Abstract
CD4 T cell activation, essential for productive HIV infection, is provided initially in acute HIV infection by innate immune system secretion of activating cytokines. This cytokine response wanes with time and long-term activation of CD4 cells is maintained by HIV Tat protein secreted by HIV infected cells. Structured treatment interruption (STI) in well-controlled antiretroviral-treated (ART) subjects was explored a decade ago with a consensus finding that, in most subjects, HIV levels rebounded within four weeks to pre-ART levels. Based on these observations we initiated a randomized placebo-controlled study of a universal anti-Tat epitope vaccine, TUTI-16, to determine if immunological blockade of Tat would prevent HIV rebound after ART cessation. TUTI-16 immunization was safe, with predominantly mild local and systemic injection-related adverse reactions. TUTI-16 was also immunogenic, with high levels of anti-Tat antibodies compared with levels previously shown to reduce HIV replication in vivo. Of 21 subjects analyzed, 13 (62%) had HIV rebounds vs. 8 (38%) that remained aviremia, but this distribution was not vaccine-related (p = 0.61 log-rank (Mantel-Cox) test), nullifying our hypothesis that anti-Tat antibodies would block rebound of Tat-dependent set-point HIV viremia after ART cessation. Our present findings are consistent with recent molecular findings that rebounding virus following STI is homogeneous and unrelated to previous circulating HIV, suggesting that rebounding HIV represents new founder virus, akin to the original acute HIV infection. We propose, therefore, that STI may have potential as a practical and economical approach to testing the safety and efficacy of candidate prophylactic HIV vaccines.
Keywords: acute HIV infection, antiretroviral, clinical trial, HIV-1, human vaccine, prophylactic, therapeutic, rebound, Tat, TUTI-16
Introduction
Thirty years after the first clinical identification of HIV infection there is widespread agreement that a preventative vaccine will be needed to control the epidemic but no vaccine is yet at hand. Basic studies provide ample evidence of severe barriers to vaccine development. Initial infection is by one or a small number of founder virus(es), a vigorous innate immune system response ensues with high levels of activating cytokines ensuring rapid HIV replication, and an active cellular immune response initially lowers viral levels but leads to selection of variants that evade the initial response but, in turn, trigger responses to these new variants.1-3 A steady-state chronic infection is the usual outcome that, untreated, leads to forward HIV transmission and slow progression to AIDS and death. A conventional vaccine anticipating the antigenicity of the founder viruses is improbable considering the high antigenic variation of HIV and, after infection is established, host immune response and viral mutation seem to have evolved a programmed interplay that usually maintains viral chronicity and does not eliminate HIV.
Activated CD4 T cells are the major site of productive HIV replication and the blockade of mechanisms that induce and maintain CD4 cell activation may offer a potential alternate point for vaccine interdiction. During acute HIV infection the innate immune system responds with a storm of pro-inflammatory activating cytokines including, notably, TNFα, a response not seen in other viral infections such as hepatitis B and C.3 Additionally, HIV Tat protein, produced in, and secreted from, cells supporting active HIV replication,4 amplifies CD4 activation further once HIV is established during acute infection.5,6 In time, the innate immune system production of pro-inflammatory activating cytokines subsides, leaving Tat protein to maintain the chronic activated state of the adaptive immune system and the set point HIV viremia characteristic of chronic HIV infection.
Anti-TNFα therapy is clinically effective in rheumatoid arthritis,7 acting by lowering not only TNFα but also a wide array of elevated pro-inflammatory cytokines.8 By analogy, a corresponding vaccine could be considered for HIV but this is probably inadvisable because this would not only block soluble TNFα, responsible for CD4 cell activation, but also transmembrane TNFα, that favors induction of apoptosis in HIV-infected cells rather than virus production9 and is critical for specific cellular immune responses controlling intracellular infections including HIV.10,11 A Tat based vaccine for HIV was proposed in 1996,12 directed at immunologic control of chronic HIV infection and particularly targeted at prevention of forward transmission. Immunization with whole native Tat or Tat toxoid has been reported to induce modest immunogenicity but no evidence of control of plasma HIV counts in humans.13-15 Because the Tat sequence shares the high variability of most Tat proteins we have focused on a Tat epitope vaccine that has allowed us to include the small number of variants within a short epitope sequence and develop a universally reactive vaccine, termed TUTI-16.16,17
The clinical exploration of the utility of Tat epitope vaccination to control set point HIV viremia was initiated in ART-naïve asymptomatic HIV subjects.18 TUTI-16 was safe but the antibody response was barely detectable. Nevertheless a statistically significant decrease in HIV viral count was detected in the low dose 30 μg treated group but not in the higher dose groups. We attributed absence of efficacy at the higher doses to adjuvant activation and demonstrated, in small open label groups that TUTI-16 was fully immunogenic in healthy HIV seronegative subjects and in aviremic ART controlled subjects. HIV viremia was not provoked in the presence of active ART therapy. A dose of 1 μg TUTI-16 appeared to be optimal.
The present study was based on these exploratory findings and on the extensive literature on supervised treatment interruption (STI) studies dating from 1999 to 2003.19-26 In eight reports encompassing 84 subjects well controlled by ART 76/84 subjects (90%) had rebounds of HIV viral load within several days to 4 weeks, with viral loads reaching or surpassing set point levels before ART initiation. Rebound after STI was considered to represent a restoration of persistent low level HIV replication27 in ART controlled subjects27 to the pre-treatment set point level and the hypothesis was that TUTI-16 would be immunogenic in ART controlled subjects, that adjuvant-induced activation would not obscure anti-Tat blockade of Tat-driven CD4 T cell activation, and that rebound would be prevented. A randomized placebo-controlled double-blind study of TUTI-16 immunization was initiated to garner further safety and immunogenicity data and to determine if Tat epitope immunization would prevent HIV rebound to set point viremia following ART cessation. The primary endpoint was safety and the secondary endpoints were immunogenicity and prevention of HIV rebound following ART cessation.
Results
Study population and safety evaluations
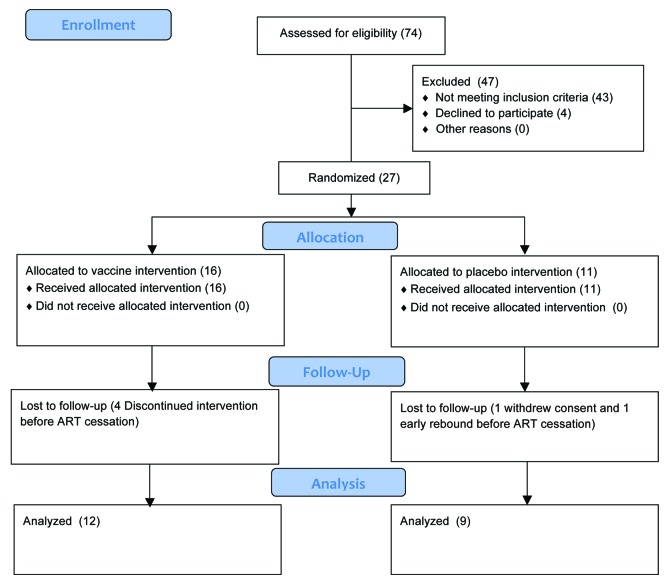
Twenty-seven subjects were randomized and, of these, 12 vaccine and 9 placebo subjects progressed through ART cessation and were evaluated (Fig. 1). The demographics are displayed in Table 1. CD4 cell counts at baseline were not statistically different between the two groups (p = 0.17, T test, two-sided) (Fig. 2), supporting effective randomization. Duration of ART at baseline was not statistically different between the two groups (p = 0.61, T test, two-sided). 13/16 (81%) of vaccinated subjects had adverse reactions that were possibly injection-related vs. 4/11 (36%) in the placebo group (p = 0.04, Fisher’s exact two-way test, two-sided). There were no serious adverse reactions with all adverse reactions being classified as mild with the exception of one moderate reaction.
Figure 1. CONSORT subject disposition chart.
Table 1. Baseline demographics and injection related adverse reactions.
| Treatment Groups | ||
|---|---|---|
| |
Vaccine (n = 16) |
Placebo (n = 11) |
|
Age (years) | ||
| Mean (SD) |
43 (8) |
42 (9) |
| Range |
27–55 |
24–54 |
|
Gender | ||
| Male |
4 |
9 |
| Female |
12 |
2 |
|
Race | ||
| Caucasian |
0 |
2 |
| Black |
9 |
8 |
| Latino/Hispanic |
6 |
1 |
| Asian |
1 |
0 |
|
Plasma HIV level (RNA copies/mL) | ||
| Mean < 20 < 20 |
|
|
|
CD4 cell count (cells/µL) | ||
| Mean (SD) |
858 (203) |
718 (229) |
| Range |
570–1176 |
415–1093 |
|
Duration of ART (months) | ||
| Mean (SD) |
59 (52) |
73 (73) |
| Range |
8–187 |
17–216 |
|
Antiretroviral Therapy (Number of subjects) | ||
| Atripla |
7 |
6 |
| Other combinations* |
9 |
5 |
|
Injection related reactions (incidence)** | ||
| None |
3/16 (19%) |
7/11 (64%) |
| Mild |
12/16 (75%) |
4/11 (36%) |
| Moderate | 1/16 (6%) | 0 |
Including (vaccine, placebo): Truvada (8,6), Norvir (3,1), Kaletra (3,1), Regataz (2,1), Combivir (0,1), Epizicon (0,2), Isentress (1,1), Prezista (1,1), Intelence (1,1), Viramun (1,1), Lexiva (0,1) Viread (1,0) and Ziagen (1,0). **Including (vaccine, placebo): Pain at injection site (5,0), flu like symptoms, (3,0), chills (2,0) dizziness (2,0) myalgia (2,0), fatigue (1,0), nausea (1,1), pyrexia (1,1), rash (1,1), headache (1,2) abdominal pain (0,1), headache (0,2)
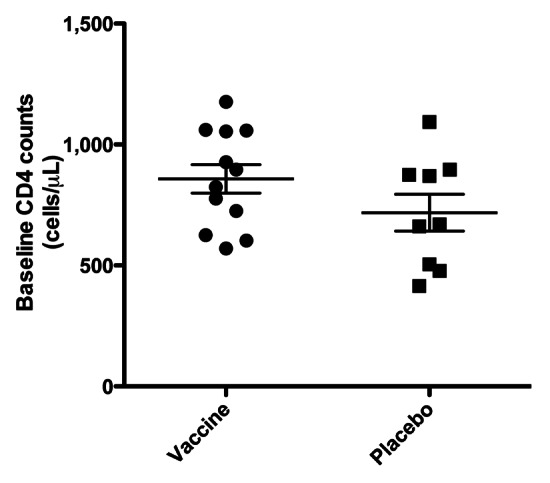
Figure 2. Baseline CD4 T cell counts (mean ± SEM) demonstrating successful randomization at study entry.
Anti-Tat antibodies
Anti-Tat antibodies were absent at baseline with the exception of two serums with levels of 44 and 86 ng/mL, barely above the 40 ng/mL assay limit of detection. There were strong primary and secondary antibody responses in 10 of 12 vaccinated subjects at 3 (159 ± 30) (mean ± SEM) ng/mL and 6 (670 ± 222) weeks post immunization vs. no antibody responses in the placebo group 0/9 (p < 0.0001, Student’s exact T test, two-tailed).
HIV plasma HIV levels prior to ART cessation
One placebo subject escaped ART control at week 6 (17,180 HIV RNA copies/mL), prior to ART cessation, and was not included in the subsequent analysis. Other than this, HIV remained largely undetectable except for sporadic low increases in vaccinated (up to 126 HIV RNA copies/mL) and placebo (up to 1,000 HIV RNA copies/mL) subjects. There were no statistically significantly differences between the groups (data not shown).
Rebounds in HIV plasma HIV levels following ART cessation
Rebounds occurred in 8/12 (67%) vaccinated subjects and 5/9 (56%) placebo subjects, as shown in the Kaplan-Meier plot (Fig. 4). There was no statistically significant difference between the two groups (p = 0.61, Log Rank (Mantel-Cox) Test). The Hazard Ratio was 0.73 (95% Cl 0.22 to 2.06). The median time from ART cessation to rebound was 6 weeks for the vaccine group vs. 4.7 weeks for placebo subjects, ratio 1.28 (95% Cl 0.95 to 1.60). Consolidating both arms the time to rebound was 5.5 ± 0.7 (mean ± SEM) and the mean rebound free time at study closure was 8.2 ± 1.5 (Fig. 5). Less than half the subjects rebounded in less than 4 weeks, in contrast to earlier reported studies (see above).
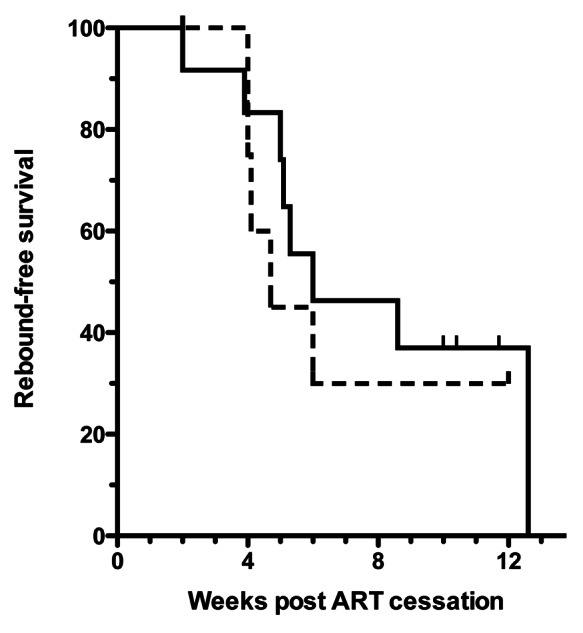
Figure 4. Kaplan-Meier rebound-free survival graph. Solid line denotes vaccine, interrupted line placebo. Rebound was defined as viral load greater than 3,000 HIV RNA copies/mL plasma.
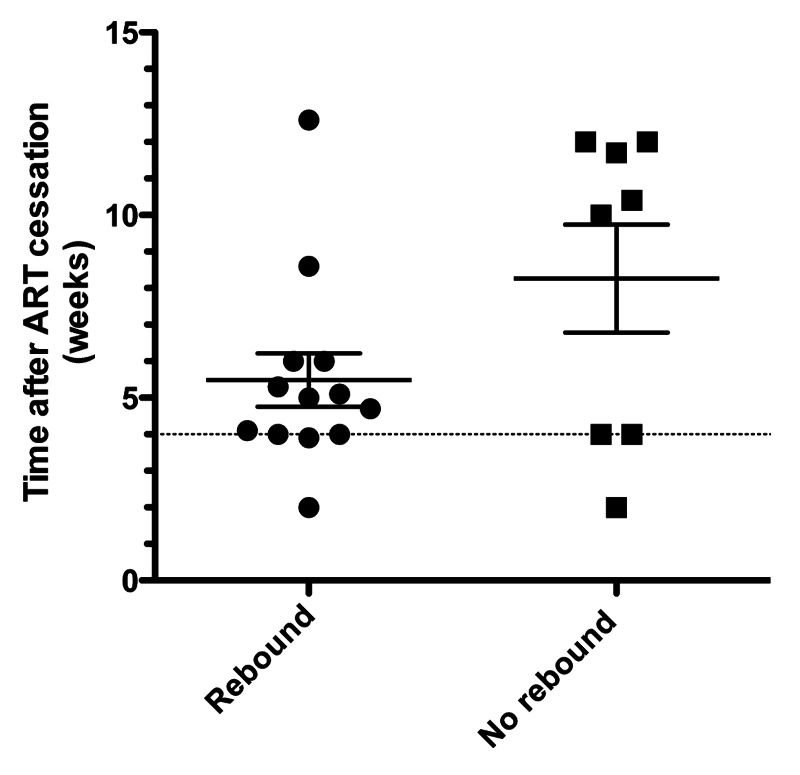
Figure 5. Consolidated times from ART cessation to rebound or to non-rebound status at study termination for all analyzed subjects. Dotted horizontal line at 4 weeks indicates maximal time to rebound for 90% of subjects in earlier STI studies. Horizontal bars denote mean ± SEM.
CD4 levels
These did not change significantly prior to ART cessation and post ART cessation CD4 counts dropped with viral rebound but rebounded after re-institution of ART. No between-group differences were detected (data not shown).
Discussion
The safety profile of TUTI-16 remained excellent, as seen in a previous study,18 fulfilling the primary endpoint of the study. Randomization was achieved as judged by the CD4 cell counts at entry (Fig. 2). TUTI-16-induced anti-Tat epitope antibodies lower free Tat concentrations in vitro17 and lower HIV plasma RNA levels in asymptomatic treatment naïve HIV subjects at antibody levels below 40 ng/mL,18 so the high levels of functional anti-Tat antibody levels achieved in the present study, after the single boost (Fig. 3), fulfilled the first secondary endpoint of immunogenicity. Interestingly, the low incidence of antibodies in the aviremic ART treated subjects at entry contrasted with the 45% incidence of anti-Tat antibodies, ranging from 43–485 ng/mL, found in treatment-naïve subjects in a previous study.18 This suggests that circulating Tat in treatment-naïve subjects induces anti-Tat antibodies but that these subside once ART control of HIV viremia and Tat production is established.
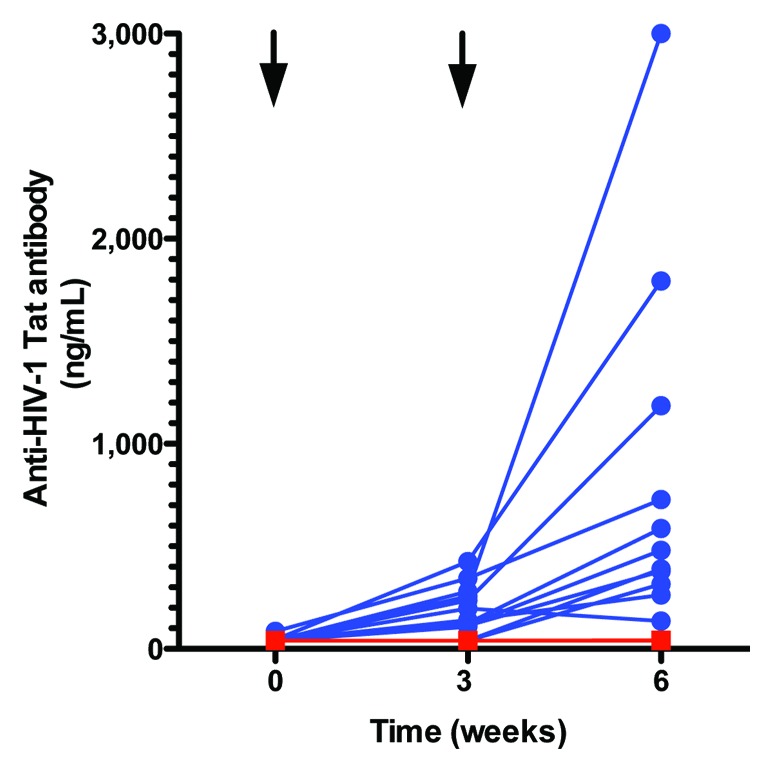
Figure 3. Changes in anti-Tat antibody levels after TUTI-16 (blue) or placebo (red) injections. Arrows denote injection times. The blue lines and symbols represent individual vaccinated subjects. The red line and symbols show the non-responsiveness of the placebo group and two non-responding vaccine subjects are concealed behind this line. TUTI-16 induced antibody levels < 40 ng/mL were previously shown to lower plasma HIV levels in asymptomatic ART naïve HIV infected subjects.18
The secondary endpoint of prevention of HIV rebound following ART cessation was not attained: there were no statistically significant differences between the number, frequency or timing of HIV rebounds between the vaccinated and placebo subjects (Fig. 4). Consolidating the two groups, the time to rebound or censoring varied from 2 weeks to 12.6 weeks in 13/21 (62%) rebounding subjects and 8/21 (38%) in subjects that had not rebounded when the study was terminated (Fig. 5). We speculate that the delayed rebounds and high incidence of non-rebounders in the present study are likely related to improvements in ART since the 1999 through 2003 STI studies previously reported.19-26 Modification of timing of initiation of ART has also been shown to affect the incidence of rebound and the establishment of HIV reservoirs28 but we had not gathered data to evaluate this potential effect. Importantly, a recent molecular study of rebounding HIV after STI in effective long-term ART subjects29 provides a new perspective for interpretation of the present findings: rebounding virus during multiple two-week treatment interruptions in long-term ART-controlled subjects showed molecular homogeneity and were not related to the diverse pre-ART virus nor to rebounding virus in other treatment interruptions in the same subject.29 Consistent with these findings, we speculate that the stochastic resurgence of new founder viruses could explain the delayed times to rebound and the higher proportion of non-rebounders within the time constraints of the present study. Acute HIV infection is associated with high levels of activating cytokines that enable HIV to become established. We further speculate that initiation of infection with new founder virus from the latent HIV reservoir may similarly require cytokine mediated activation; this would not be blocked by anti-Tat antibodies.
In conclusion, anti-Tat epitope vaccination of ART-controlled HIV-infected subjects was safe and immunogenic but was ineffectual in controlling HIV rebound after ART cessation. We conclude that there is no evidence that HIV rebound after STI is due to restoration of Tat-dependent HIV set point viremia. On the other hand our findings are consistent with the report that, by molecular analysis, the rebounding virus is homogeneous and develops heterogeneity over time, as does the founding virus in acute HIV infection. We speculate that HIV rebound after STI may represent a fresh HIV infection from internal reservoirs analogous to acute HIV infection following external transmission. STI may represent a potentially valid and economical approach to accelerate the testing of safety and efficacy of candidate prophylactic HIV vaccines.
Methods
Participants, blinding and protocol design
HIV infected male or female subjects on effective ART for greater than one year, with undetectable plasma HIV levels (< 20 copies HIV RNA/mL) and with CD4 cell counts > 500/μL were selected between July and November 2011. After clinical and laboratory screening, eligible subjects were randomized into vaccine and placebo groups according to a 1:1 randomization code developed by Damiano Consulting Associates, Inc. The assignments remained unknown to the sponsor, patients and all personnel involved with the study at the clinic except for the clinical pharmacist. Immunizations were at time 0 and week 3 and subject visits were also scheduled for week 6, at which time ART was discontinued and for post-ART weeks 2, 4, 6 and 8, with 3 subjects reaching post ART week 12. At each visit subjects were examined and safety histories taken and blood drawn for safety hematology and biochemistry evaluations and for plasma HIV levels and CD4 counts. Subjects with plasma HIV levels > 3,000 RNA copies/mL were censored from the study and returned to their prior effective ART regimen.
Vaccine preparation, formulation and injection
TUTI-16 was synthesized under cGMP by Bachem AB and provided as a lyophilized powder. It was formulated weekly at 2 mg/mL in 5% mannitol in water for injection and passed through a medical Millex-GV filter, 0.2 µm, 33 mm for sterile storage at 4°C. for up to one week. The randomization occurred at the time of injection, with 0.5 mL being injected through a Millex-GV filter subcutaneously in the deltoid muscle region. Vaccine stability was validated for lyophilized and solution storage and for passage through a Millex-GV filter.
Laboratory parameters
Spectra Laboratories, performed the biochemistry and hematology safety panels and urinalysis and provided plasma HIV RNA levels and flow cytometric analyses of CD4 T cells. Anti-Tat antibody levels were measured at Molecular Diagnostic Services, Inc., as reported previously.18
Statistical Analysis
GraphPad Prism 5 (Version 5.0d) for Mac OSX was used for statistical computations. A comparison of Kaplan-Meier curves of survival with < 3,000 HIV RNA copies/mL was analyzed by log-rank (Mantel-Cox) test and median survival and survival ratio and hazard ratio were calculated by the same program. Duration of ART treatment and baseline CD4 cell count comparisons was made by an unpaired T test, two-tailed. Incidence of antibody responses were compared with Fisher’s exact test, two-sided, as were the incidence of injection-related adverse reactions. P values < 0.05 were considered statistically significant. Power calculations based on an assumption of 90% rebounds in controls were rendered moot by the actual results in placebo controls.
Acknowledgments
We thank the study subjects for their participation in this study, Ray Ramondi, for formulations; and Michael Willet for data monitoring and assembling data summaries. Alan Fisher kindly reviewed the statistical methods. This study was performed under IND 1374 from CBER, USFDA and ethical review from the New England Institutional Review Board. All subjects signed approved informed consent forms before admission into the study. The study was performed at CliniLabs, Inc. Clinicaltrials.gov identifier #NCT01335191.
Glossary
Abbreviations:
- ART
antiretroviral therapy
- HIV
HIV-1
- STI
structured treatment interruption
- SD
standard deviation
- SEM
standard error of mean
- TNFα
tumor necrosis factor α
- TUTI-16
Thymon Universal Tat Immunogen-16
Disclosure of Potential Conflict of Interest
None of the authors declared potential conflicts of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21616
References
- 1.Brumme ZL, Walker BD. Tracking the culprit: HIV-1 evolution and immune selection revealed by single-genome amplification. J Exp Med. 2009;206:1215–8. doi: 10.1084/jem.20091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–31. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Li CJ, Ueda Y, Shi B, Borodyansky L, Huang L, Li YZ, et al. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc Natl Acad Sci USA. 1997;94:8116–20. doi: 10.1073/pnas.94.15.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahaf B, Atkuri K, Heydari K, Malipatlolla M, Rappaport J, Regulier E, et al. Culturing of human peripheral blood cells reveals unsuspected lymphocyte responses relevant to HIV disease. Proc Natl Acad Sci USA. 2008;105:5111–6. doi: 10.1073/pnas.0712363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 8.Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–8. [PubMed] [Google Scholar]
- 9.Lazdins JK, Grell M, Walker MR, Woods-Cook K, Scheurich P, Pfizenmaier K. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFR60 and TNFR80 favors induction of cell death rather than virus production in HIV-infected T cells. J Exp Med. 1997;185:81–90. doi: 10.1084/jem.185.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49:1215–28. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein G. HIV-1 Tat protein as a potential AIDS vaccine. Nat Med. 1996;2:960–4. doi: 10.1038/nm0996-960. [DOI] [PubMed] [Google Scholar]
- 13.Longo O, Tripiciano A, Fiorelli V, Bellino S, Scoglio A, Collacchi B, et al. Phase I therapeutic trial of the HIV-1 Tat protein and long term follow-up. Vaccine. 2009;27:3306–12. doi: 10.1016/j.vaccine.2009.01.090. [DOI] [PubMed] [Google Scholar]
- 14.Ensoli B, Fiorelli V, Ensoli F, Lazzarin A, Visintini R, Narciso P, et al. The preventive phase I trial with the HIV-1 Tat-based vaccine. Vaccine. 2009;28:371–8. doi: 10.1016/j.vaccine.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Ensoli B, Bellino S, Tripiciano A, Longo O, Francavilla V, Marcotullio S, et al. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PLoS ONE. 2010;5:e13540. doi: 10.1371/journal.pone.0013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein G, Tribbick G, Manson K. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine. 2001;19:1738–46. doi: 10.1016/S0264-410X(00)00393-5. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein G, Chicca JJ., 2nd A universal anti-HIV-1 Tat epitope vaccine that is fully synthetic and self-adjuvanting. Vaccine. 2010;28:1008–14. doi: 10.1016/j.vaccine.2009.10.129. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein G, Chicca J. Exploratory clinical studies of a synthetic HIV-1 Tat epitope vaccine in asymptomatic treatment-naïve and antiretroviral-controlled HIV-1 infected subjects plus healthy uninfected subjects. Hum Vaccin Immunother. 2012;8:8. doi: 10.4161/hv.19184. [DOI] [PubMed] [Google Scholar]
- 19.García F, Plana M, Vidal C, Cruceta A, O’Brien WA, Pantaleo G, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 20.Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS. 1999;13:F59–62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz GM, Nixon DF, Trkola A, Binley J, Jin X, Bonhoeffer S, et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest. 1999;104:R13–8. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann AU, Tubiana R, Calvez V, Robert C, Li TS, Agut H, et al. Comet Study Group HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. AIDS. 1999;13:677–83. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 23.Davey RT, Jr., Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García F, Plana M, Ortiz GM, Bonhoeffer S, Soriano A, Vidal C, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F29–40. doi: 10.1097/00002030-200106150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz L, Carcelain G, Martínez-Picado J, Frost S, Marfil S, Paredes R, et al. HIV dynamics and T-cell immunity after three structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F19–27. doi: 10.1097/00002030-200106150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Fischer M, Hafner R, Schneider C, Trkola A, Joos B, Joller H, et al. Swiss HIV Cohort Study HIV RNA in plasma rebounds within days during structured treatment interruptions. AIDS. 2003;17:195–9. doi: 10.1097/00002030-200301240-00009. [DOI] [PubMed] [Google Scholar]
- 27.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steingrover R, Pogány K, Fernandez Garcia E, Jurriaans S, Brinkman K, Schuitemaker H, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS. 2008;22:1583–8. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
- 29.Joos B, Fischer M, Kuster H, Pillai SK, Wong JK, Böni J, et al. Swiss HIV Cohort Study HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci USA. 2008;105:16725–30. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]