Abstract
Allergen specific immunotherapy involves the repeated administration of allergen products in order to induce clinical and immunologic tolerance to the offending allergen. Immunotherapy is the only etiology-based treatment that has the potential for disease modification, as reflected by longterm remission following its discontinuation and possibly prevention of disease progression and onset of new allergic sensitizations. Whereas subcutaneous immunotherapy is of proven value in allergic rhinitis and asthma there is a risk of untoward side effects including rarely anaphylaxis. Recently the sublingual route has emerged as an effective and safer alternative. Whereas the efficacy of SLIT in seasonal allergy is now well-documented in adults and children, the available data for perennial allergies and asthma is less reliable and particularly lacking in children. This review evaluates the efficacy, safety and longterm benefits of SCIT and SLIT and highlights new findings regarding mechanisms, potential biomarkers and recent novel approaches for allergen immunotherapy.
Keywords: adjuvant, allergen vaccine, immunopotentiator, sublingual immunotherapy, vector system
Introduction
Allergen immunotherapy (also termed hyposensitization therapy, immunologic desensitization) involves the gradual administration of increasing amounts of allergen to which the patient is sensitive, for the purpose of modulating the immune response to that allergen and alleviating allergic symptoms. It is the only treatment strategy which treats the underlying cause of the allergic disorder, by induction of a state of immunologic tolerance. It is a cost-effective treatment strategy that results in a reduction in symptoms of allergic-rhinitis and allergen-related asthma, as well as an improved quality of life and a decrease in absenteeism from school or work.1,2 Immunotherapy is able to provide long-term remission of allergic symptoms and may reduce the chance of developing new sensitization to other allergens.1-3 A decision whether to treat with immunotherapy will depend on a variety of personal and organizational factors which determine whether one type of immunotherapy is more suitable then another.4 Indeed there are two commonly used types of immunotherapy: subcutaneous (SCIT) and sublingual (SLIT).
One hundred years ago, Leonard Noon published in The Lancet the first paper on allergen subcutaneous immunotherapy.5 This paper documented Dr Noon’s experiments in which he injected patients with allergic rhinitis with grass pollen every few days, initially in minute quantities then gradually increasing the dose. He demonstrated that the injections were associated with an increase in tolerance to grass pollen. 50 y later a double blind, placebo-controlled study was undertaken by William Franklin, which proved beyond doubt that subcutaneous immunotherapy was effective.6 The first description of the sublingual route was from GK Scadding and J Brostoff and the first clinical attempts with this administration were performed only a few years later.7-9
In 1986 The British Committee for the Safety of Medicines reported “since 1957, 26 patients in the United Kingdom have died from anaphylaxis induced by subcutaneous immunotherapy”10. In this context the interest in a non-injection route increased. In 1986 the first randomized controlled trial of the sublingual (SLIT) was published.90 In 1993 the European Academy of Allergy and Clinical Immunology (EAACI) immunotherapy position paper considered SLIT as a “promising route” for hyposensitization”11. The World Health Organisation (WHO) in 1998 and the WHO-supported ARIA document (Allergic Rhinitis and its Impact on Asthma) in 2001 vindicated SLIT as an alternative route to SCIT in adults and children.12,13 Subsequently several randomized, double-blind, placebo-controlled trials (DBPC-RCT) demonstrated the efficacy and safety of SLIT. These included studies of SLIT for grass and house dust mite (HDM) allergy. Formulations for SLIT included drops and subsequently sublingual tablets. Indications were for allergic rhinitis and included the establishment of dose-response relationships for these allergens. Grass pollen allergen tablets were registered in Europe for sublingual use in seasonal allergic rhinitis in 2007.
The main indications for immunotherapy are: • IgE-mediated seasonal pollinosis, if symptoms have not responded adequately to optimal pharmacotherapy. • Selected patients with animal dander or house dust mite allergy in whom allergen avoidance and pharmacotherapy fail to control symptoms6.
Subcutaneous immunotherapy (SCIT)
Efficacy in rhinitis and asthma
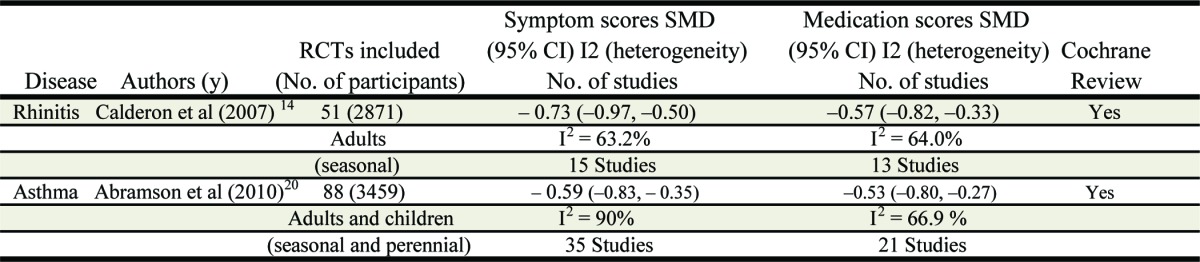
A recent Cochrane systematic review focused on the efficacy of SCIT in seasonal allergic rhinitis (SAR).14 Of 1111 abstracts identified, 51 full papers satisfied the inclusion criteria, representing a total of 2871 participants: 1645 active and 1226 placebo, each receiving on average of 18 injections. Primary outcomes focused on efficacy (symptom scores, medication scores and rhinoconjunctivitis quality of life questionnaires). The efficacy of SCIT in patients with SAR was demonstrated by a significant reduction in all these outcomes, compared with placebo treatment (Table 1). One multi-center study assessed the efficacy of subcutaneous in over 400 patients. The study involved an alum-adsorbed grass pollen extract (Alutard SQ grass pollen, ALK-Abéllo, Horsholm Denmark).15 Mean symptom and medication scores (reduced by) of 29% and 32%, respectively in the high dose group (100,000 SQ units monthly maintenance dose containing 20 µg of major allergen Phleum p1) compared with placebo treatment (both p < 0.001). This study included polysensitized subjects and improvement was observed despite free access to optimal conventional anti-allergic medication. Thus this study shows that SCIT conferred clinical benefit over and above that achievable with standard drug therapy. Further trials that compare head-to-head the effectiveness of SCIT and pharmacotherapy are needed to confirm this possibility. There is no current review and meta-analysis on SCIT for perennial rhinitis, although individual studies have shown efficacy for mite allergy. A major reason to account for possible reduced efficacy of mite immunotherapy is that multiple trigger factors other than house dust mite either allergic or non-allergic could be responsible for perennial nasal symptoms in patients with perennial symptoms and an SPT positive to mite. In one trial16, after 1 y of mite immunotherapy, symptom and medication scores were reduced by 58% (p < 0.002) and 20%, respectively.
Table 1. Summary Synopsis of Cochrane meta-analyses for SCIT.
* Allergens used in the trials evaluated: ragweed (12), mixed grass (16), timothy (5), parietaria (6), birch (4), orchard (2), cedar (3), bermuda (1), juniperus ashei (1) and cocos (1) ** Allergens used in the trials evaluated: house mite allergy (42); pollen (27) ; animal dander (10) ; Cladosporium mold (2), latex (2)and multiple allergens (5)
In allergic asthma, the clinical efficacy of SCIT has been demonstrated by studies in adults and children sensitized to seasonal and perennial allergens.17-19 A recent Cochrane meta-analysis assessed allergen specific immunotherapy for asthma.20 Eighty-eight clinical trials fulfilled the criteria for inclusion. A total of 3792 patients (3459 with asthma) were involved. There was a significant improvement in asthma symptom scores and a significant reduction in medication following immunotherapy (Table 1). For Lung function outcomes there was heterogeneity among studies although overall there was a trend for improvement in lung function. There was a marginal improvement in non-specific bronchial hyper-reactivity (SMD -0.35, 95% CI -0.59 to -0.11), and a significant reduction in allergen specific BHR following immunotherapy (SMD -0.61 95% CI -0.79 to -0.43). The finding that allergen immunotherapy significantly improves allergen specific bronchial BHR is clinically important, since patients with allergic asthma are at risk of sudden deterioration when exposed to increased levels of an aeroallergen to which they are sensitive. Thus, in clinical terms, successful immunotherapy for asthma may result in not only a reduction in asthma symptoms and use of rescue medication, but also a lowering the risk of asthma attack upon unexpected or inevitable allergen exposure. One study21 assessed the effects of specific immunotherapy as an add-on to pharmacologic treatment and allergen avoidance, in patients with mild-to-moderate asthma and allergy to house dust mite. After 3 y of administration of an alum-adsorbed HDM SCIT vaccine, a significant decrease in the number of subjects requiring rescue bronchodilators was observed. Another study demonstrated corticosteroid sparing in children when an alum-adsorbed HDM SCIT vaccine was added to asthma therapy.22
Safety of SCIT
All preparations that are currently available (standardized extract, allergoids and recombinant allergen) may trigger side effects. A Cochrane meta-analysis of SCIT for SAR showed 8% of grade II and 7% of grade III systemic reactions (according to the European Academy of Allergy and Clinical immunology for adverse event). Adrenaline/epinephrine was given in 0.13% of injections (14). A higher risk is detected in subjects with accelerated dosing schedules, and in subjects with asthma.23,24
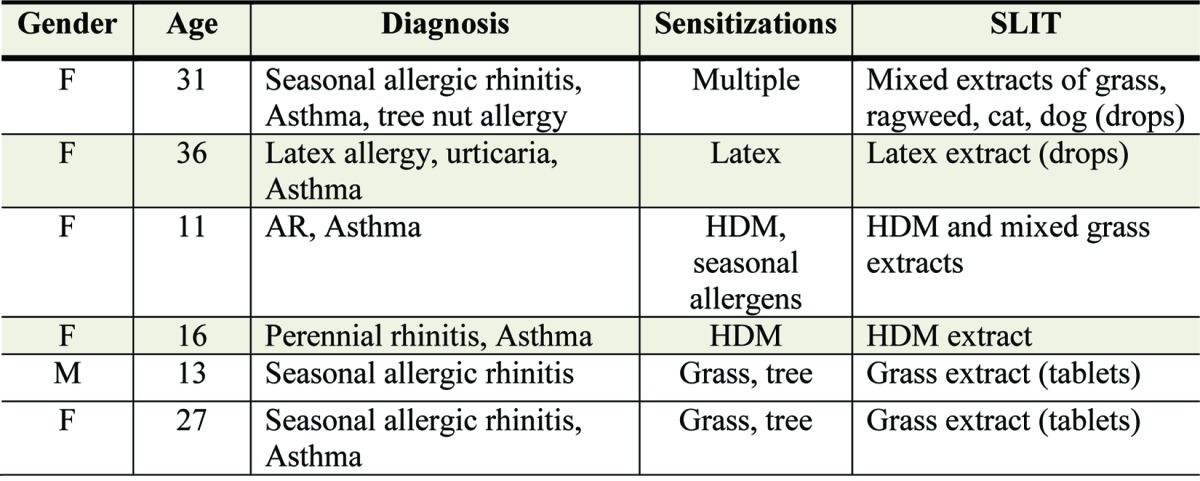
A Cochrane meta-analysis of SCIT for asthma patients (20) reported large local adverse and systemic reactions where the pooled relative risk was 1.4 (95% CI 0.97 to 2.02) and 2.45 (95% CI 1.91 to 3.13) respectively. The incidence of systemic reactions (SR) was greater than that previously reported in the literature (19.9% compared with 5% to 7%). The prevalence of near fatal reactions in this meta-analysis were reported as 1 in 1.0 million injections and fatal reactions as 1 in 2.5 million injections (Abramson). This compares with the absence of any reports of anaphylaxis in the recently updated Cochrane review of Sublingual immunotherapy (Radulovic), although this was primarily in patients with rhinitis. There have been 5 isolated reports worldwide of anaphylaxis following sublingual treatment (reported below and Table 4)
Table 4. Individual case reports of anaphylaxis after SLIT treatment.
Risks factors for severe adverse reaction during SCIT have been identified as follow: • Co-existing asthma • Poorly controlled asthma • History of previous systemic reaction(s) to immunotherapy • Delay or omission of the use of adrenaline in treating anaphylaxis • Inappropriate selection of candidates for injection immunotherapy • Dosing errors • Changeover between batches of allergen; reaction to the first dose of a new vial • Lack of cardio-respiratory resuscitation facilities • Commencing an updosing immunotherapy regimen during concomitant high environmental allergen exposure, for example during the pollen season.
Although safe when performed in a specialist clinic by trained staff, with immediate presence of a doctor experienced in immunotherapy and access to resuscitative measures, subcutaneous immunotherapy carries a small risk of significant adverse effects. In view of these risks, within UK, SCIT is not recommended for the treatment of perennial asthma in the United Kingdom. In contrast the presence of seasonal asthma in those with severe seasonal pollinosis is not a contraindication when updosing is performed out of the pollen season with appropriate dosage adjustment in season as recommended in international guidelines.4,25
Sublingual Immunotherapy
Efficacy in rhinitis and asthma
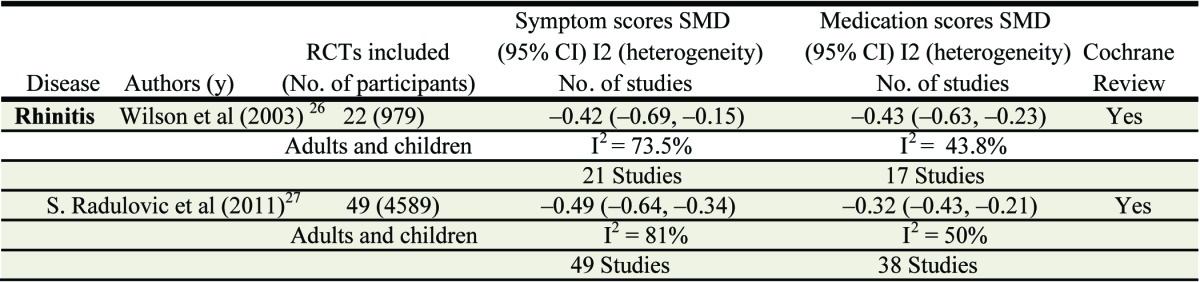
SLIT involves the regular self-administration of allergen extract (prepared as drops or tablets) that are retained under the tongue for 1–2 min and then swallowed. SLIT is in general taken once daily, in contrast to the usual 4–6 weekly maintenance administration of SCIT after up dosing. A recent Cochrane review (an update of a previous review, see ref.26) evaluated the efficacy of SLIT in subjects affected by AR (with or without asthma or conjunctivitis), compared with placebo.27 The efficacy of SLIT was compared with placebo by analysis of symptoms and/or medication scores of patients of any age (children and adults) affected by allergic rhinitis, with or without allergic conjunctivitis, with or without allergic asthma. Subgroup analysis was performed according to the following criteria: (1) seasonal vs. perennial allergens; (2) children vs. adults; (3) major allergen content of vaccine contained in a monthly maintenance dose(< 5mcg vs. 5–20 µg vs. > 20 µg); (4) duration of immunotherapy (< 6 mo vs. 6–12 mo vs. 12 mo); (5) sublingual spit vs. sublingual swallow immunotherapy protocols; (6) sublingual drops vs. tablets.
60 DBPC RC T were considered, with 49 being suitable for meta-analysis, comprise of a total of 2333 on active treatment (SLIT) and 2256 on placebo. Most trials were performed with grass pollen (23 studies). Other allergens used were Parietaria (5 trials), ragweed (2 trials), trees (9 trials: 2 olive, 3 cypress, 2 birch pollen, 2 mixed trees), 8 house dust mite and 1 cat immunotherapy trial. One trial investigated the efficacy of grass pollen and birch pollen immunotherapy. 34 studies were performed in adults and 15 investigated efficacy and safety in children. The overall results of the meta-analysis differ little from those seen in the original review in 2003, with the overall effect for symptom scores SMD being of a similar magnitude, with tighter confidence intervals reflecting the greatly increased number of study subjects. The same is true for the analysis of medication scores (Table 2). These data continue to support the clinical efficacy of SLIT for allergic rhinitis.
Table 2. Summary Synopsis of Cochrane meta-analyses for SLIT.
Allergens used in the trials evaluated: House Dust Mite allergy (6), Grass Pollen (5), Parietaria (5), Olive (2), Ragweed (1), Cat (1), Tree (1) and Cupressus (1). ** Allergens used in the trials evaluated:: grass pollen (23), Parietaria (5), ragweed (2), trees (9: 2 olive, 3 cypress, 2birch pollen, 2 mixed trees), house dust mite (8) and cat (1). One of the trials investigated the efficacy of grass and birch pollen immunotherapy.
In contrast to the original review, the greater number of studies has allowed more meaningful analyses of some of the predetermined subgroups. For example there are now 15 studies looking exclusively at children.28,29 The treatment effect within this subgroup of trials appears to be similar to that seen in adults. When subgroups of seasonal verses perennial allergens are considered the standardized mean difference for perennial mite allergy appears greater than for pollinosis, whereas this data must be interpreted with great caution since the data involve few studies with low participant numbers and the means are accompanied by very wide confidence intervals.
Other systematic reviews and meta-analyses (independent of the Cochrane center) assessed the efficacy of SLIT in children with allergic rhinitis (Table 3).30,31,37 Penagos31 evaluated ten articles that enrolled participants 18 y or younger, with a history of allergic rhinitis (with or without asthma or conjunctivitis). 484 participants were analyzed. Results showed a significant reduction in nasal symptoms compared with placebo (Table 3). Subgroup analysis showed a significance reduction in symptom scores for pollen SLIT (SMD, 0.53; 95% CI, 0.94–0.12; p = 0.01) but not for mite SLIT (SMD, 0.76; 95% CI, 1.77– 0.72; p = 0.41). One possible explanation may be differences in doses employed. In one study that involved a cumulative dose of 12 µg of major allergen, clinical efficacy appeared greater than in those trials that employed lower doses.32,,33-35 In a meta-analysis of 7 studies, Olaguibel et al.30 identified significant efficacy for SLIT (including mite allergen) for respiratory allergy in children (Table 3). However the small size and high heterogeneity make a generalisable interpretation difficult. Data in favor of sublingual immunotherapy in children are less convincing and hence more definitive trials are needed. For example, a recent study in United States evaluated the efficacy and safety of grass pollen allergen tablet sublingual immunotherapy containing 15 µg Phleum p5 in 345 children and adolescents. The subjects had predominant seasonal symptoms whereas 90% were polysensitized, and one quarter had asthma.36 The total combined score improved 26% (p = 0.001), the daily symptom score improved 25% (p = 0.005) and the daily medication score improved 81% (p = 0.006) compared with placebo. These results are comparable to those reported in an independent study of grass sublingual tablet treatment in European children.28 Rescue medication use was lower than that reported in the European study.28 This outcome is probably due to the lower peak pollen count observed in the US study and consequently reduced symptom severity (and rescue medication use). SLIT was equally effective for rhinoconjunctivitis outcomes in multisensitized compared with monosensitized participants and no worsening of asthma was observed.
Table 3. Summary of meta-analyses for SLIT.
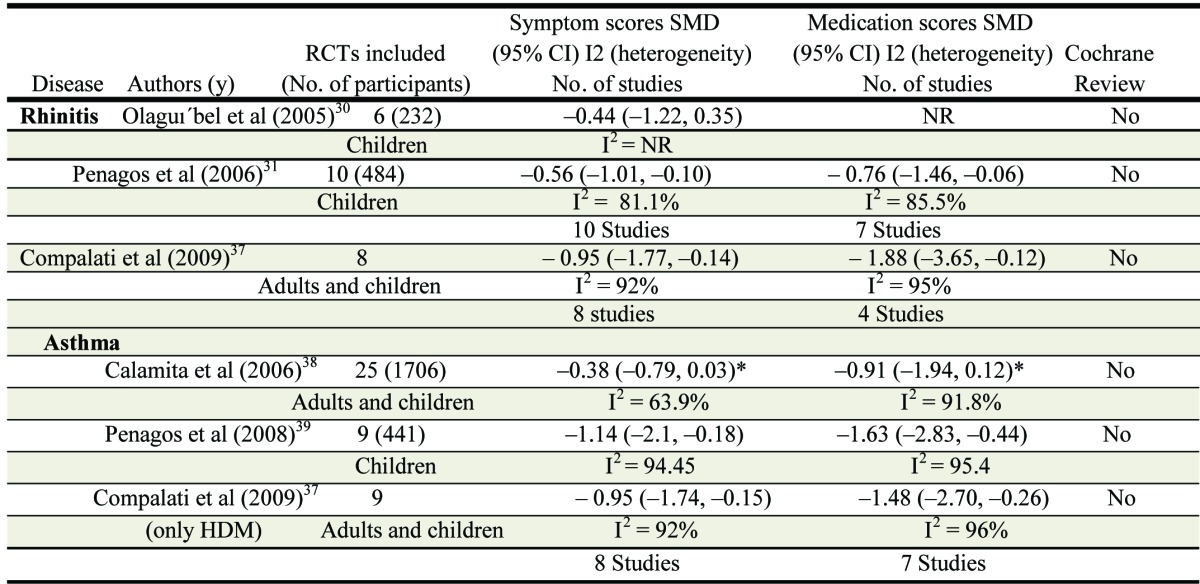
Allergens used: house dust mite (3), Parietaria (1), Olive (1), grass mix (1) ** Allergens used: house dust mite (4), grass mix (3), Parietaria (1), Olive (1), Pollen mix (1) ° Allergens used: house dust mite (8), Pollens (14), allergen mix (2), latex (1) °° Allergen used: house dust mite (6), Grass mix (1), Olive (1), Pollen mix (1)
Compalati et al.37 performed a meta-analysis of SLIT in mite sensitive adults and children. Whereas overall statistical significance was observed for the combined studies this was lacking within the pediatric studies and the small numbers and high heterogeneity prevent firm conclusions. Similarly a review by Calamita38 evaluated 25 randomized-controlled studies of SLIT in participants with allergic asthma. Results showed a significant reduction in symptom score and medication use and a significant improvement in respiratory function (FEV1, FEV1%, FEF25–75%) in adults and children. Again wide variability and heterogeneity was observed. In 2008 Penagos et al.39 reported a meta- analysis of efficacy of SLIT in children with asthma with similar findings. Unfortunately all these meta-analyses represented only small numbers of studies, with few participants. Furthermore the large heterogeneity questions the reliability of the overall conclusions that can be drawn, as acknowledged by the authors of these reviews. Whereas the efficacy of SLIT in seasonal allergy in both adults and children is well-documented, the available data for SLIT for perennial allergens and in asthma is less reliable and particularly lacking in children, thereby highlighting the need for more definitive large trials, as currently available for seasonal grass pollen allergy. The success of SLIT for seasonal grass pollen allergy and the high safety margin observed for SLIT compared with SCIT provides impetus for these studies to be performed with high priority.
Studies of sublingual immunotherapy have consistently shown that provided the allergen used for immunotherapy is historically the predominant cause of symptoms, the beneficial effect of SLIT in polysensitized participants is similar to that observed for monosensitized subjects, for example Malling.40 This is of importance since the majority of allergic patients are polysensitized. In one study participants were treated with sublingual grass allergen tablets containing 100 IR, 200 and 500 IR (in-house arbitrary units). There were comparable improvements in participants receiving 300 IR and 500 IR tablets (37% and 35%reductions in total rhinoconjunctivitis scores compared with placebo treatment. Adverse events were mild-to-moderate in severity and in general did not require any intervention. No serious systemic events or anaphylactic shock were observed. The authors concluded that daily 300 IR tablets (containing 15–25 µg Phleum p5) were optimal and efficacy was achieved regardless of the presence of polysensitization or associated seasonal asthma.
Safety of sublingual immunotherapy
One of the purported advantages of SLIT over SCIT is greater safety, which permits the administration of this treatment outside of the medical setting. Many clinical trials have shown that SLIT (drops or tablets) is well tolerated in both adult and children. None-the-less 40–85% of patients experience local side effects, such as mild itching and mild swelling of the lips. These symptoms develop quickly within minutes after ingestion and only last minutes. Furthermore symptoms usually settle within 1–2 weeks of continued treatment.41-45 Adverse reactions have occurred in 10–15% of patients receiving SLIT and have been mainly classified as local, non-life-threatening, self remitting episodes. Rarely are these reactions troublesome enough to result in discontinuation of treatment. An important unmet need is a standardised reporting system for SLIT local side effects, whereas SLIT systemic effects may be reported using the same system as recently recommended by WAO for SCIT.46 Other adverse reactions described include nausea, abdominal pain, rhinitis, conjunctivitis, and cough. Very rarely systemic reactions have been reported such as urticaria, angioedema, and asthma. No clear predictable factors were identified for SLIT adverse events, although they appear to be more common in severely allergic patients, at the height of season, and with a history of a previous systemic reaction, including to subcutaneous immunotherapy. Five of the 6 recent individual case reports of severe adverse events after SLIT administration had asthma, a further potential risk factor as for subcutaneous treatment47-51 (Table 4). The first case involved a mixture of multiple extracts of different allergens. The second case involved a rush treatment schedule. In the third case anaphylaxis occurred during the peak spring pollen season. In the fourth case the girl, for reasons unknown, self-administered herself a considerable overdose of the allergen extract after a long period of discontinuation. The latter two cases occurred after the first dose of a sublingual grass pollen allergen tablet taken unsupervised in the participant’s home and not as recommended under medical supervision for 30 min after the first dose. Patients should be provided with specific instructions regarding how to manage adverse reactions and how to deal with unplanned treatment interruptions. Furthermore it is important to store the allergen tablets or drops in a secure place away from children.
Long-term efficacy
Recently data have highlighted specific immunotherapy not only as an effective therapeutic agent but also as having disease modifying properties, inducing disease remission. Although less robust at the present time, evidence is also accumulating that immunotherapy may represent a preventive strategy capable of reducing the onset of new sensitizations to unrelated allergens, and to reduce the likelihood of disease progression from allergic rhinitis to asthma.
In a previous study in mite-sensitive children with asthma who received HDM SCIT for one year, most of the participants relapsed on blinded withdrawal in the subsequent year. In a separate study of mite immunotherapy in children, treatment for 3 y, but not 1–2 y was associated with more prolonged remission for up to 3 y after stopping treatment.52,53 This study and others54 demonstrated that SCIT given for a short time was associated with a faster relapse of symptoms after discontinuation (1–3 y) compared with treatment given for 3–4 y. A DBPC-RCT study in grass pollen SCIT showed, after 3–4 y of treatment, no significant difference in symptom or medication score in the subsequent three years.55
These data suggest that 3 y of grass pollen SCIT gives benefit that may persist for at least three years after the cessation of the treatment. These conclusions have been extended to include a recent study of HDM immunotherapy.56 Recently two studies57,58 enrolled children with allergic rhinoconjunctivitis and evaluated the long-term efficacy of SCIT given for three years. After 10 and 12 y of follow up a significant improvement in symptoms and a reduced onset of asthma was observed in the treated group. However, interpretation of these studies is limited by a non-randomized design and/or unblinded follow up. For SLIT, the available long-term data have either consisted of small numbers of participants, have not been placebo-controlled, or have not shown significant benefit after the end of treatment.59-61
A recent study in adults with moderate-severe seasonal allergic rhinitis investigated the sustained efficacy of 3 y sublingual immunotherapy using grass allergen tablets.62 The mean rhinoconjunctivitis score was reduced by 25% to 36% in the grass allergen tablet group relative to placebo over the 5 y (all p < 0.01) that included 2 y of blinded withdrawal following 3 y treatment (Table 5).63 The daily rhinoconjunctivitis medication score was similarly reduced by 20% to 45% and the combined score 27% to 41% relative to placebo. A second study64 evaluated the long-term efficacy of sublingual liquid extract of 5-grass pollen mixture using co-seasonal ultra rush protocol for three consecutive years. Moreover the participants were followed one year after discontinuation. There was a trend for a difference in terms of symptoms and medication scores, compared with placebo, but without reaching statistical significance. Few studies have evaluated the clinical effects of immunotherapy for more than 5 y.65 In one study, 198 patients were divided in four groups (drug therapy alone or SLIT for 3, 4 or 5 y) and followed for 15 y. The difference in clinical efficacy compared with the control group after SLIT discontinuation remained significant for 6 y in the group receiving the immunotherapy for 3 y and for 7 y in the remaining group suggesting that 4 y duration of SLIT may be the optimal choice. However the small numbers studied, only partially randomized design and inevitable high dropout rates over such a prolonged period are a major limitation and further long-term studies are needed.
Table 5. Seasonal rhinoconjunctivitis symptom and medication scores during 5 y treatment with grass pollen allergy tablet sublingual immunotherapy.
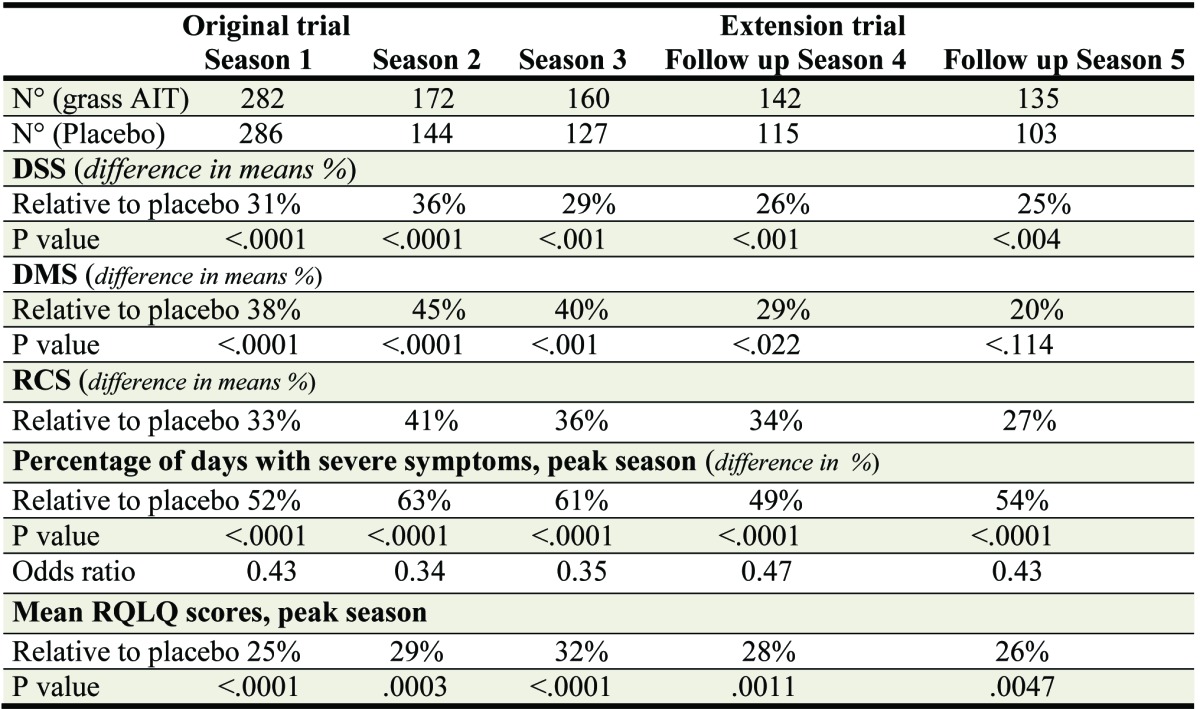
DSS, daily rhinoconjunctivitis symptom score; DMS, daily rhinoconjunctivitis medication score; RCS, Rhinoconjunctivitis combined score; RQLQ, rhinoconjunctivitis quality of life questionnaire
Mechanism of immunotherapy
Studies have confirmed a reduction in target organ sensitivity in the skin, conjunctiva, nose and lung after immunotherapy66-68 including inhibition or early and late responses. Moreover skin biopsies following allergen-SIT reported reduced numbers of T lymphocytes and suppression of eosinophil, basophil and neutrophil infiltration.67,69 Some studies demonstrated that SCIT is able to deviate the immune system by shifting the T- helper type 2 (Th2) dominated immune response to allergen toward a T-helper 1 (Th1) response.70,71 Furthermore generation of regulatory T-cells secreting IL-10 and TGF-β has been observed in studies of peripheral blood.72,73 Although other studies failed to confirm these findings in blood.74,75 The production of Th1 and T- regulatory cell-dependent cytokines (IL-10 and interferon gamma has also been confirmed in local target tissues.70,76 SIT may influence also the innate immune system. It is known that dendritic cells (DCs) produce INF-α in response TLR-9 receptor stimulation and that this is significantly impaired in allergic subjects. Tversky et al. demonstrated that SCIT may help re-establish a more balanced immune response by restoring the DC capacity to respond to innate immune receptor stimuli. Allergen immunotherapy resulted in a three- to 5-fold increase in DC production of IFN-α in response to a TLR9 agonist, CpG. Peripheral T cell tolerance following SIT is characterized by the induction of allergen-specific regulatory T cells (T reg).77 Phenotypically T reg are characterized by the expression of surface proteins CD4 and high CD 25 expression. Novel biomarkers include the increased expression of the intracellular transcription factor FOXP3 and reduced expression of the IL-7 cell surface receptor CD127. The capacity of these cells to produce IL-10 and TGF-β has been reported in several studies.72,75,78,79 These cytokine are essential for the induction of immunologic tolerance. IL-10 is a general inhibitor of proliferative response in T cells. Moreover IL-10 may reduce Th2 cytokine production (IL-4, IL-5, IL-13) and associated allergic inflammation. IL-10 is produced also by mononuclear phagocytes,80,81 natural killer cells,82 B lymphocytes and by both Th1 and Th2 type lymphocytes.83 Elevated levels of IL-10 production have been demonstrated in patients treated with SIT compared with placebo.84 T reg cells are responsible for producing this cytokine, following SCIT.
TGF-β is a pleiotropic cytokine with several regulatory functions in the immune system. These include downregulation of naive T cell differentiation into effector cells. Specifically TGF-β blocks the differentiation of Th1 and Th2 cells by inhibiting expression of the transcription factors T-BET and GATA-3, respectively.85,86Thus the regulation of both Th1 and Th2 polarization may at least in part be due to the effects of T reg cells. Furthermore it has been show that TGF-β can convert peripheral CD4+CD25- Naive T cells to CD4+CD25+ regulatory T cells by induction of FOXP3, a transcription factor characteristic of regulatory T cells. Moreover, TGF-β modulates IgE and FcεRI expression on Langerhans cells, is a class switch factor inducing B cell production of non inflammatory antibody IgA87 and also induces CTLA-4 expression on T regs.88,89
Recent studies have also demonstrated immunologic changes in the local tissue, for example, elevated numbers of IL-10+ and TGF-b+ T cells and FoxP3+CD4+ and FoxP3+CD25+ phenotypic regulatory T cells within the nasal mucosa.90,91 These local increases in phenotypic regulatory T cells within the target organ paralleled clinical improvement and were accompanied by reduced inflammatory allergic responses (a reduction in mast cells,92 basophils93 and eosinophils94 in the nasal mucosa).
Immunoglobulin responses
During immunotherapy for seasonal pollinosis there is an Initial increase of serum allergen-specific IgE without apparent ill effects, followed by blunting of seasonal increases in IgE.23,95,96 Furthermore quantitative measurements of allergen-specific IgG subclasses in these patients showed an increase in allergen-specific IgG1 and IgG4 in the serum. However, there is a poor correlation between immunoreactive IgG and IgG4 levels as determined by ELISA and the clinical response to immunotherapy. Functional studies of post immunotherapy IgG-containing serum has shown that IgG1 and IgG4 may contribute to induce immunologic tolerance by inhibiting basophil histamine release, competing with the specific IgE and preventing its interaction with the allergen – so called IgG ‘blocking activity’ by direct competition with IgE for the allergen, thereby inhibiting IgE receptor crosslinking and mast cell/basophil activation in response to allergen.97-99
An alternative mechanism of functional inhibitory activity for IgG1 and IgG4 is their ability to compete with IgE for allergen binding and thereby prevent IgE-facilitated allergen presentation by B-cells to allergic-specific T cells (IgE –FAB), thus resulting in a decrease in T cell activation, proliferation and Th2 cytokine production100,84,101-104. It is likely that the increase in IL-10 production within weeks and at low allergen concentrations in patients treated with SCIT is responsible for isotype class switching in favor of IgG4.84,105 One study addressed the role of IgA in immunotherapy. IgA2 increased in response to allergen subcutaneous treatment and when purified from post- immunotherapy serum, triggered IL-10 secretion by monocytes.106 These findings implicate a possible role for IgA antibodies in the induction of tolerance following SIT. Quantitative measurements of allergen-specific IgG subclasses in SIT-treated patients have also revealed increases in allergen-specific IgG1 and IgG4 antibody concentrations in the local target organ107-109
Sublingual Immunotherapy
The oral mucosa is a site of natural immune tolerance induction.110 Allergen uptake by specialized antigen presenting cells (APCs) within the oral mucosa is presumed to be the first step in successful SLIT. Dendritic cells (DCs) are abundant within the oral mucosa.111 Moreover there are resident professional APCs [oral Langerhans cells (oLC)] that express high levels of MHC class I and II, costimulatory molecules such as CD40, CD80, CD86 and high levels of the IgE receptor FcεRI. These cells are able to release IL10 in a TLR4 dependent manner112 and induce T cells with a regulatory phenotype in vitro, after contact with allergen. Current models of SLIT suggest) an uptake of allergen by APCs in the oral mucosa, followed by migration to regional lymph node.113-115
Oral mucosal DCs bear high amounts of IgE bound to the high affinity receptor for IgE (FceRI) on their cell surface and thus are highly efficient at capturing and taking up allergens applied to the oral mucosa during SLIT. Paradoxically, FceRI cross-linking on DCs in the oral mucosa results in induction of anti-inflammatory mediators such as IL-10116 and indoleamine 2,3-dioxygenase (IDO),117 molecules that may play a role in immunologic tolerance and in inhibiting antigen-specific T cells responses. It has been showed in the nasal mucosa in a murine model that antigen-specific regulatory T cells are inducted by activation of the IgG receptor CD32B, which contains an immunoreceptor tyrosine inhibitory motif (ITIM). Thus signaling by way of FceRI, CD32B or both on mucosal DC might be a fundamental mechanism to achieve tolerance within the oral mucosa in vivo.
During their migration to the regional lymph glands DC’s upregulate surface proteins such as CD83 and CCR7 and may prime T cells in oral lymphoid foci, reducing T cell proliferative responses and effector function118 while also inducing Foxp3+ regulatory T cells. Recent studies have demonstrated an increase of Foxp3 cells in the oral epithelium119 and in peripheral blood, within months after starting SLIT.120,121 Other studies of SLIT observed a suppression of T cells proliferative responses, elevated level of INF-gamma and a reduction in Th2 cytokines122-126 whereas some studies did not confirm this last finding in peripheral blood cells.127-129
Other immunological changes in peripheral blood mononuclear cells from SLIT treated patients include increased expression of the programmed cell death ligand (PD-L1) on B cells and monocytes.130 A significant reduction in the number of eosinophils and neutrophils in the nasal mucosa131 has been reported. These immunological changes following SLIT may be associated with induction of allergen-specific tolerance and imply a network of Langerhans cells monocytes and oral DCs and possibly B cells that are capable of producing IL-10 and priming T reg cells that play a major role in the induction of allergen-specific tolerance and suppression of allergic inflammation in target organs.
Serum immunoglobulin responses during SLIT
During SLIT for seasonal pollinosis, there is a paradoxic increased in allergen-specific IgE, followed by blunting of seasonal increases in serum IgE without any apparent association with adverse events. Thereafter, allergen-specific IgG1 and IgG4 concentrations in serum increase in a time and allergen-dose dependent manner132 and remain elevated for at least 2 y following discontinuation,133 although of a lower magnitude than that observed after SCIT.15 Even though some studies have shown no difference in the IgG level or in IgG4 efficacy, recent studies linked increases in serum specific IgG1 and IgG4 antibody level with an increase in IgG- associated serum inhibitory activity for allergen-IgE binding to B cells.134,135 Moreover there was a trend for a relationship between the size of the late-phase skin response and the inhibition of IgE-FAB.119 A likely consequence is the suppression of Th2 responses at mucosal surfaces with a consequent suppression of allergic inflammation and associated symptoms during the pollen season.
Increases in specific IgA1 and IgA2 have also been observed after SLIT.136 This may be the result of enhanced TGF-β expression from either antigen presenting cells137or T reg cells induced following SLIT. In conclusion even though local mechanism are additionally involved and need to be investigated in more depth, there are marked similarities in the mechanisms of SLIT and SCIT that may explain, at least in part, the efficacy of SLIT.
Subcutaneous vs. Sublingual Immunotherapy
It is apparent that the subcutaneous and sublingual routes of administration share common mechanisms of action in terms of T cell shift and induction of ‘blocking’ antibodies (although the latter appears less pronounced after SLIT119 compared with SCIT15). However as mentioned above, the oral mucosa is a ‘privileged’ site110 for induction of tolerance and the nasal and sublingual mucosa have a common lymphatic drainage to the cervical lymphatic chain such that it is possible that additional local mechanisms for SLIT are involved.71
Whether or not this translates into altered efficacy between SCIT and SLIT will depend on the results of adequately powered head-to-head controlled trials. There have been several comparisons performed although interpretation has been difficult on account of the small numbers and open design of these studies (Mungan, Saporta Antunez). Two studies, one involving pollen immunotherapy in adults (Kinchi) and one involving house dust mite immunotherapy in children (Yukselen) were randomized and double blind. Both confirmed efficacy of SCIT and SLIT against the placebo arm, whereas neither were adequately powered to show a difference between the two routes, if one existed. Perhaps the best information to date comes from 2 large 4–600 patient) controlled trials of SLIT tablet immunotherapy (Dahl R, JACI 2008 2yr results o0f GT-08 and Didier 2006–8) and one large (> 400 patient) subcutaneous trial with an alum-adsorbed vaccine15 for grass pollen induced seasonal pollinosis where an approximate 30–35% reduction in median symptom scores and 40–45% reduction in medication scores was comparably observed in all 3 trials.
Predictive biomarkers for clinical response
Careful patient selection and early identification of responders is necessary in order to target intervention at those who will benefit and to exclude those who are less likely to respond to immunotherapy. The current best way to engineer a favorable outcome of SCIT or SLIT is to ensure that patients are desensitized to those allergens responsible for their symptoms, as identified in the history and with objective confirmation of IgE sensitivity. Even when this is done, there is a wide spectrum in clinical response.
Any biomarker should have good positive and negative predictive value, be robust, reproducible, acceptable and convenient for the patient and feasible and economic in the context of multi-center trials and, ultimately, in usual daily practice. Unfortunately no such marker currently exists at present.
Based on knowledge of mechanisms of immunotherapy, potential biomarkers include the following: • Allergen-specific serum IgG4. • Serum functional IgG responses: inhibition of IgE-FAB, inhibition of basophil histamine release. • Immediate and late-phase skin test response to allergen provocation. • Eosinophil cationic protein in nasal lavage • T cell proliferation and IL-10 production ex vivo from peripheral blood mononuclear cells following allergen stimulation • Bronchial responsiveness to allergen and methacoline challenge.
So far none of these markers have been shown to be predictive of the clinical response to SIT in individual patients. Realistically, such a marker would require being serum-based rather than relying on complex cellular assays or provocation testing with allergen or nonspecific triggers, which are complex to standardize even between academic centers, let alone in clinical practice. Serum allergen-specific IgG4 antibodies is the most consistent immunological marker whereas IgG-associated functional assays have been shown to correlate better with clinical parameters138 Further studies are needed that include pre and post-immunotherapy measurements for clinical parameters as well as for biomarker measurements so as to allow a within participant change in the biomarker to be related to the individual alterations in clinical response in order to identify individual responders and non responders.
Novel approaches to allergen immunotherapy
Allergen immunotherapy is currently performed with crude allergen extracts that usually contain non allergenic and potentially toxic unwanted proteins, which may possibly induce side effects on therapeutic administration.139 Moreover, the three-dimensional structure of the native allergen may be recognized by specific IgE with potential to cause occasional severe and even life-threatening, systemic allergic reactions. For this reason, the optimally efficient higher dose of allergen required for successful SIT can sometimes not be achieved. Immunotherapy is thought to act mainly through the mechanism of allergen-specific peripheral T-cell tolerance. One approach to make effective allergen vaccines safer is to conserve T-cell epitopes while removing or disabling IgE antibody-binding sites that are responsible for mediating allergic/anaphylactic side effects.139,140
Most new strategies are based on the perceived need to modify allergen-specific T cell function (by skewing the cytokine profile of Th2 effector cells or inducing allergen-specific T regulatory cells) while abolishing or reducing binding of the injected substance to IgE, responsible for adverse events. This strategy also allows much higher quantities of allergen to be administered safely, which may be an important factor for tolerance induction.
Allergen variants with hypoallergenic activity following chemical modification are already in clinical practice. Formaldehyde- and glutaraldehyde-treated allergen extracts termed ‘allergoids’ display low IgE-binding capacity, and are better tolerated than the native allergen extract. Allergoids are hypoallergenic, but IgE epitopes are not fully destroyed and they still retain the risk of anaphylaxis.141 Some of the currently available hypoallergenic vaccines (allergoids) are chemically modified using formaldehyde or glutaraldehyde. The fusion of major allergens then expressed as a recombinant has also been shown to be effective and potentially safer.151
Other systems used to decrease the binding efficiency of B cell epitopes while preserving T cell epitopes include the generation of chimeric, polymeric or unrefolded molecules, by fusion of fragments of allergen, or polymerization and denaturation of major allergen, respectively.142-148 Another strategy is the fragmentation of allergens to alter their structure while preserving T cell epitopes, or immunization with identified T cell epitopes.137 This latter strategy may be problematic in that many allergenic substances (such as grass pollen) contain a mixture of many proteins to which most individuals (major allergens) or only a minority of individuals (minor allergens) may respond.
T cell epitope vaccination has been most successful with allergen products containing one or a few major allergens, such as cat dander, but even then there will be rare individuals whose MHC haplotype precludes their T cells from recognizing the particular epitopes in a vaccine. A compromise is simply to fragment the allergens into small peptides. Another strategy proposed for shifting the Th1/Th2 balance consists in modifying the function of APC through the use of Toll like receptor ligands in combination with allergens. In some studies139 synthetic DNA sequences that contain CpG and the glycolipid monophosphoryl lipid A were used as a Th1-inducing adjuvant in association with allergen for subcutaneous immunotherapy in ragweed and grass allergic patients. Although limited data support the effectiveness and safety of this approach, confirmatory studies are needed.
An alternative solution is to produce recombinant allergens at defined concentrations and in complete purity to produce vaccines which would then be universally standardized. While this has been achieved successfully for some mixtures of allergens,142 it is still problematic when extracts comprise many major and minor allergens. Although attractive in terms of allergen standardization, this strategy is likely to be expensive and not necessarily of therapeutic advantage.
Humanized monoclonal anti-IgE antibody, omalizumab, is available for use in moderate-severe allergic asthma. Omalizumab is well tolerated and has been shown to reduce the requirement for inhaled corticosteroids and the risk of asthma exacerbations Moreover, omalizumab is able to reduce circulating levels of free IgE, inhibit early- and late-phase responses to allergen, and reduce eosinophils, lymphocytes and IL-4- producing cells in tissues. Therefore a combination of anti-IgE therapy and allergen immunotherapy might offer advantages that neither method provides separately.149
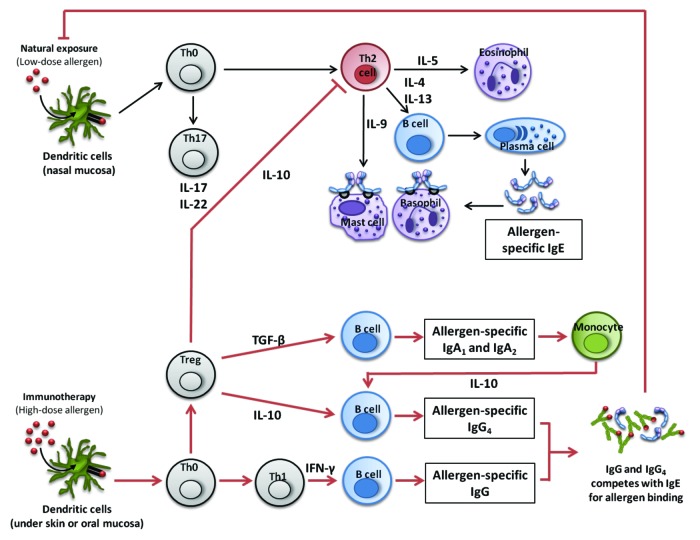
Intralymphatic allergen administration is an alternative route of SIT that may potentially be safe and effective.150 This procedure involves the apparently painless administration of allergen by injection directly into a lymph node in the groin. Absence of mast cells in lymph nodes potentially eliminates the risk of anaphylaxis. A short updosing protocol for intralymphatic treatment (3 injections in 8 weeks) has been shown to suppress target organ responses in the nose and has potential to improve patient compliance in view of the lower risk of adverse events and a shortened protocol of injections compared with conventional SCIT. (Fig. 1)
Figure 1. Immunological mechanisms of immunotherapy to aeroallergens (SCITandSLIT). Reproduced with kind permission of Dr M Shamji and Clin Exp Allergy.77 Low-dose and repeated allergen exposure at mucosal surfaces in atopic individuals results in Th2-T lymphocyte-driven, IgE-mediated allergic responses in tyarget organs. In contrast, high-allergen exposure that occurs with immunotherapy results in a shift of T cell polarization. The induction of T regulatory cells [inducible Treg cells (iTreg) and natural Treg cells (nTreg)] and cytokines such as IL-10 and TGF-beta suppress effector T cell responses. There is also observed a shift from T helper 2 (Th2) to T helper 1 (Th1) T lymphocyte responses. These altered T cell responses contribute to the induction of allergen-specific IgG, and in particular IgG4 antibody responses, and also an increase in IgA. IgG antibodies compete with IgE for allergen and suppress both FceRI- mediated basophil/mast cell activation and IgE-Facilitated CD23-dependent allergen presentation to T cells. There is good evidence that these mechanisms operate for immunotherapy via both the subcutaneous and sublingual routes. It is likely that for SLIT there are additional local mechanisms involving interactions between recirculating dendritic cells and T cells in the regional lymph glands and nasal mucosa – since the cervical lymph chain serve both the nasal mucosa and the floor of the mouth.
Acknowledgments
Dr. Durham is a member of the steering committee of the Immune Tolerance Network, NIAID.
Glossary
Abbreviations:
- SLIT
sublingual immunotherapy
- SCIT
subcutaneous immunotherapy
- EAACI
European Academy of Allergy and Clinical Immunology
- WHO
The World Health Organisation
- ARIA
Allergic Rhinitis and its Impact on Asthma
- DBPC-RCT
randomized, double-blind, placebo-controlled trials
- HDM
house dust mite
- SAR
seasonal allergic rhinitis
- SQ-U
Standardized quality unit
- RCT
randomized clinical trials
- SMD
standard median deviation
- DSS
daily rhinoconjunctivitis symptom score
- DMS
daily rhinoconjunctivitis medication score
- RCS
Rhinoconjunctivitis combined score
- RQLQ
rhinoconjunctivitis quality of life questionnaire
- IR
index of reactivity
- BHR
bronchial hyper-reactivity
- Th1
T-helper 1
- Th2
T-helper 2
- DCs
dendritic cells
- T reg
regulatory T cells
- IgE-FAB
IgE-facilitated allergen binding
- APCs
antigen presenting cells
- oLC
oral Langerhans cells
- ITIM
immunoreceptor tyrosine inhibitory motif
- PD-L1
programmed cell death ligand
Disclosures
Dr. Durham has received research funding from industry via Imperial College from Glaxo Smith Kline, ALK Abello, Novartis. He has received honoraria for consulting and /or lectures from ALK Abello, Circassia, Merck and Boehringer Ingelheim.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21629
References
- 1.Eifan AO, Shamji MH, Durham SR. Long-term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:586–93. doi: 10.1097/ACI.0b013e32834cb994. [DOI] [PubMed] [Google Scholar]
- 2.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125(Suppl 2):S306–13. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 3.Calderón M, Brandt T. Treatment of grass pollen allergy: focus on a standardized grass allergen extract - Grazax®. Ther Clin Risk Manag. 2008;4:1255–60. doi: 10.2147/tcrm.s3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, et al. British Society for Allergy and Clinical Immunology Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177–200. doi: 10.1111/j.1365-2222.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 5.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572–3. doi: 10.1016/S0140-6736(00)78276-6. [DOI] [Google Scholar]
- 6.Lowell FC, Franklin W. A double-blind study of the effectiveness and specificity of injecton therapy in ragweed hay fever. N Engl J Med. 1965;273:675–9. doi: 10.1056/NEJM196509232731302. [DOI] [PubMed] [Google Scholar]
- 7.Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16:483–91. doi: 10.1111/j.1365-2222.1986.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 8.Black JH. The oral administration of pollen. J Lab Clin Med. 1927;12:1156. [Google Scholar]
- 9.Black JH. The oral administration of pollen: clinical report. J Lab Clin Med. 1928;13:709. [Google Scholar]
- 10.Committee on the safety of medicines. CSM update. Desensitizing vaccines. BMJ. 1986;293:948. doi: 10.1136/bmj.293.6552.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malling H, Weeke B, Position Paper of the European Academy of Allergy and Clinical Immunology Position paper: Immunotherapy. Allergy. 1993;48(Suppl):9–35. doi: 10.1111/j.1398-9995.1993.tb04754.x. [DOI] [PubMed] [Google Scholar]
- 12.Bousquet J, Lockey R, Malling HJ. World Health Organization Position Paper. Allergen immunotherapy: therapeutical vaccines for allergic diseases. Allergy. 1998;53:1–42. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. World Health Organization. GA(2)LEN. AllerGen Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 14.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007:CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frew AJ, Powell RJ, Corrigan CJ, Durham SR, UK Immunotherapy Study Group Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–25. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003;33:1076–82. doi: 10.1046/j.1365-2222.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 17.Abramson M, Puy R, Weiner J. Immunotherapy in asthma: an updated systematic review. Allergy. 1999;54:1022–41. doi: 10.1034/j.1398-9995.1999.00102.x. [DOI] [PubMed] [Google Scholar]
- 18.Bousquet J, Hejjaoui A, Michel FB. Specific immunotherapy in asthma. J Allergy Clin Immunol. 1990;86:292–305. doi: 10.1016/S0091-6749(05)80091-0. [DOI] [PubMed] [Google Scholar]
- 19.Cantani A, Arcese G, Lucenti P, Gagliesi D, Bartolucci M. A three-year prospective study of specific immunotherapy to inhalant allergens: evidence of safety and efficacy in 300 children with allergic asthma. J Investig Allergol Clin Immunol. 1997;7:90–7. [PubMed] [Google Scholar]
- 20.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010:CD001186. doi: 10.1002/14651858.CD001186. [DOI] [PubMed] [Google Scholar]
- 21.Maestrelli P, Zanolla L, Pozzan M, Fabbri LM, Regione Veneto Study Group on the “Effect of immunotherapy in allergic asthma” Effect of specific immunotherapy added to pharmacologic treatment and allergen avoidance in asthmatic patients allergic to house dust mite. J Allergy Clin Immunol. 2004;113:643–9. doi: 10.1016/j.jaci.2003.12.586. [DOI] [PubMed] [Google Scholar]
- 22.Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126:942–9. doi: 10.1016/j.jaci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/S0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 24.Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST) J Allergy Clin Immunol. 1987;79:660–77. doi: 10.1016/S0091-6749(87)80164-1. [DOI] [PubMed] [Google Scholar]
- 25.Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. GA² LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30. doi: 10.1111/j.1398-9995.2010.02474.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003:CD002893. doi: 10.1002/14651858.CD002893. [DOI] [PubMed] [Google Scholar]
- 27.Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT) Allergy. 2011;66:740–52. doi: 10.1111/j.1398-9995.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 28.Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–73.e7. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 29.Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. SLIT Study Group Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–6.e3. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Olaguíbel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol. 2005;15:9–16. [PubMed] [Google Scholar]
- 31.Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol. 2006;97:141–8. doi: 10.1016/S1081-1206(10)60004-X. [DOI] [PubMed] [Google Scholar]
- 32.Ippoliti F, De Santis W, Volterrani A, Lenti L, Canitano N, Lucarelli S, et al. Immunomodulation during sublingual therapy in allergic children. Pediatr Allergy Immunol. 2003;14:216–21. doi: 10.1034/j.1399-3038.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 33.Tari MG, Mancino M, Monti G. Efficacy of sublingual immunotherapy in patients with rhinitis and asthma due to house dust mite. A double-blind study. Allergol Immunopathol (Madr) 1990;18:277–84. [PubMed] [Google Scholar]
- 34.Hirsch Th, Sähn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol. 1997;8:21–7. doi: 10.1111/j.1399-3038.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 35.Bahçeciler NN, Işik U, Barlan IB, Başaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double-blind, placebo-controlled study. Pediatr Pulmonol. 2001;32:49–55. doi: 10.1002/ppul.1088. [DOI] [PubMed] [Google Scholar]
- 36.Blaiss M, Maloney J. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. American Academy of Allergy, Asthma & Immunology. doi: 10.1016/j.jaci.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 37.Compalati E, Passalacqua G, Bonini M, Canonica GW. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta-analysis. Allergy. 2009;64:1570–9. doi: 10.1111/j.1398-9995.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 38.Calamita Z, Saconato H, Pelá AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: systematic review of randomized-clinical trials using the Cochrane Collaboration method. Allergy. 2006;61:1162–72. doi: 10.1111/j.1398-9995.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- 39.Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol. 2006;97:141–8. doi: 10.1016/S1081-1206(10)60004-X. [DOI] [PubMed] [Google Scholar]
- 40.Malling HJ, Montagut A, Melac M, Patriarca G, Panzner P, Seberova E, et al. Efficacy and safety of 5-grass pollen sublingual immunotherapy tablets in patients with different clinical profiles of allergic rhinoconjunctivitis. Clin Exp Allergy. 2009;39:387–93. doi: 10.1111/j.1365-2222.2008.03152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gidaro GB, Marcucci F, Sensi L, Incorvaia C, Frati F, Ciprandi G. The safety of sublingual-swallow immunotherapy: an analysis of published studies. Clin Exp Allergy. 2005;35:565–71. doi: 10.1111/j.1365-2222.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- 42.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–35. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 43.Lombardi C, Gargioni S, Cottini M, Canonica GW, Passalacqua G. The safety of sublingual immunotherapy with one or more allergens in adults. Allergy. 2008;63:375–6. doi: 10.1111/j.1398-9995.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 44.Passalacqua G, Pawankar R, Baena-Cagnani CE, Canonica GW. Sublingual immunotherapy: where do we stand? Present and future. Curr Opin Allergy Clin Immunol. 2009;9:1–3. doi: 10.1097/ACI.0b013e3283196a9b. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Pérez N, Ambriz-Moreno MdeJ, Canonica GW, Penagos M. Frequency of acute systemic reactions in patients with allergic rhinitis and asthma treated with sublingual immunotherapy. Ann Allergy Asthma Immunol. 2008;101:304–10. doi: 10.1016/S1081-1206(10)60496-6. [DOI] [PubMed] [Google Scholar]
- 46.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010;125:569–74, 574.e1-574.e7. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 47.Dunsky EH, Goldstein MF, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy. 2006;61:1235. doi: 10.1111/j.1398-9995.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 48.Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immunotherapy. Allergy. 2006;61:1236–7. doi: 10.1111/j.1398-9995.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 49.Eifan AO, Keles S, Bahceciler NN, Barlan IB. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy. 2007;62:567–8. doi: 10.1111/j.1398-9995.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 50.Blazowski L. Anaphylactic shock because of sublingual immunotherapy overdose during third year of maintenance dose. Allergy. 2008;63:374. doi: 10.1111/j.1398-9995.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 51.de Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass pollen tablet. Allergy. 2009;64:963–4. doi: 10.1111/j.1398-9995.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 52.Des Roches A, Paradis L, Knani J, Hejjaoui A, Dhivert H, Chanez P, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. V. Duration of the efficacy of immunotherapy after its cessation. Allergy. 1996;51:430–3. doi: 10.1111/j.1398-9995.1996.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 53.Price JF, Warner JO, Hey EN, Turner MW, Soothill JF. A controlled trial of hyposensitization with adsorbed tyrosine Dermatophagoides pteronyssinus antigen in childhood asthma: in vivo aspects. Clin Allergy. 1984;14:209–19. doi: 10.1111/j.1365-2222.1984.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 54.Naclerio RM, Proud D, Moylan B, Balcer S, Freidhoff L, Kagey-Sobotka A, et al. A double-blind study of the discontinuation of ragweed immunotherapy. J Allergy Clin Immunol. 1997;100:293–300. doi: 10.1016/S0091-6749(97)70240-9. [DOI] [PubMed] [Google Scholar]
- 55.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 56.Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011;127:57–63. e1-3. doi: 10.1016/j.jaci.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 57.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. The PAT investigator group Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 58.Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. doi: 10.1111/j.1398-9995.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 59.Di Rienzo V, Marcucci F, Puccinelli P, Parmiani S, Frati F, Sensi L, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003;33:206–10. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 60.Madonini E, Agostinis F, Barra R, Berra A, Donadio D, Pappacoda A, et al. Long-term and preventive effects of sublingual allergen-specific immunotherapy: a retrospective, multicentric study. Int J Immunopathol Pharmacol. 2003;16:73–9. doi: 10.1177/039463200301600111. [DOI] [PubMed] [Google Scholar]
- 61.Ott H, Sieber J, Brehler R, Fölster-Holst R, Kapp A, Klimek L, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:179–86. doi: 10.1111/j.1398-9995.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 62.Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–25. e5. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 63. Missing Reference Info. [Google Scholar]
- 64.Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. SLIT Study Group Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–6.e3. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126:969–75. doi: 10.1016/j.jaci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 66.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 67.Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993;92:644–51. doi: 10.1172/JCI116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benjaponpitak S, Oro A, Maguire P, Marinkovich V, DeKruyff RH, Umetsu DT. The kinetics of change in cytokine production by CD4 T cells during conventional allergen immunotherapy. J Allergy Clin Immunol. 1999;103:468–75. doi: 10.1016/S0091-6749(99)70473-2. [DOI] [PubMed] [Google Scholar]
- 69.Nish WA, Charlesworth EN, Davis TL, Whisman BA, Valtier S, Charlesworth MG, et al. The effect of immunotherapy on the cutaneous late phase response to antigen. J Allergy Clin Immunol. 1994;93:484–93. doi: 10.1016/0091-6749(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 70.Wachholz PA, Nouri-Aria KT, Wilson DR, Walker SM, Verhoef A, Till SJ, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology. 2002;105:56–62. doi: 10.1046/j.1365-2567.2002.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scadding G, Durham S. Mechanisms of sublingual immunotherapy. J Asthma. 2009;46:322–34. doi: 10.1080/02770900902785729. [DOI] [PubMed] [Google Scholar]
- 72.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 73.Faith A, Richards DF, Verhoef A, Lamb JR, Lee TH, Hawrylowicz CM. Impaired secretion of interleukin-4 and interleukin-13 by allergen-specific T cells correlates with defective nuclear expression of NF-AT2 and jun B: relevance to immunotherapy. Clin Exp Allergy. 2003;33:1209–15. doi: 10.1046/j.1365-2222.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- 74.Wachholz PA, Nouri-Aria KT, Wilson DR, Walker SM, Verhoef A, Till SJ, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology. 2002;105:56–62. doi: 10.1046/j.1365-2567.2002.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–61. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 76.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol. 1996;97:1356–65. doi: 10.1016/S0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 77.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–46. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- 78.de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 81.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu DH, Moore KW, Spits H. Differential effects of interleukin-4 and -10 on interleukin-2 induced interferon- + synthesis and lymphokine activated killer activity. Int Immunol. 1992;4:563–9. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 83.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 84.Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121:1120–5.e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 85.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–7. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 87.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178:4658–66. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 88.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 89.Oida T, Xu L, Weiner HL, Kitani A, Strober W. TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J Immunol. 2006;177:2331–9. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- 90.Grindebacke H, Larsson P, Wing K, Rak S, Rudin A. Specific immunotherapy to birch allergen does not enhance suppression of Th2 cells by CD4(+)CD25(+) regulatory T cells during pollen season. J Clin Immunol. 2009;29:752–60. doi: 10.1007/s10875-009-9312-x. [DOI] [PubMed] [Google Scholar]
- 91.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–72, 1472.e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178:4658–66. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 93.Nouri-Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 2005;116:73–9. doi: 10.1016/j.jaci.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Wilson DR, Irani AM, Walker SM, Jacobson MR, Mackay IS, Schwartz LB, et al. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clin Exp Allergy. 2001;31:1705–13. doi: 10.1046/j.1365-2222.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 95.Van Ree R, Van Leeuwen WA, Dieges PH, Van Wijk RG, De Jong N, Brewczyski PZ, et al. Measurement of IgE antibodies against purified grass pollen allergens (Lol p 1, 2, 3 and 5) during immunotherapy. Clin Exp Allergy. 1997;27:68–74. doi: 10.1046/j.1365-2222.1997.d01-416.x. [DOI] [PubMed] [Google Scholar]
- 96.Lichtenstein LM, Ishizaka K, Norman PS, Sobotka AK, Hill BM. IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest. 1973;52:472–82. doi: 10.1172/JCI107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Devey ME, Wilson DV, Wheeler AW. The IgG subclasses of antibodies to grass pollen allergens produced in hay fever patients during hyposensitization. Clin Allergy. 1976;6:227–36. doi: 10.1111/j.1365-2222.1976.tb01901.x. [DOI] [PubMed] [Google Scholar]
- 98.Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 99.García BE, Sanz ML, Diéguez I, de las Marinas MD, Oehling A. Modifications in IgG subclasses in the course of immunotherapy with grass pollen. J Investig Allergol Clin Immunol. 1993;3:19–25. [PubMed] [Google Scholar]
- 100.Ejrnaes AM, Svenson M, Lund G, Larsen JN, Jacobi H. Inhibition of rBet v 1-induced basophil histamine release with specific immunotherapy -induced serum immunoglobulin G: no evidence that FcgammaRIIB signalling is important. Clin Exp Allergy. 2006;36:273–82. doi: 10.1111/j.1365-2222.2006.02442.x. [DOI] [PubMed] [Google Scholar]
- 101.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–22. doi: 10.1016/S0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 102.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 103.Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010;40:1346–52. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- 104.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 105.Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–26. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 106.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178:4658–66. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 107.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 108.Movérare R, Elfman L, Vesterinen E, Metso T, Haahtela T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy. 2002;57:423–30. doi: 10.1034/j.1398-9995.2002.13248.x. [DOI] [PubMed] [Google Scholar]
- 109.Gehlar K, Schlaak M, Becker W-M, Buffe A. Monitoring allergen Immunotherapy Mechanisms of immunotherapy to aeroallergens of pollen-allergic patients: The ration of allergen-specificIgG4 to IgG1 correlates with clinical outcome. Clin Exp Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 110.Bellinghausen I, Knop J, Saloga J. The role of interleukin 10 in the regulation of allergic immune responses. Int Arch Allergy Immunol. 2001;126:97–101. doi: 10.1159/000049499. [DOI] [PubMed] [Google Scholar]
- 111.Allam JP, Stojanovski G, Friedrichs N, Peng W, Bieber T, Wenzel J, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63:720–7. doi: 10.1111/j.1398-9995.2007.01611.x. [DOI] [PubMed] [Google Scholar]
- 112.Allam JP, Novak N, Fuchs C, Asen S, Bergé S, Appel T, et al. Characterization of dendritic cells from human oral mucosa: a new Langerhans’ cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:141–8. doi: 10.1067/mai.2003.1607. [DOI] [PubMed] [Google Scholar]
- 113.Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61:151–65. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 114.Novak N, Haberstok J, Bieber T, Allam JP. The immune privilege of the oral mucosa. Trends Mol Med. 2008;14:191–8. doi: 10.1016/j.molmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–9.e5. doi: 10.1016/j.jaci.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 116.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 117.von Bubnoff D, Matz H, Frahnert C, Rao ML, Hanau D, de la Salle H, et al. FcepsilonRI induces the tryptophan degradation pathway involved in regulating T cell responses. J Immunol. 2002;169:1810–6. doi: 10.4049/jimmunol.169.4.1810. [DOI] [PubMed] [Google Scholar]
- 118.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 119.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 120.Nieminen K, Laaksonen K, Savolainen J. Three-year follow-up study of allergen-induced in vitro cytokine and signalling lymphocytic activation molecule mRNA responses in peripheral blood mononuclear cells of allergic rhinitis patients undergoing specific immunotherapy. Int Arch Allergy Immunol. 2009;150:370–6. doi: 10.1159/000226238. [DOI] [PubMed] [Google Scholar]
- 121.Laaksonen K, Junikka M, Lahesmaa R, Terho EO, Savolainen J. In vitro allergen-induced mRNA expression of signaling lymphocytic activation molecule by PBMC of patients with allergic rhinitis is increased during specific pollen immunotherapy. J Allergy Clin Immunol. 2003;112:1171–7. doi: 10.1016/j.jaci.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 122.Passalacqua G, Albano M, Fregonese L, Riccio A, Pronzato C, Mela GS, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite-induced rhinoconjunctivitis. Lancet. 1998;351:629–32. doi: 10.1016/S0140-6736(97)07055-4. [DOI] [PubMed] [Google Scholar]
- 123.Fanta C, Bohle B, Hirt W, Siemann U, Horak F, Kraft D, et al. Systemic immunological changes induced by administration of grass pollen allergens via the oral mucosa during sublingual immunotherapy. Int Arch Allergy Immunol. 1999;120:218–24. doi: 10.1159/000024270. [DOI] [PubMed] [Google Scholar]
- 124.Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–15. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 125.Cosmi L, Santarlasci V, Angeli R, Liotta F, Maggi L, Frosali F, et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-gamma- and interleukin-10-production. Clin Exp Allergy. 2006;36:261–72. doi: 10.1111/j.1365-2222.2006.02429.x. [DOI] [PubMed] [Google Scholar]
- 126.Savolainen J, Jacobsen L, Valovirta E. Sublingual immunotherapy in children modulates allergen-induced in vitro expression of cytokine mRNA in PBMC. Allergy. 2006;61:1184–90. doi: 10.1111/j.1398-9995.2006.01206.x. [DOI] [PubMed] [Google Scholar]
- 127.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 128.Savolainen J, Nieminen K, Laaksonen K, Laiho T, Jacobsen L, Lahesmaa R, et al. Allergen-induced in vitro expression of IL-18, SLAM and GATA-3 mRNA in PBMC during sublingual immunotherapy. Allergy. 2007;62:949–53. doi: 10.1111/j.1398-9995.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 129.Rolinck-Werninghaus C, Kopp M, Liebke C, Lange J, Wahn U, Niggemann B. Lack of detectable alterations in immune responses during sublingual immunotherapy in children with seasonal allergic rhinoconjunctivitis to grass pollen. Int Arch Allergy Immunol. 2005;136:134–41. doi: 10.1159/000083320. [DOI] [PubMed] [Google Scholar]
- 130.Piconi S, Trabattoni D, Rainone V, Borgonovo L, Passerini S, Rizzardini G, et al. Immunological effects of sublingual immunotherapy: clinical efficacy is associated with modulation of programmed cell death ligand 1, IL-10, and IgG4. J Immunol. 2010;185:7723–30. doi: 10.4049/jimmunol.1002465. [DOI] [PubMed] [Google Scholar]
- 131.Passalacqua G, Albano M, Riccio A, Fregonese L, Puccinelli P, Parmiani S, et al. Clinical and immunologic effects of a rush sublingual immunotherapy to Parietaria species: A double-blind, placebo-controlled trial. J Allergy Clin Immunol. 1999;104:964–8. doi: 10.1016/S0091-6749(99)70076-X. [DOI] [PubMed] [Google Scholar]
- 132.Durham SR, Riis B. Grass allergen tablet immunotherapy relieves individual seasonal eye and nasal symptoms, including nasal blockage. Allergy. 2007;62:954–7. doi: 10.1111/j.1398-9995.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 133.Calderon MA, Birk AO, Andersen JS, Durham SR. Prolonged preseasonal treatment phase with Grazax sublingual immunotherapy increases clinical efficacy. Allergy. 2007;62:958–61. doi: 10.1111/j.1398-9995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 134.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–22. doi: 10.1016/S0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 135.Ejrnaes AM, Svenson M, Lund G, Larsen JN, Jacobi H. Inhibition of rBet v 1-induced basophil histamine release with specific immunotherapy -induced serum immunoglobulin G: no evidence that FcgammaRIIB signalling is important. Clin Exp Allergy. 2006;36:273–82. doi: 10.1111/j.1365-2222.2006.02442.x. [DOI] [PubMed] [Google Scholar]
- 136.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 137.O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180:936–47. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 138.Akdis CA, Blaser K. Bypassing IgE and targeting T cells for specific immunotherapy of allergy. Trends Immunol. 2001;22:175–8. doi: 10.1016/S1471-4906(01)01862-2. [DOI] [PubMed] [Google Scholar]
- 139.Akdis CA, Blaser K. Bypassing IgE and targeting T cells for specific immunotherapy of allergy. Trends Immunol. 2001;22:175–8. doi: 10.1016/S1471-4906(01)01862-2. [DOI] [PubMed] [Google Scholar]
- 140.Kussebi F, Karamloo F, Rhyner C, Schmid-Grendelmeier P, Salagianni M, Mannhart C, et al. A major allergen gene-fusion protein for potential usage in allergen-specific immunotherapy. J Allergy Clin Immunol. 2005;115:323–9. doi: 10.1016/j.jaci.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 141.Marsh DG, Norman PS, Roebber M, Lichtenstein LM. Studies on allergoids from naturally occurring allergens. III. Preparation of ragweed pollen allergoids by aldehyde modification in two steps. J Allergy Clin Immunol. 1981;68:449–59. doi: 10.1016/0091-6749(81)90199-8. [DOI] [PubMed] [Google Scholar]
- 142.Karamloo F, Schmid-Grendelmeier P, Kussebi F, Akdis M, Salagianni M, von Beust BR, et al. Prevention of allergy by a recombinant multi-allergen vaccine with reduced IgE binding and preserved T cell epitopes. Eur J Immunol. 2005;35:3268–76. doi: 10.1002/eji.200425522. [DOI] [PubMed] [Google Scholar]
- 143.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Larché M. Immunoregulation by targeting T cells in the treatment of allergy and asthma. Curr Opin Immunol. 2006;18:745–50. doi: 10.1016/j.coi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 145.Simons FE, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol. 2004;113:1144–51. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 146.Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, Lavigne F, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004;113:235–41. doi: 10.1016/j.jaci.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 147.Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 148.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 149.Klunker S, Saggar LR, Seyfert-Margolis V, Asare AL, Casale TB, Durham SR, et al. Immune Tolerance Network Group Combination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: Inhibition of IgE-facilitated allergen binding. J Allergy Clin Immunol. 2007;120:688–95. doi: 10.1016/j.jaci.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 150.Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci USA. 2008;105:17908–12. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]