Abstract
Bateman’s experimental study of Drosophila melanogaster produced conclusions that are now part of the bedrock premises of modern sexual selection. Today it is the most cited experimental study in sexual selection, and famous as the first experimental demonstration of sex differences in the relationship between number of mates and relative reproductive success. We repeated the experimental methodology of the original to evaluate its reliability. The results indicate that Bateman’s methodology of visible mutations to assign parentage and reproductive success to subject adults is significantly biased. When combined in offspring, the mutations decrease offspring survival, so that counts of mate number and reproductive success are mismeasured. Bateman’s method overestimates the number of subjects with no mates and underestimates the number with one or more mates for both sexes. Here we discuss why Bateman’s paper is important and present additional analyses of data from our monogamy trials. Monogamy trials can inform inferences about the force of sexual selection in populations because in monogamy trials male–male competition and female choice are absent. Monogamy trials also would have provided Bateman with an a priori test of the fit of his data to Mendel’s laws, an unstated, but vital assumption of his methodology for assigning parentage from which he inferred the number of mates per individual subject and their reproductive success. Even under enforced monogamous mating, offspring frequencies of double mutant, single mutant and no mutant offspring were significantly different from Mendelian expectations proving that Bateman’s method was inappropriate for answering the questions he posed. Double mutant offspring (those with a mutation from each parent) suffered significant inviability as did single mutant offspring whenever they inherited their mother’s marker but the wild-type allele at their father’s marker locus. These inviability effects produced two important inaccuracies in Bateman’s results and conclusions. (1) Some matings that actually occurred were invisible and (2) reproductive success of some mothers was under-estimated. Both observations show that Bateman’s conclusions about sex differences in number of mates and reproductive success were unwarranted, based on biased observations. We speculate about why Bateman’s classic study remained without replication for so long, and we discuss why repetition almost 60 years after the original is still timely, necessary and critical to the scientific enterprise. We highlight overlooked alternative hypotheses to urge that modern tests of Bateman’s conclusions go beyond confirmatory studies to test alternative hypotheses to explain the relationship between mate number and reproductive success.
Keywords: Drosophila melanogaster, A.J. Bateman, Mendel’s rules, fitness variances, genetic parentage tests, number of mates, number of offspring, reproductive success
The Paper That Shaped Studies in Sexual Selection
A.J. Bateman’s experimental study of Drosophila melanogaster1 has had a huge impact on empirical and theoretical studies in modern sexual selection.2-7 Bateman’s conclusions are now part of the bedrock premises of modern sexual selection and they are foundational ideas in the evolutionary study of sex differences.2 Bateman’s results were that (1) male number of different mates (NM) was greater than female NM, (2) males had greater variation in number of adult offspring, i.e., “reproductive success” (RS) than females, and (3) because of the number of mates, which Bateman inferred from plots of RS against NM (now known as “Bateman gradients”). Bateman said that his data showed that sex difference in the variance in number of offspring was the sign (italicized in the original) of “intra-masculine selection” while the cause of selection among males was a “stronger correlation” in males between NM and RS. His conclusions were consistent with Darwin’s discussion8 of the evolution of elaborate male traits via female choice and male–male competition.
In Bateman’s time it was impossible to do the carefully controlled parentage assignment study he attempted on any organism other than D. melanogaster. The work of the great early 20th century drosophilist, Thomas Hunt Morgan, provided the necessary tools when he discovered, cultured, and maintained lines of flies with spontaneously arising and phenotypically dramatic mutations and then explored Mendelian inheritance patterns according to the basic rules of Mendelian genetics (see ref. 9). Bateman’s study simply could not have been imagined before there was a solid understanding of the origins and inheritance of phenotypically-obvious mutations in an experimentally tractable model organism, putting Bateman’s “discoveries” in the very large bin of experimental firsts with D. melanogaster.
Trivers’s rediscovery10 of Bateman’s previously little-cited paper on D. melanogaster1 propelled Bateman’s inferences onto center stage in studies of sexual selection and sex differences. Deservedly so, as it was the first study that seemed to experimentally demonstrate what Darwin hypothesized about the force of female choice and male–male combat8 on variation among male rivals in their number of mates. It was the first experimental study to conclude what Fisher proposed: a greater variance in fitness among males associated with sexual selection on males (measured as number of eclosed offspring).11 Until 2007,12 it was unrecognized that the paper also provided the first experimental demonstration that mating with more than one male enhances females’ number of offspring. Bateman claimed he had confirmed Darwin’s ideas about the force of female choice and male–male competition1 on male fitness variance.8 He said he had shown “….that sexual selection is more effective in males than females,”1 (p. 363) which he argued was consistent with the idea that males are not as discriminating in mating behavior as females, an argument that was interpreted for a very long time as though there was no evidence for fitness payouts to females who mated with more than one male or for the fact that males are choosy too. Bateman also argued that egg number limits female reproduction, which “…causes a severe strain on their nutrition” so that reproductive capacity limits female reproductive success, but sperm limitation was unlikely to limit male reproduction. In other words, Bateman argued that resources limit females, but that females limit males. “With intramasculine selection males will be expected to show polygamous tendencies whereas in females there would be selection in favour of obtaining only one mate after which they would become relatively indifferent” (p. 367). These ideas are ones that took hold and spread.
The parental investment (PI) paper of Trivers10 inspired a generation when it linked sex differences in PI to the evolution of genes for choosy behavior in females and indiscriminate behavior in males. Among the most important results of the PI idea was its prediction that when PI is greater in males, males would be “the choosy sex” and females the indiscriminate sex.
After Trivers, Arnold seized on Bateman’s ideas and called the results “principles”2: truths to count on or at least important assumptions to test. In a nutshell, what Bateman did with the help of these authors was anchor within-sex variance in number of mates (VNM) as the key predictor of variance in reproductive success (VRS),” which is sometimes considered the only measure of sexual selection. Bateman’s paper is part of the standard historical trajectory in the controversial paradigm of the origins of sex differences.3,7
Why Did We Replicate Bateman (1948)?
Given that replication is a pillar of the scientific method, the previous lack of strict repetition seemed odd to us. A few previous investigators had evaluated and questioned Bateman’s methods,12 his results,7 the sweep of his conclusions3 and the implications others took from his study.3,7,13 In particular, we wondered if the obvious failure of data in Bateman’s Table 41 (reproduced in Fig. 1) to match Mendel’s expectations was a systematic problem associated with all of his populations. We wondered: Would a strict repetition of Bateman’s methods calm our concerns and those of earlier critics?
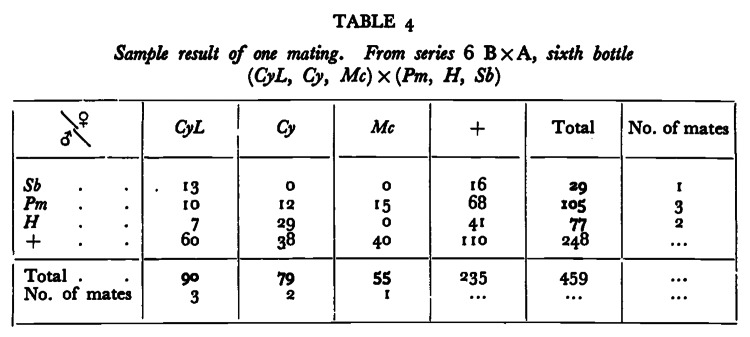
Figure 1. Bateman’s (1948) Table 4, p. 357 (reprinted by permission from Macmillan Publishers Ltd. License Number: 2960430598991), showed the logic of Bateman’s method for assigning parentage and thus inferring NM and RS per adult in a given population. The table is the only completely displayed data for any population in Bateman’s study. The tabled values are the number of offspring that carried a parental marker. The only offspring that allowed inference of NM were the double mutants—M♀M♂. In the table M♀M♂ offspring include the 13 that inherited the mother’s CyL dominant gene and the father’s Sb dominant gene, and so on. To estimate the number of offspring that each mother produced, he took the sum of their M♀M♂ + M♀w♂ offspring and for each father he took the sum of their M♀M♂ + w♀M♂ offspring. Most important, however, is that from the data in this table one can compute the frequencies of M♀M♂, M♀w♂, w♀M♂ and w♀w♂ and compare them to Mendel’s expectations under the assumption that each adult was a dominant heterozygote at a unique locus and homozygous wild type at every other adults marker loci. The values are a significant departure from Mendelian expectations with M♀M♂ significantly fewer than 25%.12
We also wondered if other explanations might explain the key results as well as or better than sexual selection seemed to do, so we planned to test the results against the predictions of sexual selection and stochastic demography (i.e., chance variation in individual survival and/or chance encounters with potential mates).14,15
In addition, we were interested in following up the questions about the validity of Bateman’s method that arose when we12 were reading his paper to ensure that our repetition was as faithful as possible to its methodology, which is what we mean by “strict.” Our reading identified several problems with the methodology. The most important was obvious from Bateman’s Table 4 (Fig. 1), which he included as an example of how he inferred numbers of mates and number of offspring per subject. The frequency of offspring inheriting and phenotypically expressing the dominant marker gene from each parent was significantly lower than the ¼ that Mendel’s rules require (Fig. 2), suggesting that in combination, the marker mutations lowered offspring viability and potentially could have biased observations of NM and RS.
Figure 2. A cartoon of the even frequencies of offspring phenotypes when parents are unique heterozygote dominants each at a unique locus. Drosophilist Sergio Castrezana, PhD, painted the image styled as Mayan-like hieroglyphs in a Mexican bark painting in black ink and acrylic on amate paper.
“Repetitions” using modern molecular genetic studies, of course, exist. However, these are not repetitions in the strict sense, but rather stand-alone tests of “Bateman’s principles.” Tang-Martinez16 has recently reviewed these studies and shown that while many studies confirm Bateman’s principles, others do not. Studies reporting results inconsistent with Bateman’s principles17-20 and the many studies showing a benefit of polyandry21-24 for females implicitly reject Bateman’s conclusion that selection does not favor female multiple mating. The studies that demonstrated consistency with Bateman’s principles4,6,25-28 piqued our interest further, as alternative explanations for Bateman’s observations besides sexual selection have also been available for at least 25 years; yet few workers have tested their observations against these alternatives.15,29 A repetition could have put our minds to rest about the validity of Bateman’s conclusions and might have provided an unbiased evaluation of mate number and reproductive success that would allow robust tests of the currently obvious alternatives.15,29 It seemed to us way past time to replicate Bateman’s study using his original methodology.
What the Repetition Discovered
Our repetition appears to be unique in that we tried to replicate exactly Bateman’s methodology of parentage assignment as far as we could. We used the same mutant lines Bateman used. In our study, we cultured adult subjects as Bateman had done, so that each expressed a unique genetically-determined marker phenotype (Fig. 3A and B). Table S1 in our original report30 illustrates that each adult subject was genetically and phenotypically distinct; that is, (regardless of their sex) in each of our replicated populations, each adult carried a single allele at its marker locus, while having only wild-type alleles at all other subjects’ marker loci. We included the cultured adults in experimental populations in the same combinations of sexes, markers per sex, age of males and females and duration of the period during which mating could occur that Bateman reported (see Table S1 in ref.30).
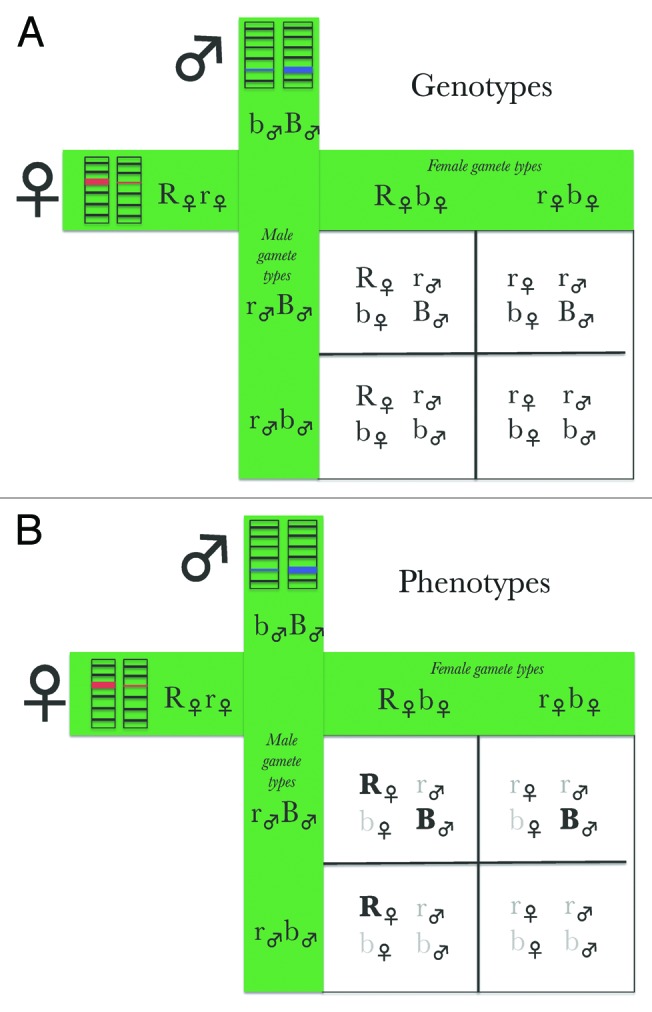
Figure 3. A stylized view of offspring genotypes (A) and observable offspring phenotypes (B) when each parent is a heterozygote dominant at a unique marker locus and wild type at the other parent’s marker locus. In both panels capital letters indicate dominant alleles and lower case indicates wild-type alleles for two parents each with a dominant marker allele each at a different locus. The male’s maker locus is indicated by “B” and the female’s by “R.” Wild-type alleles at mother’s marker locus are indicated by lowercase, “r” and at father’s marker locus by lowercase “b.” In (B) the bolded letters indicate visible mutations.
Following Bateman’s method closely, we counted the number of mates using only the offspring with a mutation from each parent, the double mutant offspring, M♀M♂s. According to Bateman, M♀M♂s allowed an unbiased count of how many mates the subject adults had. Like Bateman we used the sum of the double mutant offspring plus the single mutant offspring (those with a marker mutation from one parent only) as an estimate of the number of offspring for each subject adult (see Fig. 1).
Like Bateman we did not watch the mating behavior of our subjects, so we had no better estimate of who mated with whom than Bateman did. Thus, the veracity of Bateman’s study and our replication turned on the “fairness” of the parental markers: Were they neutral with respect to Bateman’s goals of inferring number of mates and number of offspring for each subject? This question is one that modern forensic scientists and those studying genetic parentage also must do and do in fact address. If markers fail to fit Mendel’s rules or Hardy-Weinberg expectations or are otherwise non-neutral, modern geneticists exclude particular loci from use in their studies of forensics, kinship, and genetic parentage.31,32 Bateman-era geneticists would have used the simple expectations from a consideration of Mendelian principles to test the fairness of the markers (Figs. 2 and 3): Bateman did test if half the offspring could be assigned to mothers and half to fathers, and concluded that there was no statistical difference in the representation among offspring of mothers’ vs. fathers’ marker phenotypes. However, if he tested whether the frequency of double mutant offspring was unbiased, he did not report it. It appears he did not test his data against Mendel’s expected frequencies as his Table 4 (Fig. 1) data clearly significantly depart from Mendelian frequencies. Such a test of the frequency of adult markers in offspring is the decisive one required for demonstration that estimates of number of mates per individual and the VNM were unbiased, fairly representing who did and did not mate.
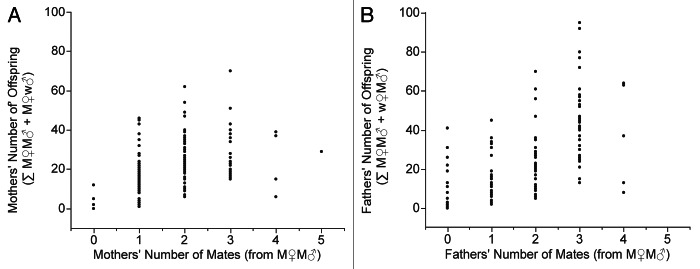
Assuming Mendelian inheritance of alleles when each parent is heterozygous dominant at unique loci (Fig. 1), there should be 25% of offspring with both parental mutations, 25% with the dominant allele at mother’s marker locus but a wild-type allele at father’s marker locus, 25% with the wild-type allele at mother’s marker locus and the dominant allele at father’s marker locus and 25% with the wild-type allele at each of their parents’ marker loci (Figs. 2 and 3). We generalize these types of offspring phenotypically as M♀M♂, M♀w♂, w♀M♂ and w♀w♂, respectively. The frequency of M♀M♂ offspring was significantly lower than expected overall.30 The fatal flaw that our repetition revealed is that the method miscounts the number of mates for each sex—key variables in Bateman’s study—to an unknown degree, because in Bateman’s study the only information about who mated with whom was from the phenotypes of the M♀M♂ offspring. Using Bateman’s method we coded some subjects as having zero mates, when they in fact had one or more mates, which was clear when we observed “zero mated subjects” whose phenotypes appeared in their single mutant—M♀w♂ and w♀M♂—offspring (Fig. 4). Thus the method of assigning parentage miscounted the number of subjects with 0, 1, 2, 3, 4 or 5 mates. The error in counts of zero mates was systematically larger for males than for females as Figure 4 shows. Thus counts of NM were inappropriate, and by necessity the estimates of VNM were inaccurate and perhaps misleading to an unknown extent.
Figure 4. Subjects seemingly without mates had offspring, which is highly unlikely in a sexually reproducing diploid species. Reproductive success counted as the sum of M♀M♂ plus M♀w♂ for female subjects (A) and as M♀M♂ plus w♀M♂ for male subjects (B) against the number of mates counted from M♀M♂ offspring for females (A) and for males (B) exposes a biological impossibility. Bateman’s method overestimates the number of individuals with zero mates (21 subjects among females and 43 among males), thereby underestimating the number with one or more mates. The magnitude of error among male subjects (43 males out of a total N of 166 males) was greater than among female subjects (21 females out of a total N of 166 females), which would have falsely increased the VNM estimates of males relative to females. We use these plots to illustrate one of the most egregious errors in Bateman’s method: subjects who seemed to have no mates had offspring.
The second flaw arose because significantly more single mutant offspring survived when they had their mother’s wild-type allele and their father’s mutant allele (that is, the w♀M♂s) than offspring with their mother’s marker allele and their father’s wild-type allele (the M♀w♂s), the sex differences in reproductive success were also biased, showing higher RS for fathers than for mothers. Although Bateman did report that the numbers of offspring from M♀w♂ and w♀M♂ were about equal and his Table 4 (Fig. 1) suggested statistical equality in RS for assigned parents, he did not report the frequency of M♀M♂s or the test for parental equality of RS in his entire experiment. Had he reported a test of observed frequencies of M♀M♂, M♀w♂, w♀M♂ and w♀w♂ against Mendel’s expectations of M♀M♂, M♀w♂, w♀M♂ and w♀w♂, he would have provided the information that his contemporaries and modern readers needed to evaluate the reliability of his markers given his questions. Although it appeared to be “cutting edge” in its day, the methodology of Bateman’s study appears to have been fatally flawed.
Our replication showed that using Bateman’s method produced two observations that are biologically impossible or at least extremely unlikely. Figure 4 shows the first by examining inferences about NM and RS for the 166 female subjects (A) and 166 male subjects (B) in the replication. The x-axis is the number of mates for subjects counted from M♀M♂ offspring, the only offspring providing information about who mated with whom. The y-axis is reproductive success counted as the ∑ = M♀M♂ + M♀w♂ (for female subjects, the mothers) or ∑ = M♀M♂ + w♀M♂ (for male subjects, the fathers). A single fact exposes the bias in Bateman’s method: Some individuals that we counted using Bateman’s method as having zero mates nevertheless, using Bateman’s method, had offspring (Fig. 4). The mismatch between information in double mutant and single mutant offspring was due in our experiment to significantly lower viability of M♀M♂ offspring. Remember that single mutant offspring inform questions about how many offspring each subject had, but they are silent about who mated with whom. Because of the inviability of double mutant offspring, some adults were scored as having zero mates, when in fact that had some unknown number of mates. The resultant miscount incorrectly increased the number of males in the class having zero mates, and the number with ≥ 1 was consequently underestimated. Using the method Bateman used, Figure 4 shows that Bateman would have incorrectly assigned many more males than females to the zero class of number of mates, so that the effect would be to increase the male VNM relative to the effect on female VNM. There is no way to know what effect the incorrect assignment of subjects to the zero mating class has on the under-estimation of the subjects in the classes with ≥ 1 mate (Fig. 4).
The second biological impossibility that our replication revealed was that there was a systematic bias in counts of offspring with fathers vs. mothers. That is, using Bateman’s method we were able to assign genetic paternity significantly more often than genetic maternity, making it seem as though more offspring had fathers than mothers, verifying that there was something wrong with the methodology, just as we12 had earlier suspected. More paternal assignments occurred because the number of w♀M♂ offspring was greater than the number of M♀w♂ offspring. We tested for significance differences with a plot of the difference scores in the apparent number of offspring with fathers minus the apparent number with mothers (see Fig. 1 in ref. 30). Because the bias resulted in assigning more fathers than mothers as parents, the estimate of RS was greater for fathers than mothers, demonstrably because of mismeasurements derived from Bateman’s method: single mutant offspring who inherited the dominant allele from their mother’s marker locus plus a wild-type allele at their fathers had lower viability than offspring who inherited a dominant allele from their father’s marker locus but a wild-type from their mother’s marker locus. Systematic, methodological mismeasurement of number of mates and number of offspring leaves open to question Bateman’s conclusion that there is an enhanced effect of RS as a function of NM for males, but not females. Because of the bias in the methodology, our repetition could not address the main questions that Bateman set out to answer. If his overall data, not just the data in his Table 4 (Fig. 1), were inadequate to his questions as our repetition suggests, his answers to his questions would be unreliable as well.
M♀M♂ Offspring from Monogamous Pairs Also Were Inviable
A control that was available to Bateman, but that he did not report having done was to test the frequencies of M♀M♂, M♀w♂, w♀M♂ and w♀w♂ in trials of monogamous pairs representing each possible combination of parents. Such a test a priori would have informed Bateman of the fairness of the markers as unbiased indicators of parentage. Notably because sexual selection is absent in strict monogamy, it would also have provided a strong comparative test of the force of sexual selection in his populations.
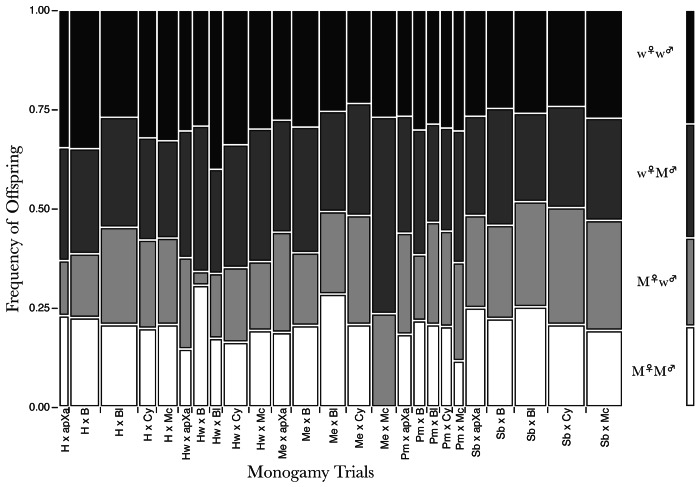
We reported monogamy trials in our original report along with the tabled counts and frequencies of M♀M♂, M♀w♂, w♀M♂ and w♀w♂ for each combination of heterozygote dominant female and male (see Table S4 in ref. 30). We showed that even under monogamy that only four out of 25 of the pair combinations (each with five trials) had M♀M♂ at or near 25% (see tabled data, Table S14 in ref. 30). The vast majority were under 25%. Figure 5 shows graphically the distribution of M♀M♂, M♀w♂, w♀M♂ and w♀w♂ of offspring for each set of pairs in the monogamy trials that included analysis of 12,005 offspring phenotypes. Overall M♀M♂ were significantly less frequent than the Mendelian expected frequency of 25%. Had Bateman performed trials of this sort, he would have known that his methodology—at least with the mutations he used as marker “nametags” for his potentially breeding subjects—was inappropriate for and incapable of answering his questions about who mated with whom, which provided in his experiment the only data on the number of mates for each subject.
Figure 5. Frequencies of M♀M♂, M♀w♂, w♀M♂ and w♀w♂ offspring from monogamy trials (5 sets for each parental marker combination). The column on the far left identifies the offspring types: White bars = M♀M♂, light gray bars = M♀w♂, dark gray bars = w♀M♂ and black bars = w♀w♂.
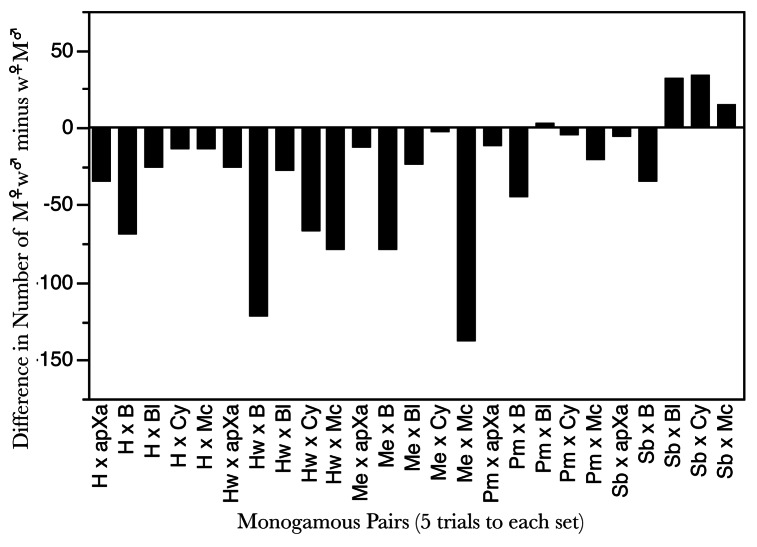
In addition, had Bateman performed monogamy trials, he a priori could have observed that his methodology—at least with the mutations he used—was also inappropriate for evaluating reproductive success. Consider that counts of M♀M♂ offspring are necessarily equal for mothers and fathers. However, counts of M♀w♂ and w♀M♂ may differ. The question then arises: Are counts of M♀w♂ and w♀M♂ significantly different from 50% of offspring with fathers and 50% with mothers? A simple way to answer this question is in Figure 6 that shows the frequency of difference scores for numbers of M♀w♂ and w♀M♂ in each set of the monogamy trials. Single mutant offspring reveal only one of their parent’s identities; thus on average for each parental set in the monogamy trials averaged over the five trials per set, we expect there would be no systematic differences in the number of offspring that were scored as having mothers and fathers. Figure 6 shows that more offspring apparently had fathers than had mothers, which is a biological impossibility. The reasons for the bias in the inferences appear to be that in M♀w♂ offspring, the father’s wild-type alleles may fail to rescue offspring from the deleterious effects of the mother’s marker allele. Alternatively, in w♀M♂, the mother’s wild-type alleles may have rescued offspring from the deleterious effects of the father’s marker allele. This is speculation, of course. There is little way to know what explains the apparent difference in the number of offspring with fathers compared with the offspring with mothers without additional experiments on the effects of the mutations singly and in combination.
Figure 6. The difference in the number of offspring assigned to mothers and fathers computed from M♀w♂ and w♀M♂ offspring that individual adults confined in monogamous pairs produced. There were five trials of monogamous pairs in each parental combination of unique markers. The distribution of difference scores was significantly different from 0 (t-test = −3.7004, DF = 24, p > |t| = 0.0011).
In sum, the monogamy trials did reveal that the marker genes Bateman used were deleterious to offspring when inherited together. It is worth noting that half of the markers Bateman used and that we used were homozygous lethal (see Table 1 in Bateman1 and Table 1 in ref. 30); however, no potential parent in either Bateman’s or our experiment was a homozygote for their marker allele. More important, because each parent had a unique marker locus, no offspring could be homozygous dominant for any single one of the parental marker loci. Thus the deleterious effects on offspring viability are from inheritance of the parental marker alleles in combination (Fig. 5).
What Can the Replication Say About the Original Study?
What can we make of Bateman’s original study and his claims given our study? First and most important, if throughout his study double mutant offspring occurred in significantly lower frequencies than 25% as they did in the one population for which he displayed all the offspring phenotypes (Fig. 1), his entire study, not just one population, would have been biased. Our replication emphasizes the likelihood of that possibility.
The replicated results show that in all but one of our 46 populations M♀M♂ offspring were significantly less frequent than expected. If that also were true for Bateman’s original study, Bateman too would have overestimated the number of adults that failed to mate, underestimated the number who mated one or more times and perhaps may also have assigned parentage to more fathers than mothers, a biological impossibility in species in which offspring have both a mother and a father. If Bateman’s method was flawed as our repetition suggests it was, he had no valid evidence for sex differences in numbers of mates or reproductive success, much less for VNM and VRS. In keeping with Bateman’s method, because we did not watch behavior, we have no basis for claiming that males mated indiscriminately or that females were indifferent to mating more than once.
Critics may claim that the mutant lines we used may have undergone significant evolutionary change since Bateman’s day that affected the results. That, of course, is a possibility, yet the hypothesis that guided our research was the evidence in Bateman’s own study (Fig. 1) that double mutant offspring were significantly less frequent than Mendel’s expected frequency of ¼. Our results are very like the results in the only bit of data in Bateman’s study (Fig. 1) that allowed a test against Mendel’s expectations. And, it remains the case that without a time machine, it would be difficult for anyone today to replicate Bateman’s method. We think we did the best that anyone could do by using the lines of flies Bateman used that are still available today.
Thus, our repetition raises potentially unanswerable questions about Bateman’s original studies. Were the double mutant offspring in his experiment as likely to die as in the repetition and as frequently as the offspring must have done in his Table 4 (Fig. 1) population? Bateman did seem to assign more offspring to fathers than to mothers in his overall study;12 however, the investigators reporting that observation worked only from Bateman’s published data, which Bateman included in summary tables that lacked some details. These are questions that only a time machine can conveniently answer now. And, perhaps that is the main value of our repetition: It may tell us all that we can know now about Bateman’s original study.
Given that Bateman did his study 60 years ago and given that its citation frequency soared in the mid-1970s, it remains curious that it was not repeated post-1972. We continue to wonder why. Were Bateman’s results so intuitive and comfortable that investigators didn’t think to retest it? It is true that textbook authors “simplified” a description of Bateman’s gradients leaving out more than half the information available,3,7 something that could have misled students, increasing their “comfort zone” with the material, but surely could not account for the lack of vigilance of professionals. Was a repetition just too hard to do? We admit that it was a challenging, complex, time-consuming experiment to complete, so we can imagine that others began but did not complete a repetition. We also wonder if perhaps other strict repetitions do exist but remain unpublished. Are there strict replications whose authors never offered them up for publication? Are there strict replications that were rejected? That too may be a question whose answer is lost to time. But, the unanswered questions continue to beg why our field did not repeat the classic sooner.
Our field might profitably do some soul-searching: Why were Bateman’s obvious errors overlooked for so long? As we said30 in our primary report, legions of graduate students have for the past 40 years read and discussed Bateman. Why did they not bring attention to the errors? Surely all of them, among biologists at least, understand the elements of mutation, inheritance and Mendelian genetics. Why did their professors not challenge Bateman’s results? We are inclined to the idea that Bateman’s results and conclusions are so similar to status quo, dominating world-views (competitive males, dependent females) that pre-existing cultural biases of readers may have dampened skepticism and objectivity. Perhaps lack of repetition is simply due to lack of professional incentives such as funding for repetitions.
Why Does the Repetition Challenge “the Paradigm”?
It seems that the modern bedrock of sexual selection may have been quicksand. But does a single replication of an important study invalidate an entire field? We do not think so.
Yet in the company of other challenges to the competitive male and discriminating female paradigm, the repetition gives pause,33 particularly in light of the “problems with paradigms.”7 “…paradigms act as a “lingua franca” that facilitates communication among scientists. On the negative side, paradigms can lead to simplification, can blind us to phenomena that do not fit the accepted world-view, can guide us to accept hypotheses that are unfounded, and can prevent us from considering alternative hypotheses and explanations. In such cases paradigms become dogma and have detrimental effects on the development of scientific ideas”7 (p. 821) Perhaps we should routinely urge our students to challenge paradigmatic material. Perhaps we need new rules such as “if it’s intuitive, test it.” Are we as a field asking the right questions? For example, could both males and females in most species assess others’ quality as mates before accepting or rejecting potential mates,14,34 as happens in D. pseudoobscura?35 Could males and females in all species be simultaneously choosy and competitive?14,34 Might the mechanisms of sexual selection act on number of mates among males, but on quality of mates among females?36 Or more radically, might both females and males enjoy significant fitness benefits through mate assessment and adaptively flexible mate choice based on their constantly updated predictions of the viability of potential offspring?37 What exactly does a Bateman gradient tell about among-female competition over the quality of mates? What if female and male reproductive competition is mediated in one sex via within sex variation in quality of mates and in the other via within sex variation in number of mates? Would the metric of sexual selection then be the same for females and males? If females and males do compete over different things, perhaps we need a new metric for evaluating the effect on VRS of variance in mate quality (VMQ).
Questions about Modern Studies of Bateman Gradients
A probable rejoinder from some readers of our recent report30 is that the repetition fails to weaken Bateman’s principles, because other studies have validated them (see above for examples). Even in the face of validation from other studies in other species, the repetition does matter, because it puts in a different, more interesting light the published studies with results inconsistent with Bateman’s conclusions, and it demands that investigators with results consistent with Bateman’s conclusions take care of at least two imperative remaining concerns: (1) Observation bias and (2) failure to test alternative hypotheses for fitness variances.
Genetic parentage studies allow inferences about mating systems when copulations and identification of individuals are hard to observe. Some, but far from most, modern investigators of genetic parentage watch copulations and therefore have an independent estimate of the possibility that this individual mated with this and that potential mate. But, copulations that actually occur may be invisible to molecular genetic testing even when genetic markers are neutral, fit Hardy-Weinberg expectations, or have low mutation frequencies, because sperm may be inviable or females might not use the sperm. Thus, investigators must watch behavior of individually identified subjects and simultaneously account for “detection bias” in the field38,39 and in the laboratory.
It is rarely possible to identify all potential breeders in a wild-living population, something that challenges all genetic parentage studies in the field, particularly of organisms that fly, flies and birds being notable examples. Laboratory populations have walls around them; wild-living populations do not. But, in truth it is just plain hard to identify individual fruit flies in a jar without intrusive manipulations, which could bias subjects’ mate assessments, or contests among rivals for resources or mate access. Thus, it is a real possibility that observational biases are near ubiquitous in parentage studies on wild and captive organisms as well. We suggest caution as it is likely that estimates of fitness variances are often invisibly biased, with consequent effects on the VNM that many consider the key mechanism of sexual selection.
Acknowledgments
We thank Stephen P. Hubbell for comments on the manuscript and Margaret Anderson for technical support and advice. We gratefully acknowledge funding from NSF grants IBN-9631801, IBN-0911606, IOS-1121797 to P.A.G. and W.W.A., which provided support for various aspects of the work reported here. Attributions: P.A.G., W.W.A. and Y.K.K. designed the original research; technicians blind to the purpose of our study, under the supervision of W.W.A. and Y.K.K., performed the experiments; P.A.G. performed analyses, made figures and wrote the papers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/23505
References
- 1.Bateman AJ. Intra-sexual selection in Drosophila. Heredity (Edinb) 1948;2:349–68. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 2.Arnold SJ. Bateman principles and the measurement of sexual selection in plants and animals. Am Nat. 1994;144:S126–49. doi: 10.1086/285656. [DOI] [Google Scholar]
- 3.Dewsbury DA. The darwin-bateman paradigm in historical context. Integr Comp Biol. 2005;45:831–7. doi: 10.1093/icb/45.5.831. [DOI] [PubMed] [Google Scholar]
- 4.Jones AG, Arguello JR, Arnold SJ. Validation of Bateman’s principles: a genetic study of sexual selection and mating patterns in the rough-skinned newt. Proc Biol Sci. 2002;269:2533–9. doi: 10.1098/rspb.2002.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones AG, Ratterman NL. Mate choice and sexual selection: what have we learned since Darwin? Proc Natl Acad Sci U S A. 2009;106(Suppl 1):10001–8. doi: 10.1073/pnas.0901129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc Biol Sci. 2000;267:677–80. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang-Martinez Z, Ryder TB. The Problem with Paradigms: Bateman’s Worldview as a Case Study. Integr Comp Biol. 2005;45:821–30. doi: 10.1093/icb/45.5.821. [DOI] [PubMed] [Google Scholar]
- 8.Darwin C. The descent of man, and selection in relation to sex. London: John Murray, 1871. [Google Scholar]
- 9.Kohler RE. Lords of the fly: Drosophia genetics and the experimental life. University of Chicago Press, 1994. [Google Scholar]
- 10.Trivers RL. Parental investment and sexual selection. In: Campbell B, ed. Sexual Selection and the Descent of Man. Chicago, IL: Aldine, 1972: 136-179. [Google Scholar]
- 11.Fisher RA. The genetical theory of natural selection. Oxford: Oxford Clarendon Press, 1930. [Google Scholar]
- 12.Snyder BF, Gowaty PA. A reappraisal of Bateman’s classic study of intrasexual selection. Evolution. 2007;61:2457–68. doi: 10.1111/j.1558-5646.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 13.Parker PG, Tang-Martinez Z. Bateman gradients in field and laboratory studies: a cautionary tale. Integr Comp Biol. 2005;45:895–902. doi: 10.1093/icb/45.5.895. [DOI] [PubMed] [Google Scholar]
- 14.Gowaty PA, Hubbell SP. Chance, time allocation, and the evolution of adaptively flexible sex role behavior. Integr Comp Biol. 2005;45:931–44. doi: 10.1093/icb/45.5.931. [DOI] [PubMed] [Google Scholar]
- 15.Hubbell SP, Johnson LK. Environmental variance in lifetime matng success, mate choice, and sexual selection. Am Nat. 1987;130:91–112. doi: 10.1086/284700. [DOI] [Google Scholar]
- 16.Tang-Martinez Z. Bateman's principles: Original experiment and modern data for and against. In: Breed MD & Moore J, eds. Encyclopedia of animal behavior. Oxford: Academic Press, 2010:166-176. [Google Scholar]
- 17.Williams RN, DeWoody JA. Reproductive Success and Sexual Selection in Wild Eastern Tiger Salamanders (Ambystoma t. tigrinum) Evol Biol. 2009;36:201–13. doi: 10.1007/s11692-009-9058-7. [DOI] [Google Scholar]
- 18.Lorch PD. Understanding reversals in the relative strength of sexual selection on males and females: a role for sperm competition? Am Nat. 2002;159:645–57. doi: 10.1086/339992. [DOI] [PubMed] [Google Scholar]
- 19.Ketterson ED, Parker PG, Raouf S, Nolan V, Ziegentus C. The relative impact of extra-pair fertilizations on variation in male and female reproductive success in dark-eyed juncos (Junco hyermatis) Ornithological Monogaphas. 1998;49:81–101. doi: 10.2307/40166719. [DOI] [Google Scholar]
- 20.Brown GR, Laland KN, Mulder MB. Bateman’s principles and human sex roles. Trends Ecol Evol. 2009;24:297–304. doi: 10.1016/j.tree.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becher SA, Magurran AE. Multiple mating and reproductive skew in Trinidadian guppies. Proc Biol Sci. 2004;271:1009–14. doi: 10.1098/rspb.2004.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao L. Evolution of polyandry in a communal breeding system. Behav Ecol. 1997;8:668–74. doi: 10.1093/beheco/8.6.668. [DOI] [Google Scholar]
- 23.García-González F, Simmons LW. The evolution of polyandry: intrinsic sire effects contribute to embryo viability. J Evol Biol. 2005;18:1097–103. doi: 10.1111/j.1420-9101.2005.00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Ronkainen K, Kaitala A, Kivela SM. Polyandry, multiple mating, and female fitness in a water strider Aquarius paludum. Behav Ecol Sociobiol. 2010;64:657–64. doi: 10.1007/s00265-009-0883-6. [DOI] [Google Scholar]
- 25.Balenger SL, Johnson LS, Mays HL, Masters BS. Extra-pair paternity in the socially monogamous mountain bluebird Sialia currucoides and its effect on the potential for sexual selection. J Avian Biol. 2009;40:173–80. doi: 10.1111/j.1600-048X.2009.04521.x. [DOI] [Google Scholar]
- 26.Bjork A, Pitnick S. Intensity of sexual selection along the anisogamy-isogamy continuum. Nature. 2006;441:742–5. doi: 10.1038/nature04683. [DOI] [PubMed] [Google Scholar]
- 27.Jones A. Validation of Bateman's principles: Comparative evidence from taxa with conventional and reversed sex roles. Integr Comp Biol. 2003;43:834. [Google Scholar]
- 28.Zhang M, Woodruff RC. Confirmation of the Bateman's principle: a sexual selection exercise. Drosoph Inf Serv. 2006;89:143–8. [Google Scholar]
- 29.Sutherland WJ. Chance can produce sex differences in mating success and explain Batman's data. Anim Behav. 1985;33:1349–1352. doi: 10.1016/S0003-3472(85)80197-4. [DOI] [Google Scholar]
- 30.Gowaty PAG, Kim Y-K, Anderson WW. No evidence of sexual selection in a repetition of Bateman’s classic study of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2012;109:11740–5. doi: 10.1073/pnas.1207851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler JM. Forensic DNA typing: Biology, technology, and genetics of STR markers. London: Elsevier, 2005:660. [Google Scholar]
- 32.Butler JM. Advanced topics in forensic DNA typing: Methodology. London: Academic Press, 2012:704. [Google Scholar]
- 33.Tang-Martínez Z. Repetition of Bateman challenges the paradigm. Proc Natl Acad Sci U S A. 2012;109:11476–7. doi: 10.1073/pnas.1209394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gowaty PA, Hubbell SP. Reproductive decisions under ecological constraints: it’s about time. Proc Natl Acad Sci U S A. 2009;106(Suppl 1):10017–24. doi: 10.1073/pnas.0901130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson WW, Kim YK, Gowaty PA. Experimental constraints on mate preferences in Drosophila pseudoobscura decrease offspring viability and fitness of mated pairs. Proc Natl Acad Sci U S A. 2007;104:4484–8. doi: 10.1073/pnas.0611152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altmann J. Mate choice and intrasexual reproductive competition: contributions to reproduction that go beyond acquiring more mates. In Gowaty PA, ed. Feminism and evolutionary biology: Boundaries intersections and frontiers. New York, NY: Chapman and Hall, 1997:320-333. [Google Scholar]
- 37.Gowaty PA, Hubbell SPH. The quantitative theory of Baysian animals: individually flexible reproductive decisions under ecological constraints predict fitness. Behav Brain Sci. 2013 doi: 10.1017/S0140525X12002385. In press. [DOI] [PubMed] [Google Scholar]
- 38.Gowaty PA, Bridges WC. Behavioral, demographic, and environmental correlates of extrapair fertilizations in eastern bluebirds, Sialia sialis. Behav Ecol. 1991;2:339–50. doi: 10.1093/beheco/2.4.339. [DOI] [Google Scholar]
- 39.Gowaty PA, Bridges WC. Nestbox availability affects extra-pair fertilizations and conspecific nest parasitism in eastern bluebirds, Sialia sialis. Anim Behav. 1991;41:661–75. doi: 10.1016/S0003-3472(05)80904-2. [DOI] [Google Scholar]