was injected into the hemolymph of stage 16 wild type and cora1 and wun wun2 mutants, and embryos were imaged 10 min after injection. 70 kDa dextran is visible in the trachea of cora1 and wun wun2 embryos, but not wild type embryos. Arrows indicate the tracheal dorsal trunk.
If the Serp and Verm can leak out of wun and SJ mutant trachea, this raises the question of why the 2A12 antigen remains in the tracheal lumen. Without knowing the molecular identity of the 2A12 antigen, we can only postulate that the protein is either too large to diffuse out of the trachea or it binds tightly to chitin or other trachea luminal components.
Proteins Involved in SJ Formation and Maintenance
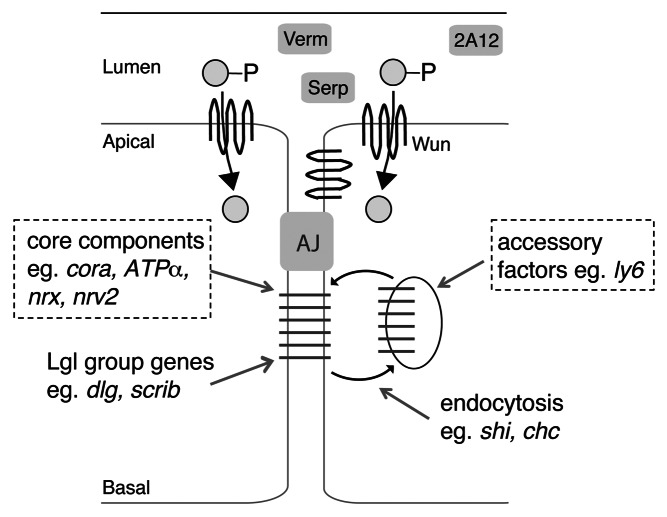
The proteins that are required for functional SJs can be divided into different classes (Fig. 2): First, there are the “core components” including Megatrachea/Pickel (Mega), Sinu, ATPα, Nrv2, Neurexin IV (NrxIV), Neuroglian (Nrg), Vari and Cora.23 These components are thought to interact to form a tight complex. Second, several cell polarity proteins, such as Lethal giant larvae (Lgl) and Discs large (Dlg), co-localize with SJ markers and are required for proper localization of SJ components and hence barrier function. However, these polarity proteins are not part of the core complex and the core SJ can form in their absence (though it mislocalizes).23 Finally, several classes of proteins that do not localize to SJs are nevertheless required for its proper formation and/or localization. One group is the Ly6 family members, which are thought to help the SJ components assemble in an internal compartment of the cell. Another group are those required for intracellular vesicle trafficking, such as Clathrin heavy chain and Dynamin (encoded by shibire), which are not required for SJ components to form a complex, but are required for the complex to localize properly.23,24
Figure 2. Pathways that contribute to SJ formation and localization. The proteins required for SJ formation and localization can be divided into four classes (see text). The proteins indicated with the dotted line box are necessary for the interaction and formation of the SJ “core”. The other groups of proteins are not required for SJ core complex formation, but are instead required for proper SJ localization. A failure in SJ complex formation or localization causes Serp and Verm to no longer accumulate in the tracheal lumen, while the 2A12 antigen is unaffected. We hypothesize that Wun acts to produce a lipid product (indicated by a gray circle), which could affect SJs via one or more of the aforementioned pathways.
Wun could be affecting SJs via one or more of above pathways. Wun itself probably does not play a strictly structural role in the SJ since a catalytically dead Wun or Wun2 (resulting from a single amino acid change) cannot rescue the SJ defects of a wun mutant (ref. 2 and our unpublished data). The presence of Wun-GFP at the apical and apicolateral surface means it can potentially reduce the luminal levels of a particular substrate. Alternatively it has been demonstrated that dephosphorylation by LPPs renders the products favorable to entering the cell.7,25 If this holds true for tracheal cells, the intracellular accumulation of the lipid product, or a metabolite arising from such a product, may be the critical function of Wun.
In the later case, a particular lipid environment may help mediate core SJ complex formation. Alternatively, Wun could provide a lipid necessary for trafficking of SJ complexes, which is necessary to restrict them in the lateral membrane. For example, sphingolipids can influence vesicle trafficking.26-28 One way to better understand the mode of action for Wun would be through fluorescence recovery after photobleaching (FRAP) studies, such as those described in reference 23. SJ core components show very little lateral membrane movement as compared with SJ-associated polarity proteins such as Lgl and Dlg, so the kinetics of Wun-GFP recovery after photobleaching could be used to determine whether it is a component of the SJ core complex. Further, when SJ core complexes do not form properly, the SJ components show a greater mobility in the membrane. Therefore, by performing FRAP on SJ core components in a wun null background, it could be determined whether Wun is required for the formation of the SJ core complex.
Potential Wun Lipid Substrates
Another question raised by our studies is which lipid(s) are the critical Wun substrate. Because enzymes that synthesize and degrade potential Wun substrates are known,8,29 genetic approaches could be used to identify lipids important in tracheal development. So far, there are no reports of tracheal phenotypes in lipid modifying enzyme mutants, however, many of these enzymes are not well studied. Additionally, because the wun tracheal phenotype is seen only in the absence of both maternal and zygotic wun, it may be necessary to look at these enzymes in maternal loss-of-function conditions.
Little is yet known about the complement of lipids present in tracheal lumen or in the Drosophila hemolymph. However, recent advances in lipid mass spectrometry have allowed identification of lipids in specific tissues of Drosophila larvae, indicating that analysis of tissue-specific embryonic lipids may soon be possible.30
While efforts to identify the particular substrates are ongoing, our work already suggests that the lipid substrate for Wun is different in germ cell migration and tracheal development. A mammalian LPP (mouse LPP2) that is capable of rescuing the wun mutant tracheal phenotype does not rescue the germ cell phenotype.2 We hypothesize that LPP2 dephosphorylates a particular lipid that is essential for tracheal formation but it cannot dephosphorylate the particular lipid that is essential for germ cell migration, implying these two lipids are different. In this scenario, Wun and Wun2 would show less substrate specificity and be able to act on both lipids. As further evidence for distinct mechanisms of Wun in germ cell migration and trachea formation, we find that germ cell migration is normal in embryos lacking maternal and zygotic Cora (our unpublished data), indicating that Wun is not acting through SJs to affect germ cells.
A General Role for LPPs in Promoting Barrier Function?
A role for LPPs in tube formation is also seen in mammals. Mice lacking LPP3 die during embryogenesis and exhibit defects in placenta and yolk vasculature formation. Though the vasculature cells are specified correctly, they are not able to form the capillary tubes.31 More recently, mice with postnatal inactivation of the LPP3 gene specifically in the vascular endothelium are viable but have impaired vascular endothelial barrier function leading to increased vascular leakage, particularly in the lungs (M. Panchatcharam, A. J. Morris and S. S. Smyth, personal communication). Vascular endothelial cells contain tight junctions as well as adherens junctions, and it will be exciting to see if either of these is affected in the mutant animals. While septate junctions and tight junctions are ultrastructurally distinct they do contain a common family of proteins (the claudins).22 A more general role for LPPs in tube formation or maintenance is suggested by their expression pattern. Mouse LPP2 is enriched in lung and kidney32 while mouse LPP3 is also expressed in the mammary gland.33
In mammals, S1P and LPA have demonstrated roles in forming and protecting vascular endothelium.34,35 Most of the known effects of these lipids are through activation of G-protein coupled receptors (GPCRs). However, there are no S1P or LPA activated GPCR homologs in Drosophila, and it is not yet clear whether there are GPCR-independent LPA and/or S1P pathways in mammals or Drosophila. The identification of either the LPP lipid substrate or its receptor in Drosophila will allow us to further examine whether LPP function is analogous in these systems.
Acknowledgments
This work was supported by the Max Planck Society.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/22938
References
- 1.Schottenfeld J, Song Y, Ghabrial AS. Tube continued: morphogenesis of the Drosophila tracheal system. Curr Opin Cell Biol. 2010;22:633–9. doi: 10.1016/j.ceb.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ile KE, Tripathy R, Goldfinger V, Renault AD. Wunen, a Drosophila lipid phosphate phosphatase, is required for septate junction-mediated barrier function. Development. 2012;139:2535–46. doi: 10.1242/dev.077289. [DOI] [PubMed] [Google Scholar]
- 3.Burnett C, Howard K. Fly and mammalian lipid phosphate phosphatase isoforms differ in activity both in vitro and in vivo. EMBO Rep. 2003;4:793–9. doi: 10.1038/sj.embor.embor900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jasinska R, Zhang QX, Pilquil C, Singh I, Xu J, Dewald J, et al. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem J. 1999;340:677–86. doi: 10.1042/0264-6021:3400677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts R, Sciorra VA, Morris AJ. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J Biol Chem. 1998;273:22059–67. doi: 10.1074/jbc.273.34.22059. [DOI] [PubMed] [Google Scholar]
- 6.Kai M, Wada I, Imai S, Sakane F, Kanoh H. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J Biol Chem. 1997;272:24572–8. doi: 10.1074/jbc.272.39.24572. [DOI] [PubMed] [Google Scholar]
- 7.Renault AD, Sigal YJ, Morris AJ, Lehmann R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 2004;305:1963–6. doi: 10.1126/science.1102421. [DOI] [PubMed] [Google Scholar]
- 8.Renault AD, Starz-Gaiano M, Lehmann R. Metabolism of sphingosine 1-phosphate and lysophosphatidic acid: a genome wide analysis of gene expression in Drosophila. Mech Dev. 2002;119(Suppl 1):S293–301. doi: 10.1016/S0925-4773(03)00131-X. [DOI] [PubMed] [Google Scholar]
- 9.Wu VM, Beitel GJ. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr Opin Cell Biol. 2004;16:493–9. doi: 10.1016/j.ceb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–74. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- 11.Laprise P, Paul SM, Boulanger J, Robbins RM, Beitel GJ, Tepass U. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr Biol. 2010;20:55–61. doi: 10.1016/j.cub.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Jayaram SA, Hemphälä J, Senti KA, Tsarouhas V, Jin H, et al. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr Biol. 2006;16:180–5. doi: 10.1016/j.cub.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, et al. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134:999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson KS, Furuse M, Beitel GJ. The Drosophila Claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010;185:831–9. doi: 10.1534/genetics.110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson KS, Khan Z, Molnár I, Mihály J, Kaschube M, Beitel GJ. Drosophila Src regulates anisotropic apical surface growth to control epithelial tube size. Nat Cell Biol. 2012;14:518–25. doi: 10.1038/ncb2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grieder NC, Caussinus E, Parker DS, Cadigan K, Affolter M, Luschnig S. gammaCOP is required for apical protein secretion and epithelial morphogenesis in Drosophila melanogaster. PLoS One. 2008;3:e3241. doi: 10.1371/journal.pone.0003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massarwa R, Schejter ED, Shilo BZ. Apical secretion in epithelial tubes of the Drosophila embryo is directed by the Formin-family protein Diaphanous. Dev Cell. 2009;16:877–88. doi: 10.1016/j.devcel.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Tsarouhas V, Senti KA, Jayaram SA, Tiklová K, Hemphälä J, Adler J, et al. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell. 2007;13:214–25. doi: 10.1016/j.devcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Forster D, Armbruster K, Luschnig S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol. 2010;20:62–8. doi: 10.1016/j.cub.2009.11.062.. [DOI] [PubMed] [Google Scholar]
- 20.Armbruster K, Luschnig S. The Drosophila Sec7 domain guanine nucleotide exchange factor protein Gartenzwerg localizes at the cis-Golgi and is essential for epithelial tube expansion. J Cell Sci. 2012;125:1318–28. doi: 10.1242/jcs.096263. [DOI] [PubMed] [Google Scholar]
- 21.Hijazi A, Masson W, Augé B, Waltzer L, Haenlin M, Roch F. boudin is required for septate junction organisation in Drosophila and codes for a diffusible protein of the Ly6 superfamily. Development. 2009;136:2199–209. doi: 10.1242/dev.033845. [DOI] [PubMed] [Google Scholar]
- 22.Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–20. doi: 10.1016/S1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 23.Oshima K, Fehon RG. Analysis of protein dynamics within the septate junction reveals a highly stable core protein complex that does not include the basolateral polarity protein Discs large. J Cell Sci. 2011;124:2861–71. doi: 10.1242/jcs.087700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiklová K, Senti KA, Wang S, Gräslund A, Samakovlis C. Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila. Nat Cell Biol. 2010;12:1071–7. doi: 10.1038/ncb2111. [DOI] [PubMed] [Google Scholar]
- 25.Roberts RZ, Morris AJ. Role of phosphatidic acid phosphatase 2a in uptake of extracellular lipid phosphate mediators. Biochim Biophys Acta. 2000;1487:33–49. doi: 10.1016/S1388-1981(00)00081-0. [DOI] [PubMed] [Google Scholar]
- 26.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 27.Acharya U, Mowen MB, Nagashima K, Acharya JK. Ceramidase expression facilitates membrane turnover and endocytosis of rhodopsin in photoreceptors. Proc Natl Acad Sci U S A. 2004;101:1922–6. doi: 10.1073/pnas.0308693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Göbel V. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol. 2011;13:1189–201. doi: 10.1038/ncb2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acharya U, Acharya JK. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol Life Sci. 2005;62:128–42. doi: 10.1007/s00018-004-4254-1. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho M, Sampaio JL, Palm W, Brankatschk M, Eaton S, Shevchenko A. Effects of diet and development on the Drosophila lipidome. Mol Syst Biol. 2012;8 doi: 10.1038/msb.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Jr-Gang-Cheng, et al. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–37. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N, Sundberg JP, Gridley T. Mice mutant for Ppap2c, a homolog of the germ cell migration regulator wunen, are viable and fertile. Genesis. 2000;27:137–40. doi: 10.1002/1526-968X(200008)27:4<137::AID-GENE10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Escalante-Alcalde D, Morales SL, Stewart CL. Generation of a reporter-null allele of Ppap2b/Lpp3and its expression during embryogenesis. Int J Dev Biol. 2009;53:139–47. doi: 10.1387/ijdb.082745de. [DOI] [PubMed] [Google Scholar]
- 34.Ren HM, Panchatcharam M, Mueller P, Escalante-Alcalde D, Morris AJ, Smyth SS. Lipid phosphate phosphatase (LPP3) and vascular development. Biochim Biophys Acta. 2012;1831:126–32. doi: 10.1016/j.bbalip.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teo ST, Yung YC, Herr DR, Chun J. Lysophosphatidic acid in vascular development and disease. IUBMB Life. 2009;61:791–9. doi: 10.1002/iub.220. [DOI] [PMC free article] [PubMed] [Google Scholar]