Abstract
Babies born with Pompe disease require life-long treatment with enzyme-replacement therapy (ERT). Despite the human origin of the therapy, recombinant human lysosomal acid α glucosidase (GAA, rhGAA), ERT unfortunately leads to the development of high titers of anti-rhGAA antibody, decreased effectiveness of ERT, and a fatal outcome for a significant number of children who have Pompe disease. The severity of disease, anti-drug antibody (ADA) development, and the consequences thereof are directly related to the degree of the enzyme deficiency. Babies born with a complete deficiency GAA are said to have cross-reactive immunologic material (CRIM)–negative Pompe disease and are highly likely to develop GAA ADA. Less frequently, GAA ADA develop in CRIM-positive individuals. Currently, GAA-ADA sero-positive babies are treated with a combination of immunosuppressive drugs to induce immunological tolerance to ERT, but the long-term effect of these regimens is unknown. Alternative approaches that might redirect the immune response toward antigen-specific tolerance without immunosuppressive agents are needed. Methods leading to the induction of antigen-specific regulatory T cells (Tregs), using peptides such as Tregitopes (T regulatory cell epitopes) are under consideration for the future treatment of CRIM-negative Pompe disease. Tregitopes are natural T cell epitopes derived from immunoglobulin G (IgG) that cause the expansion and activation of regulatory T cells (Treg). Teaching the immune system to tolerate GAA by co-delivering GAA with Tregitope peptides might dramatically improve the lives of CRIM-negative babies and could be applied to other enzyme replacement therapies to which ADA have been induced.
Keywords: Pompe Disease, Anti-drug Antibodies, ADA, Lysosomal acid alpha glucosidase (GAA), Enzyme Replacement Therapy, ERT, Tregitope, Treg, Regulatory T cell, tolerance
Introduction
Pompe disease is a lysosomal storage disorder caused by a deficiency in the enzyme acid α-glucosidase (GAA). The consequence of GAA deficiency is muscle inflammation, disruption of muscle tissue, and impaired function of heart and skeletal muscle. Although the advent of enzyme replacement therapy (ERT) for Pompe disease has had a dramatic impact on the life expectancy of babies who are born with this disorder, treatment advances are still needed. Current therapy for Pompe disease is based on early detection of the genetic defect and infusions of the recombinant human enzyme acid α-glucosidase (rhGAA) to prevent glycogen accumulation. Pompe-affected children who do not express endogenous GAA (cross-reactive immunologic material; CRIM) and undergo myozyme treatment develop high-titer anti-drug-antibodies (ADA) because they are not immunologically tolerant to GAA. ADA decrease GAA enzyme uptake by muscle and/or inhibit its activity. High ADA titers correlate with poor outcomes, and even though ERT has prolonged the life of Pompe disease babies, most CRIM-negative Pompe infants who have complete GAA deficiency will eventually succumb to the disease if they are not treated with tolerance-inducing drugs.
The development of experimental immune tolerance regimens to inhibit ADA against life-saving enzyme replacement therapy is an active area of investigation. Current approaches for mitigating GAA-ADA are based on treatment with methotrexate (MTX) to inhibit the proliferation of lymphocytes and Rituximab (Rituxan) to deplete antibody (Ab)-producing B cells.1-3 These approaches, however, share significant limitations. Namely, these treatments are not effective in eliminating long-lived plasma cells, thus the timing of intervention in patients experiencing an ADA response is critical. Additional pharmacological agents that suppress antibody production by long-lived plasma cells might be of use, such as a drug currently in use for plasma cell leukemia and multiple myeloma, Bortezomib (Velcade).4 However, the long-term effects of Rituximab and Bortezomib, that both suppress the immune system systemically, are as yet unknown. Finally, the association between the use of Rituximab and development of certain infections has been reported.5
In addition to these strategies aimed at inhibiting proliferation or eliminating lymphocyte subsets participating in the ADA response are those aimed at modulating the immune system to become tolerant to the therapeutic protein. IVIG has been shown to be associated with modulation of the regulatory T cell axis, including induction of nTregs;6 reduction of IL-17,7 and by enhancing the suppressive function of Tregs.8 It has thus been applied with much success in a number of autoimmune diseases. A recent report describes the clinical outcomes in two Pompe patients who had received prolonged Rituximab therapy for ADA who were also placed on chronic IVIG in an effort to decrease infectious complications. The addition of IVIG may have provided an additional immunomodulatory benefit in promoting tolerance to the GAA therapy.9
Therapies that safely and permanently harness the immune system to induce long-lasting and specific tolerance in Pompe disease children will address a critical unmet medical with broad-reaching implications for other replacement-protein therapies that are also limited by ADA (the Lysosomal Storage Disorders, Hemophilia A and B, etc.).10-12
Natural Regulatory T cells, Tregitopes and Tolerance Induction
Autoreactive T cells with moderate T cell receptor (TCR) affinity are known to escape deletion in the thymus to circulate in the periphery where they function as ‘natural’ regulatory T cells (nTreg) by suppressing immunity against self-antigens.13 Induced Tregs (iTregs), also known as adaptive Tregs, are generated from circulating T effector cells; these cells perform similar functions but have more plasticity. It has become increasingly clear that both nTregs and iTregs contribute to immune regulation in the periphery and that their presence, or absence, contributes to the induction of tolerance and the development of autoimmunity and inflammation, respectively.
One of the most fundamental questions about nTreg cells has been their antigen-specificity. We surmised that autologous proteins, such as immunoglobulin G (lgG), contain nTreg epitopes. The presence of nTreg epitopes in lgG might explain why immunoglobulins, which undergo somatic hypermutation in the periphery, do not elicit the expected immune response against the new ‘foreign’ hypervariable sequences. After discovering highly promiscuous MHC class II epitopes in the constant region of IgG, we proposed that these epitopes were nTreg epitopes (Tregitopes)14 that provide inherent inhibitory signals to counter-balance any stimulatory signals that might result from neo-epitopes expressed in Ab hypervariable region.15 Two independent publications support our hypothesis: Ephrem and colleagues showed that intravenous immunoglobulin G (IVIg) induces nTreg,6 and Anthony and Ravetch demonstrated the a linkage between immunoglobulin binding to surface receptors that are associated with antigen-processing pathways, and Treg induction by IVIg.16,17 We describe here our acquired knowledge on the ability of Tregitope peptides to reduce T cell and T cell-dependent antibody responses, induce regulatory T cells, and lessen disease scores in animal models of inflammatory disease.18
Tregitopes have the following four characteristics: 1) Their sequences are highly conserved in IgG sequences; 2) They bind to MHC class II promiscuously; 3) In response to Tregitopes, T cells that exhibit a T regulatory phenotype (CD4+CD25+ FoxP3+) expand in vitro and in vivo; and 4) Co-incubation of Tregitopes with target autoantigens such as pre-proinsulin inhibits effector T cell (Teff) proliferation in vitro and suppresses the secretion of effector cytokines (De Groot et al., unpublished and 18).
We have more recently demonstrated that APCs present Tregitopes to Treg, engage feedback mechanisms promoting a tolerogenic APC phenotype, induce Treg expansion, and modulate antigen-specific effector T cell responses (De Groot De Groot et al., unpublished). Proportions of APC expressing MHC II, CD80, and CD86 are suppressed, consistent with reported effects of IVIg19 and of the IgG-derived peptide hCDR1.20 Moreover, we have observed significant increases in proportions of IL-10-producing CD4+CD25+ FoxP3-expressing Treg in the presence of Tregitopes. The basic mechanism of Tregitope tolerance induction is currently proposed to be as follows: 1) APC present Tregitopes to nTreg, 2) nTreg are activated to proliferate and produce IL-10, 3) nTreg provide tolerogenic feedback signals to APC, modulating the APC phenotype, and 4) nTreg and tolerogenic APC together suppress antigen-specific T cell responses (De Groot et al., unpublished).
In Vivo Studies with Tregitopes
Modulation of T cell responses with Tregitopes may contribute to the design of new approaches for the treatment of autoimmune and inflammatory diseases via expansion of Tregs in vivo or ex vivo. Experience with IVIg gives an example of the therapeutic potential of this approach, and evidence is accumulating that Tregitopes provide beneficial immunomodulatory effects that in many respects parallel IVIg. Within our collaborative Tregitope network, we have performed a number of studies to probe potential therapeutic applications of Tregitopes in mouse models of MS (EAE), cardiac transplant, diabetes (NOD), antigen-induced airway hyper-responsiveness, and modulation of viral vector immunogenicity in adeno-associated virus (AAV)-mediated gene transfer.21 Together, the results obtained in these models show that Tregitopes co-administered with proteins suppress antigen-specific T cell and antibody responses, and induce expansion of functional Tregs. Side-by-side in vivo comparisons of Tregitope with IVIg have been performed in the autoimmune EAE model (Khoury and Elyaman, personal communication) and antigen-induced allergic airway disease (Mazer and Massoud, personal communication), demonstrating that IVIg effects can be replicated by Tregitope administration. Adaptation or incorporation of Tregitopes in drug design may aid in the design of improved tolerance-inducing therapies, and safer, more effective protein therapeutics.
Natural (n)Treg participate in central tolerance by controlling immune responses to autologous proteins, and in peripheral tolerance by stimulating induced (i)Treg cells.22 Induction of iTreg is associated with sustained tolerance to transplants, allergens and autologous proteins.13,23,24 Tolerance can be induced with non-depleting anti-CD4 antibodies25,26 and with AAV vector-mediated expression of antigens in the liver,27 including rhGAA.28-30 IVIg, a source of Tregitopes, induces tolerance in a number of different settings such as solid organ transplant,31 eradication of FVIII and FIX inhibitory antibodies in hemophilia patients,32-34 and autoimmune diseases such as systemic lupus erythematosus (SLE35), idiopathic thrombocytopenic purpura (ITP) and chronic inflammatory demyelinating polyneuropathy (CIDP).36-41 Of relevance to the goal of inducing tolerance to rhGAA, IVIg has been used to reduce ADA in Pompe patients undergoing Myozyme treatment.9 See Table 1 for a list of experiments that have been performed with Tregitope, the human disease parallel, and a comment on current therapy for that condition (highlighting the potential role of Tregitope in the future therapy of the condition).
Table 1. Overview of Tregitope in vivo experiments and results.
| Experimental Model | Finding | Human Disease Parallel | Current Clinical Therapy | In vivo controls | Source |
|---|---|---|---|---|---|
|
OVA-specific tolerance in C57BL/6 mice |
Suppression of T cell proliferation; Suppression of OVA-antibody titer |
Enzyme Replacement Therapy |
Immune Tolerance Induction |
Influenza HA (Negative CTR) No effect; IVIG CTR similar to Tregitope |
L. Cousens, A. De Groot (EpiVax) |
|
OVA-specific tolerance in DO11.10 TCR Transgenics |
Suppression of T cell proliferation; induction of antigen-specific Tregs |
Enzyme Replacement Therapy |
Immune Tolerance Induction |
Influenza HA peptide: No effect |
D.Scott, Y. Su (USUHS) |
|
EAE prevention with Tregitope In C57Bl/6 |
Reduction of EAE symptoms; induction of Tregs |
Multiple Sclerosis |
Copaxone; Interferon β Tysabri; Campath |
OVA peptide (Negative CTR) No effect; IVIG CTR similar to Tregitope |
S.Khoury; W. Elyaman (Brigham) |
|
OVA Allergy Model; Therapy with Tregitope vs. IVIG |
Reduction of allergic response to OVA and airway reactivity, increase in Treg Induction |
Allergy |
Anti-histamines, Immune Tolerance Induction |
Albumin (Negative CTR) No effect; IVIG CTR similar to Tregitope |
B. Mazer, A. Massoud (McGill) |
|
Tregitope in AAV; TNBS-induced model of inflammatory colitis |
Reduction of clinical and histological severity, increased Treg infiltration in the colon |
Crohn’s Disease |
Azathiaprine, Anti-TNF Mabs, Steroids |
Saline; No effect |
V. Ferreira, S. van der Marel (UniQure) |
|
Tregitope in AAV for Gene Transfer |
Suppressed CD8+-T cell response to AAV epitope in gene transfer model |
Gene Transfer |
Gene Transfer Vectors currently induce CTL response |
Scrambled Tregitope peptide (Negative CTR) No effect |
F. Mingozzi (CHOP) |
|
Tregitope in T1D (NOD model) with insulin peptides |
Suppressed development of diabetes; effect enhanced when Tregitope co-delivered with insulin | Type I Diabetes | Insulin Therapy | PPI peptides, Tet Tox peptide (Negative CTR) No effect | L. Cousens, A. De Groot (EpiVax) |
How Could Tregitopes be Applied to Pompe Disease Therapy?
We believe Tregitopes are natural, human regulatory T cell epitopes (“Tregitopes”) present in the Fc and Fab domains of IgG that may be responsible for tolerance to idiotypic epitopes (Fig. 1). When incubated with peripheral blood mononuclear cells (PBMCs) in vitro, Tregitopes specifically activate CD4+ T cells, increase CD25/Foxp3 expression, and increase expression of regulatory cytokines and chemokines.13 Administration of Tregitopes with protein antigens in vivo inhibits T cell proliferation and effector cytokine expression. Tregitope effects are long-lasting in a range of disease models (Table 1);duration of effect extends at least 26 weeks in NOD-T1D or 100 d in transplant (De Groot et al., unpublished).42
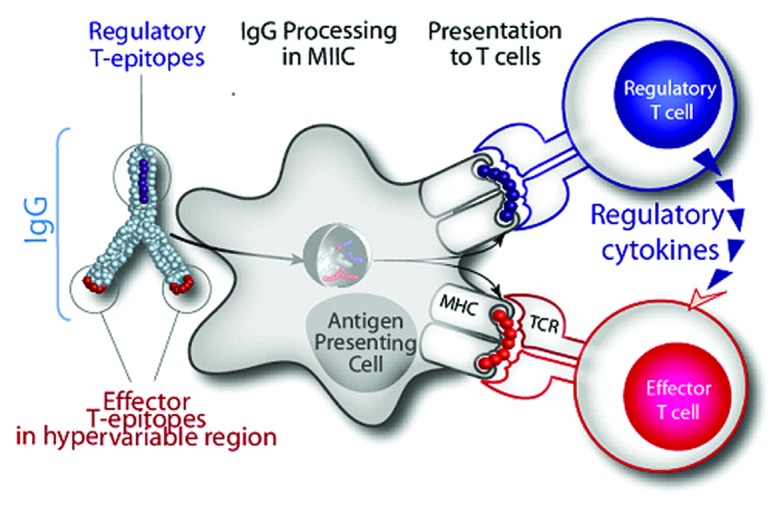
Figure 1. Hypothesized Tregitope mechanism of action. Treg epitopes (Tregitopes) present in Fc and Fab domain of IgG drive tolerance to neo-epitopes pre-sent in the Fab hypervariable domains. EpiVax discovered conserved T -cell epitopes in IgG that engage natural Treg. We hypothesize that Tregitopes (dark blue) activate Treg that lead to bystander suppression of effector T cells that recognize effector epitopes (red) and, depending on the context, induction of antigen-specific Tregs (aTregs) to these epitopes.
As shown in Table 1 for T1D (last row), co-administration of Tregitopes with the antigen leads to even greater suppression of in vivo cytokine production, T cell response, and antibody titers. The combined efficacy of target antigen and Tregitope, administered together, suggests that Tregitopes induce antigen-specific tolerance. This approach is deserving of further investigation in Pompe disease models.
Gene Transfer and Tregitopes
Gene transfer of GAA is under consideration as a therapy for Pompe disease. Several studies in GAA-KO mice testing the AAV vector-driven expression of GAA show an initial robust expression of GAA that is rapidly inactivated by ADA responses.43-45 Low dose and liver-specific expression of therapeutic enzymes may lead to Treg-mediated tolerance,29 an effect that may be enhanced by delivering GAA fused to Tregitopes. EpiVax’ Tregitope collaborators Mingozzi and High (Children’s Hospital of Philadelphia) have tested the concept of Tregitope-induced tolerance using AAV vectors in which Tregitopes are fused to the expressed transgene.
To summarize their results: an AAV vector expressing a protein antigen fused to Tregitopes (or ‘scrambled’ Tregitopes as a control) between which was engineered a PACE/furin sequence that is susceptible to cleavage by intracellular proteases. C57BL/6 mice received the Tregitope-fused vector (or the scrambled control, n = 4 per group). Four weeks later, mice were challenged with an adenoviral vector expressing the antigen to trigger a strong CD8+ T cell response against it.46 Ten days later, at the peak of T cell responses triggered by the adenoviral challenge, mice were sacrificed. Tregitope co-delivery in this gene transfer model effectively suppressed the development of effector (CD8+) T cell responses (Mingozzi and De Groot, unpublished).
Similarly, van der Marel et al. have tested an AAV-5 vector carrying a human Tregitope167-expressing cassette for its ability to reduce the development of inflammation in the TNBS model of colitis (van der Marel, personal communication). This reduction in inflammation was associated with an increase in the presence of Tregs in the intestinal mucosa, lamina propria and the thymus of AAV5-Tregitope167-treated mice. Additionally, expression of both Foxp3 and GARP were increased in the thymic CD4+ T lymphocyte population in mice pre-treated with AAV5-Tregitope167 but not in mice treated with saline alone, suggesting that Tregitope treatment increased activation of regulatory T-cells.47-51 The presence of higher numbers of activated regulatory T cells in the AAV5-Tregitope-treated mice corresponded with the prevention of fulminant intestinal inflammation in vivo, in this TNBS model of inflammatory bowel disease.
These two studies demonstrate that AAV vector-mediated co-delivery of Tregitopes with the enzyme replacement therapies could represent an alternative Tregitope-based approach to induce specific tolerance to GAA.
Conclusions
Much progress has been made in the field of enzyme-replacement therapy over the past decade. New treatments are reaching patients, and approaches that lead to the reduction of ERT-ADA are under evaluation. Despite these significant advances, the long-term effects of current immune tolerance induction regimens have yet to be defined. Proof-of-principle for Tregitopes in treating ADA in the context of Pompe disease would have an immediate impact on the field of enzyme replacement therapy and could accelerate adaptation of Tregitope therapy in the treatment of these children. Tregitopes merit consideration even if they only reduce dependence on immunosuppressive drugs, however ongoing studies appear to indicate that Tregitopes elicit antigen-specific tolerance, augmenting the potential benefits of this approach in mitigating ADA. Thus, further proof-of-principle studies with Tregitopes are likely to have a far-reaching impact on the clinical development of biologic therapies, including enzyme replacement therapy for Pompe disease.
Acknowledgments
The expert assistance of Ryan Tassone is gratefully acknowledged.
Disclosure of Potential Conflicts of Interest
Anne S. De Groot and William Martin are senior officers and majority shareholders at EpiVax, Inc., a privately owned vaccine design company located in Providence, Rhode Island, USA. Leslie P. Cousens is a senior scientist at EpiVax, Inc.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21405
References
- 1.Rohrbach M, Klein A, Köhli-Wiesner A, Veraguth D, Scheer I, Balmer C, et al. CRIM-negative infantile Pompe disease: 42-month treatment outcome. J Inherit Metab Dis. 2010;33:751–7. doi: 10.1007/s10545-010-9209-0. [DOI] [PubMed] [Google Scholar]
- 2.Joseph A, Munroe K, Housman M, Garman R, Richards S. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol. 2008;152:138–46. doi: 10.1111/j.1365-2249.2008.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garman RD, Munroe K, Richards SM. Methotrexate reduces antibody responses to recombinant human alpha-galactosidase A therapy in a mouse model of Fabry disease. Clin Exp Immunol. 2004;137:496–502. doi: 10.1111/j.1365-2249.2004.02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran E, Carbone F, Augusti V, Patrone F, Ballestrero A, Nencioni A. Proteasome inhibitors as immunosuppressants: biological rationale and clinical experience. Semin Hematol. 2012;49:270–6. doi: 10.1053/j.seminhematol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47:187–98. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–22. doi: 10.1182/blood-2007-03-079947. [DOI] [PubMed] [Google Scholar]
- 7.Maddur MS, Kaveri SV, Bayry J. Comparison of different IVIg preparations on IL-17 production by human Th17 cells. Autoimmun Rev. 2011;10:809–10. doi: 10.1016/j.autrev.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Kessel A, Ammuri H, Peri R, Pavlotzky ER, Blank M, Shoenfeld Y, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 9.Messinger YH, Mendelsohn NJ, Rhead W, Dimmock D, Hershkovitz E, Champion M, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med. 2012;14:135–42. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deegan PB. Fabry disease, enzyme replacement therapy and the significance of antibody responses. J Inherit Metab Dis 2011; PMID: 22037707 http://www.springerlink.com/content/6517140513818126/ [DOI] [PubMed]
- 11.Saint-Remy JM, Lacroix-Desmazes S, Oldenburg J. Inhibitors in haemophilia: pathophysiology. Haemophilia. 2004;10(Suppl 4):146–51. doi: 10.1111/j.1365-2516.2004.01009.x. [DOI] [PubMed] [Google Scholar]
- 12.Starzyk K, Richards S, Yee J, Smith SE, Kingma W. The long-term international safety experience of imiglucerase therapy for Gaucher disease. Mol Genet Metab. 2007;90:157–63. doi: 10.1016/j.ymgme.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 14.De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–11. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soukhareva N, Jiang Y, Scott DW. Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell Immunol. 2006;240:41–6. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–8. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–6. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cousens LP, Najafian N, Mingozzi F, Elyaman W, Mazer B, Moise L, et al. In vitro and in vivo studies of IgG-derived Treg epitopes (Tregitopes): A promising new tool for tolerance induction and treatment of autoimmunity. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9762-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–65. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 20.Sela U, Sharabi A, Dayan M, Hershkoviz R, Mozes E. The role of dendritic cells in the mechanism of action of a peptide that ameliorates lupus in murine models. Immunology. 2009;128(Suppl):e395–405. doi: 10.1111/j.1365-2567.2008.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui DJ, Basner-Tschakarjan E, Pien GC, Martin WD, De Groot AS, High KA, et al. Peptide-Induced Antigen-Specific CD4+CD25+FoxP3+ T Cells Suppress Cytotoxicity T Cell Responses Directed Against the AAV Capsid. Blood. 2010;116:3769. [Google Scholar]
- 22.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 23.Durinovic-Belló I, Rosinger S, Olson JA, Congia M, Ahmad RC, Rickert M, et al. DRB1*0401-restricted human T cell clone specific for the major proinsulin73-90 epitope expresses a down-regulatory T helper 2 phenotype. Proc Natl Acad Sci USA. 2006;103:11683–8. doi: 10.1073/pnas.0603682103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumida T, Kato T, Hasunuma T, Maeda T, Nishioka K, Matsumoto I. Regulatory T cell epitope recognized by T cells from labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 1997;40:2271–3. doi: 10.1002/art.1780401225. [DOI] [PubMed] [Google Scholar]
- 25.Cobbold SP, Qin SX, Waldmann H. Reprogramming the immune system for tolerance with monoclonal antibodies. Semin Immunol. 1990;2:377–87. [PubMed] [Google Scholar]
- 26.Winsor-Hines D, Merrill C, O’Mahony M, Rao PE, Cobbold SP, Waldmann H, et al. Induction of immunological tolerance/hyporesponsiveness in baboons with a nondepleting CD4 antibody. J Immunol. 2004;173:4715–23. doi: 10.4049/jimmunol.173.7.4715. [DOI] [PubMed] [Google Scholar]
- 27.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–56. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Bird A, Young SP, Kishnani PS, Chen YT, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet. 2007;81:1042–9. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun B, Kulis MD, Young SP, Hobeika AC, Li S, Bird A, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther. 2010;18:353–60. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler RJ, Bercury SD, Fidler J, Zhao MA, Foley J, Taksir TV, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther. 2008;19:609–21. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- 31.Jordan SC, Toyoda M, Vo AA. Regulation of immunity and inflammation by intravenous immunoglobulin: relevance to solid organ transplantation. Expert Rev Clin Immunol. 2011;7:341–8. doi: 10.1586/eci.11.10. [DOI] [PubMed] [Google Scholar]
- 32.Muzaffar J, Katragadda L, Haider S, Javed A, Anaissie E, Usmani S. Rituximab and intravenous immunoglobulin (IVIG) for the management of acquired factor VIII inhibitor in multiple myeloma: case report and review of literature. Int J Hematol. 2012;95:102–6. doi: 10.1007/s12185-011-0968-7. [DOI] [PubMed] [Google Scholar]
- 33.Kubisz P, Plamenová I, Hollý P, Stasko J. Successful immune tolerance induction with high-dose coagulation factor VIII and intravenous immunoglobulins in a patient with congenital hemophilia and high-titer inhibitor of coagulation factor VIII despite unfavorable prognosis for the therapy. Med Sci Monit. 2009;15:CS105–11. [PubMed] [Google Scholar]
- 34.Klarmann D, Martinez Saguer I, Funk MB, Knoefler R, von Hentig N, Heller C, et al. Immune tolerance induction with mycophenolate-mofetil in two children with haemophilia B and inhibitor. Haemophilia. 2008;14:44–9. doi: 10.1111/j.1365-2516.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- 35.Zandman-Goddard G, Blank M, Shoenfeld Y. Intravenous immunoglobulins in systemic lupus erythematosus: from the bench to the bedside. Lupus. 2009;18:884–8. doi: 10.1177/0961203309106921. [DOI] [PubMed] [Google Scholar]
- 36.Zandman-Goddard G, Krauthammer A, Levy Y, Langevitz P, Shoenfeld Y. Long-Term Therapy with Intravenous Immunoglobulin is Beneficial in Patients with Autoimmune Diseases. Clin Rev Allergy Immunol 2011; PMID: 21732045 http://www.springerlink.com/content/j614131732668223/ [DOI] [PubMed]
- 37.Dykes AC, Walker ID, Lowe GD, Tait RC. Combined prednisolone and intravenous immunoglobulin treatment for acquired factor VIII inhibitors: a 2-year review. Haemophilia. 2001;7:160–3. doi: 10.1046/j.1365-2516.2001.00489.x. [DOI] [PubMed] [Google Scholar]
- 38.Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46(Suppl 2):S2–14. doi: 10.1053/j.seminhematol.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provan D. Characteristics of immune thrombocytopenic purpura: a guide for clinical practice. Eur J Haematol Suppl. 2009;82:8–12. doi: 10.1111/j.1600-0609.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 40.Lozeron P, Adams D. Advances in the treatment of chronic inflammatory demyelinating neuropathies in 2010. J Neurol. 2011;258:1737–41. doi: 10.1007/s00415-011-6143-5. [DOI] [PubMed] [Google Scholar]
- 41.Rajabally YA, Mahdi-Rogers M. Overview of the pathogenesis and treatment of chronic inflammatory demyelinating polyneuropathy with intravenous immunoglobulins. Biologics. 2010;24:45–9. doi: 10.2147/btt.s4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elyaman W, Khoury SJ, Scott DW, De Groot AS. Potential application of tregitopes as immunomodulating agents in multiple sclerosis. Neurol Res Int. 2011;2011:256460. doi: 10.1155/2011/256460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raben N, Jatkar T, Lee A, Lu N, Dwivedi S, Nagaraju K, et al. Glycogen stored in skeletal but not in cardiac muscle in acid alpha-glucosidase mutant (Pompe) mice is highly resistant to transgene-encoded human enzyme. Mol Ther. 2002;6:601–8. doi: 10.1016/S1525-0016(02)90716-1. [DOI] [PubMed] [Google Scholar]
- 44.Cresawn KO, Fraites TJ, Wasserfall C, Atkinson M, Lewis M, Porvasnik S, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther. 2005;16:68–80. doi: 10.1089/hum.2005.16.68. [DOI] [PubMed] [Google Scholar]
- 45.Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12:876–84. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Sabatino DE, Mingozzi F, Hui DJ, Chen H, Colosi P, Ertl HC, et al. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol Ther. 2005;12:1023–33. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 48.Wang R, Wan Q, Kozhaya L, Fujii H, Unutmaz D. Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS ONE. 2008;3:e2705. doi: 10.1371/journal.pone.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13439–44. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 51.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]


