Abstract
BACKGROUND: Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor, usually arises in the setting of liver cirrhosis (LC), and has a poor prognosis. The recently discovered Th2-cytokine interleukin-33 (IL-33) is a possible mediator in pancreatic and gastric carcinogeneses. IL-33 binds to its receptor and to soluble ST2 (sST2), which thereby acts as a regulator. The role of IL-33 and sST2 in HCC has not been elucidated yet. METHODS: We conducted a case-control study with 130 patients and 50 healthy controls (HCs). Sixty-five patients suffered from HCC and 65 patients had LC without HCC. We assessed serum IL-33 and sST2 levels and their association with established prognostic scores, liver function parameters, and overall survival (OS). RESULTS: No significant difference in IL-33 serum levels was found in HCC compared to LC and HCs. IL-33 levels did not correlate with OS, liver function parameters, the Model for End-Stage Liver Disease (MELD) score, or the Cancer of the Liver Italian Program (CLIP) score. sST2 levels were significantly elevated in LC and HCC patients compared to HCs (P < .0001). Mean sST2 levels in LC were higher than in HCC (P < .0001), but a significant association with OS was only observed in the HCC group (P = .003). sST2 in HCC correlated with the CLIP score, the MELD score, and liver function parameters. CONCLUSION: In the present study, the serum concentration of sST2 was associated with OS of HCC. Therefore, sST2 may be considered as a new prognostic marker in HCC and is worth further evaluation.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor, usually arises in the setting of liver cirrhosis (LC), and accounts for up to 70% to 85% of all primary liver cancers [1]. More than 700,000 cases are newly diagnosed each year with a high incidence especially in Asian countries [2]. Patients with HCC often present at an advanced stage of the disease leaving limited treatment options due to underlying LC and a lack of effective systemic therapies. Therefore, prognosis is poor with a median overall survival (OS) of less than 1 year [3]. To the present day, prognosis in those patients is mainly derived from different staging systems, e.g., the Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), or Japan Integrated Scoring System [4]. New types of biomarkers (e.g., des-γ-carboxy prothrombin, α-l-fucosidase, human carbonyl reductase 2, tumor-specific growth factor, and hepatocyte growth factor) have recently been under investigation [5]. Some of them have shown good sensitivity in terms of early HCC detection but failed to show a good correlation with prognosis. New prognostic markers such as CXCL5, HMGB1, or vascular endothelial growth factor receptor-1 (VEGFR-1) have not yet been established in clinical routine [6–8]. Th1- and Th2-associated cytokines like interleukin-6 (IL-6) or IL-10 also play an important role since hepatic carcinogenesis is closely related to the host immune response and the tumor immune surveillance [9,10].
IL-33 was discovered as a novel cytokine belonging to the IL-1 family in 2005 [11]. It is expressed by various nonhematopoietic cells including endothelial cells, fibroblasts, bronchial and epithelial cells, and certain populations of immune cells, e.g., macrophages and dendritic cells [12]. Currently, it is proposed that IL-33 is released upon cellular necrosis as a 30-kDa molecule (full-length IL-33) and is processed into less active forms of 20 to 22 kDa by caspase-3 and caspase-7 cleavage [13–15]. IL-33 signaling is mediated by its receptor ST2 that exists in a soluble form (sST2) and as a transmembrane receptor (ST2L). Binding to ST2L leads to activation of nuclear factor κB and mitogen-activated protein kinase signaling pathways. sST2 is assumed to act as a decoy receptor for IL-33 and thereby attenuates its biologic activity [16,17]. In contrast to IL-1 and IL-18 triggering Th1/Th17 immune responses, IL-33 mainly promotes Th2 cytokines such as IL-4, IL-5, and IL-13 [11]. The role of the IL-33/ST2 axis has been extensively studied in Th2-driven chronic inflammatory conditions such as asthma, rheumatoid arthritis, allergic rhinitis, or inflammatory bowel disease. The role of IL-33 and ST2 in terms of carcinogenesis and tumor progression is less well understood. It is known that IL-33 contributes to alternative macrophage activation leading to recently described M2 polarization [18,19]. Tumor-associated macrophages are known to play a relevant role in different types of solid tumors. Polarization towards immune regulative M2 seems to be unfavorable since tumors are protected and escape immune surveillance. In an in vitro model, IL-33 has been described as a crucial mediator of inflammation-associated pancreatic carcinogenesis [20]. Elevated levels of IL-33 have been determined in sera and tumor tissue of gastric cancer patients and were associated with prognostic markers such as depth of tumor invasion [21]. In contrast to that, other authors have described IL-33 as a potent inducer of anticancerogenic immunity leading to enhanced activation of cytotoxic CD8+ cells [22]. ST2 levels were elevated in breast cancer patients and associated with tumor progression and metastasis [23]. In the field of hepatology, IL-33 has recently been identified as an important factor contributing to liver injury caused by chronic hepatitis C and is also associated with fibrosis in in vitro models [24].
However, the relation between IL-33 and sST2 serum levels and the severity of disease and the survival of patients suffering from LC and of patients suffering from cirrhosis and HCC has not been determined yet. In the present study, we analyzed for the first time IL-33 and sST2 serum levels in such patients and examined the association of IL-33 and sST2 with severity and prognosis of liver disease in cirrhotic patients with and without HCC.
Patients and Methods
Patients
Sixty-five patients suffering from LC with HCC and 65 patients with LC without HCC treated at the Department of Gastroenterology at Johann Wolfgang Goethe University Hospital were included in the study. Patients were matched one by one in a case-controlled manner. This was a substudy of two case-control studies performed at the Goethe University Hospital. Written informed consent was obtained from all patients before inclusion in the study. The studies were approved by the Ethics Committee of the Goethe University Hospital.
Patients with LC and HCC (HCC group). Sixty-five patients with LC and recently diagnosed HCC who were admitted to our liver unit between April 2009 and August 2011 were prospectively enrolled in the present study. Inclusion criteria were HCC diagnosed on the basis of histologic samples obtained from liver biopsies before study inclusion or on the basis of morphologic/visual criteria according to current American Association for the Study of Liver Diseases (AASLD) guidelines. When the diagnosis of HCC was confirmed by dynamic imaging, two independent dynamic imaging techniques were used including dynamic contrast-enhanced magnetic resonance imaging (MRI) or four-phase computed tomography (CT), whereas arterial hypervascularity and delayed or venous phase washout was obligatory for diagnosis. Additionally, such patients received contrast-enhanced ultrasound and/or lipiodol angiography. Patients were assigned to different BCLC stages or CLIP scores on the basis of clinical examination, laboratory tests, and CT/MRI or ultrasound findings. Exclusion criteria were defined by an age below 18 years, former liver transplantation, ongoing concomitant autoimmune disease including asthma and allergies, and a history of cancer within the last 5 years other than HCC.
Patients with LC without HCC (LC group). Sixty-five patients with LC who had been admitted to our liver unit from May 2009 to May 2010 were included in a comparison cohort and were individually matched to the HCC group according to age, sex, model for end-stage liver disease (MELD) score, etiology of liver disease, and laboratory values [C-reactive protein (CRP), sodium, creatinine, bilirubin, international normalized ratio (INR)]. Patients were included in the comparison cohort when they had LC assessed by histopathologic examination or pathognomonic results in abdominal ultrasound examination, transient elastography, CT, or MRI. Exclusion criteria were an age below 18 years, former liver transplantation, a history of cancer within the last 5 years, and clinical or morphologic signs of HCC.
At the day of informed consent and enrollment of patients, blood samples for further analysis were obtained and blood was also tested for routine parameters of clinical chemistry.
Healthy controls (HC group). Sera of 50 individuals were collected on a voluntarily basis. Individuals were free of any significant disease including asthma and allergies, nonsmokers, and had no history of concomitant liver disease.
Blood Sampling
Ten milliliters of peripheral blood was collected from each patient at the time of study inclusion (heparinized serum tubes; Sarstedt, Nümbrecht, Germany). The tube was immediately centrifuged at 3000g for 10 minutes to remove cellular components, aliquoted, and then stored at -80°C until the date of experiments. Sera of the 50 healthy individuals were collected, centrifuged, aliquoted, and stored in the same way.
Clinical Chemistry
Routine parameters of clinical chemistry were measured at the Central Laboratory of the Goethe University Hospital.
Detection of IL-33 and sST2 by ELISA
The concentrations of IL-33 and sST2 in the sera of individual patients and HCs were determined by ELISA. Commercially available human IL-33 and sST2 ELISA kits were used according to the manufacturers' instructions (R&D Systems, Minneapolis, MN). Sera of all patients and HCs were subjected to analysis and the concentrations of serum IL-33 and sST2 in individual samples were calculated according to the standard curve established by using the recombinant IL-33 and sST2 provided. The detection limit of the IL-33 and sST2 ELISA kits was 16 pg/ml and 1.6 pg/ml, respectively.
Statistical Analysis
Data were analyzed and compiled using the BiAS software for windows (version 9.11; Epsilon-Verlag, Darmstadt, Germany), SPSS version 20 (IBM, Chicago, IL), and GraphPad Prism 5 for Windows (version 5; GraphPad Software, La Jolla, CA). The primary end point of the study was OS. Death was considered as event. Follow-up time was time until death or last contact to the patient. OS rates and survival curves were calculated for all patients using the Kaplan-Meier model. Independent predictors of survival were determined with a univariate and a multivariate Cox regression analysis using forward stepwise (likelihood ratio) entry. The nonparametric Wilcoxon-Mann-Whitney and Kruskal-Wallis tests were used to determine differences between groups of patients. A Bonferroni correction was used when multiple subgroup comparisons were performed. The data are expressed as means ± SEM unless specified. P values < .05 were considered to be significant. The correlation coefficient ρ was calculated by using the Spearman correlation.
Results
A total of 130 patients were included in the study. Sixty-five of them suffered from HCC and 65 had LC without HCC. Since the groups were matched individually, no significant differences in terms of age, sex, MELD, underlying etiology of liver disease, and laboratory values were observed (Table 1). In 59 of 65 HCC patients, the diagnosis of HCC was confirmed by histopathologic examination of biopsy material, and in 6 of the 65 patients, HCC diagnosis was performed on the basis of morphologic/visual criteria according to current AASLD guidelines [25].
Table 1.
Patients' Characteristics.
| HCC Group | LC Group | P Value | HCs | |
| Number (n) | 65 | 65 | 50 | |
| Male (n) | 56 (86%) | 61 (93.8%) | 1.0 | 28 (56%) |
| Mean age (range) | 64.4 (38–83) | 62.4 (27–79) | .31 | 35 (23–57) |
| Liver cirrhosis (n) | 61 (93.8%) | 65 (100%) | 0 (0%) | |
| HBV | 9 (14.7%) | 9 (14.7) | ||
| HCV | 26 (42.6%) | 28 (43.1%) | ||
| Alcohol | 23 (37.7%) | 33 (50.7%) | ||
| Others | 7 (10.7%) | 7 (10.7%) | ||
| HCC (n) | 65 (100%) | 0 (0%) | 0 (0%) | |
| BCLC A | 9 (13.8%) | NA | ||
| BCLC B | 36 (55.4%) | NA | ||
| BCLC C | 17 (26.2%) | NA | ||
| BCLC D | 4 (6.2%) | NA | ||
| MELD score (mean ± SD) | 11.0 ± 3.96 | 11.8 ± 3.91 | .08 | |
| CLIP score (mean ± SD) | 3 ± 1.4 | NA | ||
| AFP (ng/ml, mean ± SD) | 4520 ± 1835 | NA | ||
| Bilirubin (mg/dl) | 1.94 ± 3.19 | 1.77 ± 1.73 | .44 | |
| Creatinine (mg/dl) | 1.0 ± 0.6 | 1.03 ± 0.42 | .48 | |
| INR | 1.23 ± 0.22 | 1.27 ± 0.22 | .14 | |
| Sodium (mmol/l) | 139.4 ± 4.82 | 138.7 ± 4.47 | .23 | |
| CRP (mg/dl) | 2.27 ± 3.6 | 1.35 ± 1.98 | .44 |
NA, not applicable.
sST2 Levels Are Raised in Patients with HCC and LC
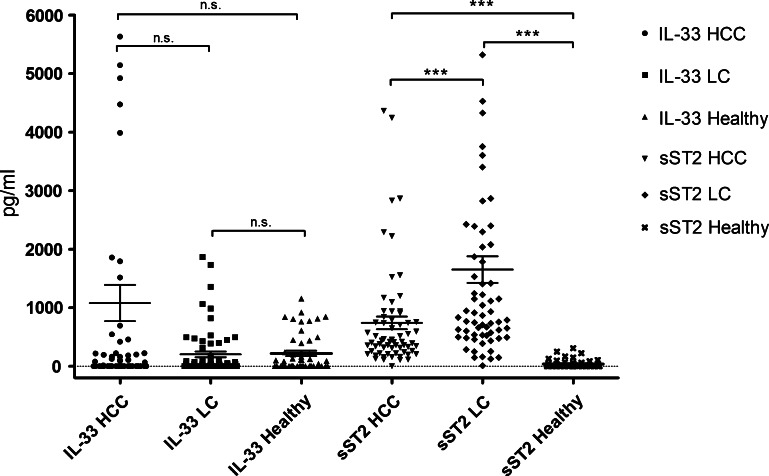
IL-33 and sST2 serum levels were assessed in patients with HCC and LC and in a cohort of HC subjects. There was a tendency to higher IL-33 levels in HCC in comparison to LC (1079.6 ± 310.1 vs 203.4 ± 49.95 pg/ml), but the difference was not statistically significant (P = .41). The IL-33 levels in patients with LC did not differ from HCs (203.4 ± 49.95 vs 218.6 ± 45.8 pg/ml, P = .51). Mean sST2 levels in HCC were 741.6 ± 109.0 pg/ml compared to 1653.0 ± 228.2 pg/ml in LC (P < .0001). sST2 levels in both HCC and LC were significantly higher than in HCs (38.5 ± 10.1 pg/ml, P < .0001). See Table 2 and Figure 1 for details.
Table 2.
IL-33 and sST2 Levels in HCC, LC, and HC.
| HCC Group | LC Group | P Value | HCs | |
| IL-33 | ||||
| Mean ± SEM (pg/ml) | 1079.6 ± 310.1 | 203.4 ± 49.95 | NS | 218.6 ± 45.8 |
| sST2 | ||||
| Mean ± SEM (pg/ml) | 741.6 ± 109.0 | 1653.0 ± 228.2 | <.0001 | 38.5 ± 10.1 |
Figure 1.
Comparison of IL-33 and sST2 serum levels in HCC, LC, and HCs. IL-33 levels in HCC tended to be higher than in LC. sST2 levels in both LC and HCC patients were significantly higher than in HCs (P < .0001).
Levels of IL-33 and sST2 in Association with Tumor Stage
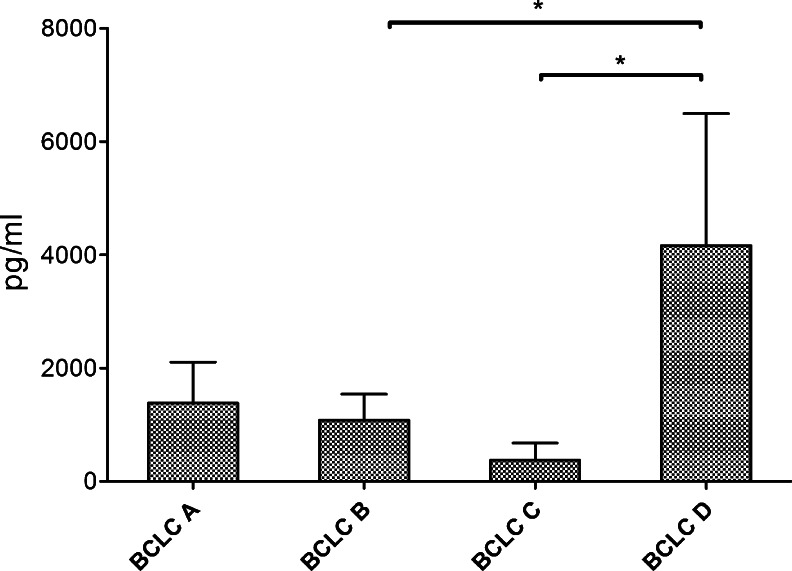
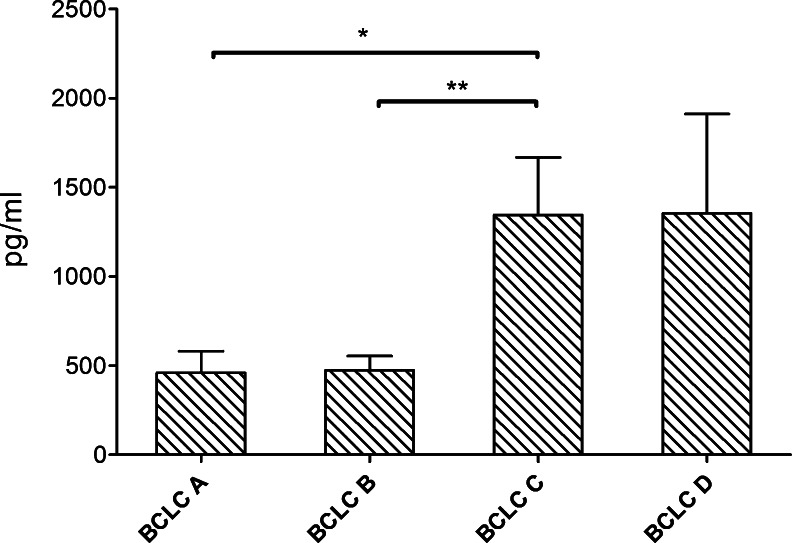
IL-33 levels in HCC patients at BCLC stage D were significantly higher than at BCLC stage C (4163 ± 2329 vs 375.1 ± 300.8 pg/ml, P = .01) and at BCLC stage B (4163 ± 2329 vs 1081 ± 460.4 pg/ml, P = .01; Figure 2). The highest sST2 levels were found in BCLC stage C and D patients (1345 ± 322.2 and 1355 ± 557 pg/ml, respectively). sST2 levels in BCLC stage A (460.3 ± 121.5 pg/ml) and B patients (476.0 ± 78.26 pg/ml) were significantly lower in comparison to BCLC stage C patients (P = .02 and P = .002; Figure 3).
Figure 2.
IL-33 levels in HCC according to BCLC stage. IL-33 levels were significantly higher at BCLC stage D than at stage B or C.
Figure 3.
sST2 levels according to BCLC stage. Significantly higher levels of sST2 were observed at BCLC stage C.
Levels of IL-33 and sST2 in Accordance to Underlying Liver Disease
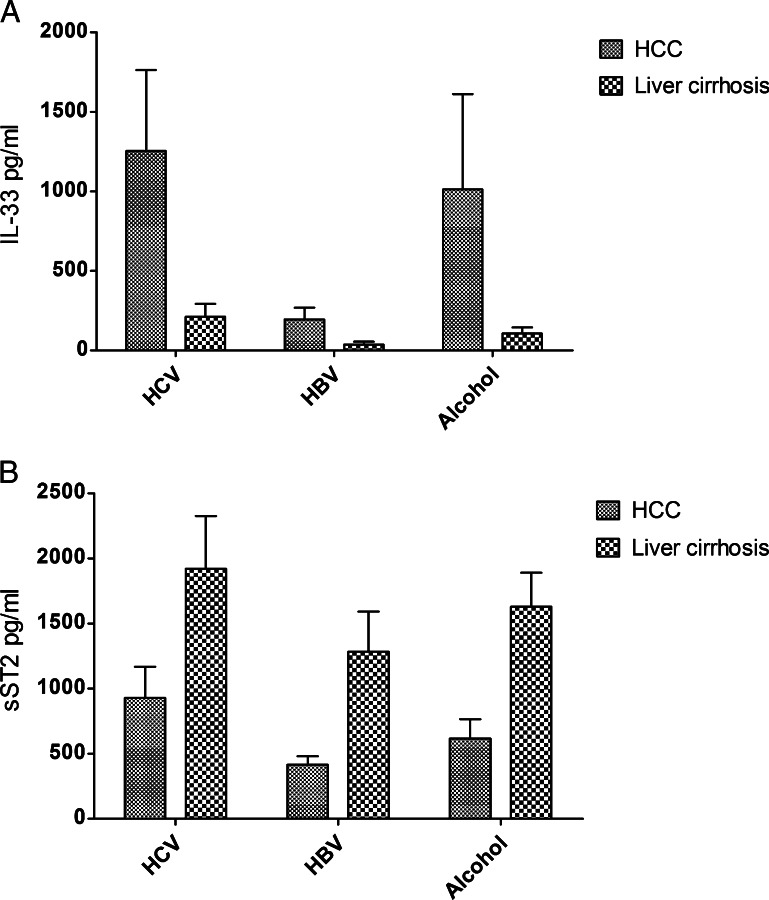
Serum levels of IL-33 and sST2 were also analyzed in accordance to the etiology of the underlying liver disease. Three subgroups consisting of patients with chronic hepatitis B (HBV) or hepatitis C (HCV) and alcohol-induced liver disease were compared. The analysis revealed higher IL-33 concentrations in the HCV and alcohol groups in comparison to the HBV group in HCC and LC patients. However, neither in the HCC group nor in the LC group the differences were statistically significant. A similar but again insignificant tendency was obtained for the sST2 levels in the HCC and LC groups with higher sST2 levels in the HCV and alcohol subgroups and lower levels in the HBV group (Figure 4).
Figure 4.
(A and B) Comparison of IL-33 and sST2 levels according to underlying liver disease. HCV patients had slightly higher IL-33 and sST2 levels than other patients in both the HCC and LC groups. Differences were not significant.
Correlation of IL-33 and sST2 with Liver Function Parameters
To investigate the possible role of IL-33 and sST2 in necroinflammation and cell turnover, the correlation between IL-33 and sST2 levels with surrogate markers of cell death, liver function, and established prognostic scores in patients with HCC and LC without HCC was analyzed. IL-33 levels did not correlate with liver enzymes [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], liver synthesis (INR), α-fetoprotein (AFP), the MELD score, or the CLIP score. In the HCC group, the sST2 serum concentration significantly correlated with AFP, CRP, the MELD score, and the CLIP score. In the LC group, sST2 was inversely correlated with IL-33 and positively correlated with AST and INR (Table 3).
Table 3.
Correlations of sST2 with Liver Function Parameters, CLIP Score, MELD Score, and IL-33.
| HCC Group | LC Group | |||
| ρ | P Value | ρ | P Value | |
| AFP | 0.32 | .008 | NA | NA |
| CRP | 0.51 | <.0001 | 0.06 | NS |
| CLIP | 0.47 | <.0001 | NA | NA |
| MELD | 0.33 | .007 | 0.17 | NS |
| AST | 0.30 | .01 | 0.27 | .031 |
| INR | 0.05 | NS | 0.31 | .01 |
| IL-33 | 0.03 | NS | -0.26 | .035 |
Survival Data
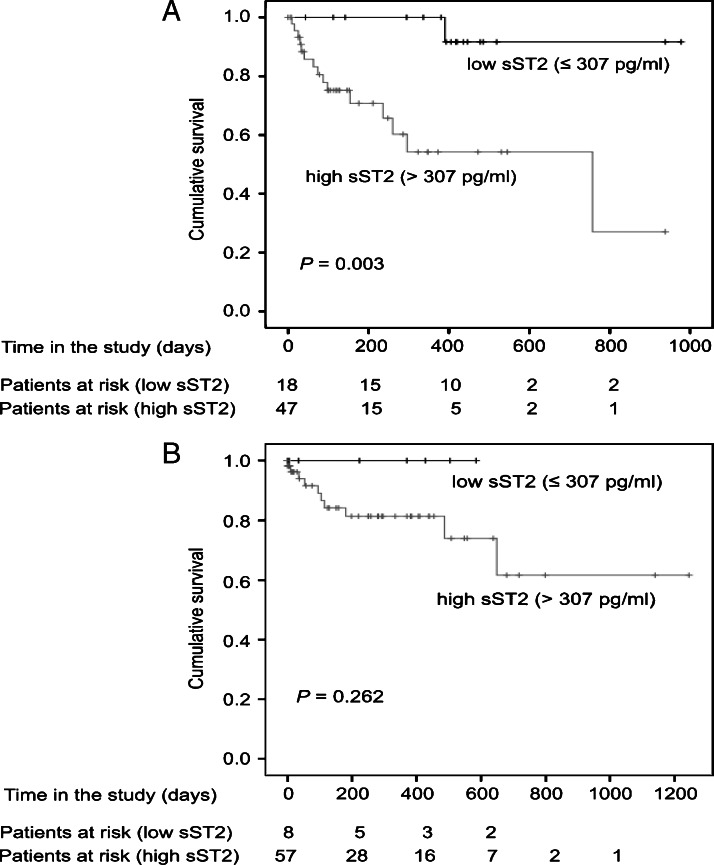
As sST2 levels correlated with parameters associated with survival (MELD and CLIP scores), the relations between sST2 and IL-33 serum levels and OS were assessed. Referring to the data obtained from HCs, the highest sST2 value found in that group was used as a cutoff (307 pg/ml). Kaplan-Meier analysis revealed a significant association between the sST2 levels and OS in HCC patients. Mean OS was 928.1 days with low sST2 levels [±46.8 days, 95% confidence interval (CI) = 836.3–1019.9] and 522.4 days with high sST2 levels (P = .003, ±77.6 days, 95% CI = 370.3–674.5). Survival curves for HCC patients according to their sST2 levels are shown in Figure 5A. For patients with LC, the same cutoff (307 pg/ml) was used and Kaplan-Meier analysis was performed. However, although patients with LC with low sST2 levels tended to show a better prognosis, the difference among both groups was not statistically significant (P = .262; Figure 5B). Furthermore, there was no significant relation between serum IL-33 levels and OS in the HCC and LC groups (P = .297 and P = .101, respectively).
Figure 5.
(A) Survival curve for HCC patients according to sST2 serum levels. Patients with high sST2 levels (cutoff = 307 pg/ml) had a significantly worse OS (P = .003). (B) Survival curve for LC patients according to sST2 serum levels. OS of LC patients with high sST2 levels tended to be worse, but the difference was not statistically significant (cutoff = 307 pg/ml, P = .262).
As sST2 was significantly associated with OS in HCC, we sought to determine association with OS independently from other parameters. First, a univariate Cox regression analysis using sST2, age, gender, the CLIP score, BCLC stage, CRP, AFP, ALT, and AST was performed. The analysis showed that only low sST2 (P = .018, hazard ratio (HR) = 0.084, 95% CI = 0.011–0.654) and a low CLIP score (P = .005, HR = 0.210, 95% CI = 0.071–0.624) were associated with OS in HCC patients. Additionally, a sex- and age-corrected multivariate analysis with forward stepwise likelihood ratio with inclusion of the CLIP score, AFP, CRP, ALT, and AST was performed. The analysis revealed that only the sST2 levels (P = .021, HR = 0.088, 95% CI = 0.011–0.693) and the CLIP score (P = .010, HR = 0.235, 95% CI = 0.077–0.711) were independently associated with OS (Table 4).
Table 4.
Univariate and Multivariate Analyses of Parameters Associated with Overall Survival.
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Low sST2 | 0.084 | 0.011–0.654 | .018 | 0.088 | 0.011–0.693 | .021 |
| Male gender | 0.495 | 0.139–1.768 | .279 | |||
| Age < 67 years | 1.832 | 0.575–5.841 | .306 | |||
| CLIP score ≤ 3 | 0.210 | 0.071–0.624 | .005 | 0.235 | 0.077–0.711 | .010 |
| BCLC stage A | 1.246 | 0.340–4.566 | .740 | |||
| Normal CRP | 0.446 | 0.100–1.994 | .290 | |||
| AFP ≤ 400 ng/ml | 0.424 | 0.146–1.232 | .115 | |||
| ALT ≤ 50 U/l | 0.258 | 0.059–1.138 | .074 | |||
| AST ≤ 50 U/l | 0.037 | 0.000–12.746 | .269 | |||
Low sST2, sST2 serum level ≤ 307 pg/ml; normal CRP, C-reactive protein ≤ 0.5 mg/dl.
IL-33/sST2 Ratio
IL-33 binds to the sST2 receptor, thereby mediating its function as a regulator. Therefore, the potential bioactivity of free IL-33 was determined by calculation of the IL-33/sST2 ratio for each patient. The mean ratio found in HCs was significantly higher than in HCC patients (149.2 ± 41.9 vs 32.61 ± 28.6, P < .0001) and in LC patients (149.2 ± 41.9 vs 0.42 ± 0.13, P < .0001). The difference of the mean IL-33/sST2 ratio between HCC and LC indicated a higher amount of unbound IL-33 in HCC patients, but the difference between those two was not statistically significant.
Discussion
IL-33 is a recently identified cytokine with diverse and context-dependent functions. IL-33 has been described as a potent inducer of Th2 immune responses as well as “alarmin” cytokine released from necrotic cells [26,27]. Together with its receptor ST2 that exists in a soluble form sST2 and a transmembrane form ST2L, the influence of the IL-33/ST2 axis reaches from protective to pathogenic in various disease conditions. In dextran sodium sulfate (DSS)-induced chronic colitis in mice, exogenously administered IL-33 has a protective effect on the epithelial barrier, whereas exogenous administration in a liver fibrosis model is pathogenic and leads to profibrotic switch of cytokine production [24]. Little is known about the function of IL-33 and its soluble receptor sST2 in patients with LC or HCC, two diseases with different conditions regarding their clinical and immunologic basis. In our work, we assessed for the first time IL-33 and sST2 serum concentrations in cirrhotic patients with and without HCC and compared it to HCs. We found significantly higher sST2 levels in cirrhotic patients with and without HCC in comparison to healthy subjects. Kaplan-Meier analysis revealed a significant association between sST2 levels and OS in patients suffering from HCC.
The finding of high sST2 levels in our patients with HCC is in line with the data of Gillibert-Duplantier et al. showing elevated serum concentrations of sST2 in patients with metastatic breast cancer [23]. High sST2 levels have also been found in malignant pleural effusions of patients with lung cancer [28]. Furthermore, it has been demonstrated that a lack of ST2 in ST-/- mice is associated with suppressed breast cancer progression and metastasis, which was accompanied by enhanced cytotoxic activity of NK cells and increased systemic Th1/Th17 cytokines [29]. Nevertheless, to the current date, it remains unclear whether sST2 is causally and directly involved in carcinogenesis or whether it is to be seen as a marker of concomitant inflammation or tissue injury. Evidence for that arises from elevated levels of sST2, which are also found in nontumor conditions, e.g., allergy, atopy, multiple sclerosis, inflammatory bowel diseases, and recently even in cardiovascular disease [30–32]. The significant correlation of the sST2 serum concentration with the levels of AST and CRP in our study indicates that sST2 is associated with liver injury and (hepatic) cell death as well as the systemic inflammatory response. The association with systemic inflammation is supported by other publications dealing with sST2 in different disease settings. The Dallas Heart Study revealed a significant association of sST2 with markers of inflammation; furthermore, sST2 was highly associated with all-cause mortality and cardiovascular mortality [33]. Another study showed elevated levels of sST2 in diabetic patients with critical limb ischemia, again predicting mortality in those patients [34]. Correspondingly, our study also showed a significant elevation of sST2 in LC, primarily a nontumor condition. This fact must be addressed considering the different cytokine environment in LC and HCC patients. Several publications clearly demonstrated an up-regulation of Th1 cytokines like IL-1β, IL-2, or tumor necrosis factor α (TNF-α) in patients with LC [9,35,36]. For many different tumor types, e.g., gastric cancer [37,38], pancreatic cancer [39], or colorectal cancer [40–42], a shift toward a Th2-dominant immune response has been shown. A local Th2 cytokine skewing in HCC was seen by Budhu et al. and was associated with a metastatic phenotype [35]. It is also known that ST2 is induced in a proinflammatory environment by tumor necrosis factor α, IL-6, and IL-1β [43–45]. We therefore hypothesize that high levels of sST2 in the sera of LC patients are to be seen in the wake of a proinflammatory cytokine environment of LC patients, thus supporting the above-mentioned hypothesis of inflammation. However, despite higher levels of sST2 in LC, differences in OS for those patients were not significant in contrast to the association of OS and sST2 levels in HCC patients.
Referring to the previously addressed context-dependent functions of IL-33, levels observed in the HCC group of our study tended to be higher in comparison to both LC patients and healthy individuals. The IL-33/sST2 ratio in HCC indicated a higher amount of unbound IL-33 in those patients compared to LC patients. Considering IL-33 as an “alarmin” similar to HMGB1 [26], elevated levels in HCC are possibly caused by its release from necrotic tumor cells, thus inducing antitumoral immunity by recruiting CD4+ and CD8+ cytotoxic lymphocytes as well as interferon-γ (IFN-γ) producing NK cells [17,22]. Neutralization of IL-33 by its soluble receptor sST2 may then lead to attenuation of antitumoral immunity. If this provides an explanation for the negative prognostic impact of sST2 in HCC is unsure and has yet to be demonstrated.
In conclusion, this is the first study to show a significant correlation of survival in HCC patients with serum levels of the IL-33 receptor sST2. Our data suggest an important role of sST2 in HCC, whereas the implicit function—promotor of carcinogenesis or reflection of systemic inflammation—remains to be clarified. Further research will be needed to clearly elucidate the source, regulation, secretion, and degradation of IL-33 and ST2 in HCC and LC and to evaluate their potential as prognostic markers.
Footnotes
A.R. and part of this investigation were funded by a grant from Else Kröner-Fresenius Foundation (TRIP) awarded to H.H.R. The authors have no conflicts of interest to declare.
References
- 1.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, Loong CC, Chiang JH, Huo TI, Lee SD. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006–3014. doi: 10.1002/cncr.25044. [DOI] [PubMed] [Google Scholar]
- 5.Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076. doi: 10.1155/2012/859076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012);56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Zhang Y, Peng Z, Gao H, Xu L, Chen M. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012;10:135. doi: 10.1186/1479-5876-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Zhu Y, Qin CY, Yang Z, Fang A, Xu S, Ren W. Expression and prognostic significance of vascular endothelial growth factor receptor 1 in hepatocellular carcinoma. J Clin Pathol. 2012;65:808–814. doi: 10.1136/jclinpath-2012-200721. [DOI] [PubMed] [Google Scholar]
- 9.Johnson C, Han Y, Hughart N, McCarra J, Alpini G, Meng F. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer. 2012;1:58–70. doi: 10.3978/j.issn.2224-4778.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan SL, Mo FK, Wong CS, Chan CM, Leung LK, Hui EP, Ma BB, Chan AT, Mok TS, Yeo W. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2011;118:3984–3992. doi: 10.1002/cncr.26726. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jovanovic IP, Pejnovic NN, Radosavljevic GD, Arsenijevic NN, Lukic ML. IL-33/ST2 axis in innate and acquired immunity to tumors. Oncoimmunology. 2012;1:229–231. doi: 10.4161/onci.1.2.18131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 18.Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, Alves-Filho JC, Togbe D, Goodyear CS, Linington C, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 19.Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, Das A, Hogaboam CM. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmieder A, Multhoff G, Radons J. Interleukin-33 acts as a pro-inflammatory cytokine and modulates its receptor gene expression in highly metastatic human pancreatic carcinoma cells. Cytokine. 2012;60:514–521. doi: 10.1016/j.cyto.2012.06.286. [DOI] [PubMed] [Google Scholar]
- 21.Sun P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Dig Dis Sci. 2011;56:3596–3601. doi: 10.1007/s10620-011-1760-5. [DOI] [PubMed] [Google Scholar]
- 22.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 23.Gillibert-Duplantier J, Duthey B, Sisirak V, Salaun D, Gargi T, Tredan O, Finetti P, Bertucci F, Birnbaum D, Bendriss-Vermare N, et al. Gene expression profiling identifies sST2 as an effector of ErbB2-driven breast carcinoma cell motility, associated with metastasis. Oncogene. 2011;31:3516–3524. doi: 10.1038/onc.2011.525. [DOI] [PubMed] [Google Scholar]
- 24.Marvie P, Lisbonne M, L'Helgoualc'h A, Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Theret N, Gascan H, Piquet-Pellorce C, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arshad MI, Piquet-Pellorce C, Samson M. IL-33 andHMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32:1200–1210. doi: 10.1111/j.1478-3231.2012.02802.x. [DOI] [PubMed] [Google Scholar]
- 27.Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5:18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oshikawa K, Yanagisawa K, Ohno S, Tominaga S, Sugiyama Y. Expression of ST2 in helper T lymphocytes of malignant pleural effusions. Am J Respir Crit Care Med. 2002;165:1005–1009. doi: 10.1164/ajrccm.165.7.2105109. [DOI] [PubMed] [Google Scholar]
- 29.Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, Jonjic S, Lukic ML. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41:1902–1912. doi: 10.1002/eji.201141417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao YS, Na SP, Zhang P, Jia XB, Liu RC, Yu CY, Mu SH, Xie RJ. Characterization of interleukin-33 and soluble ST2 in serum and their association with disease severity in patients with chronic kidney disease. J Clin Immunol. 2012;32:587–594. doi: 10.1007/s10875-011-9622-7. [DOI] [PubMed] [Google Scholar]
- 31.Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, Cheng S, Fradley MG, Kretschman D, Gao W, et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. 2012;58:1673–1681. doi: 10.1373/clinchem.2012.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AM, Purves D, McConnachie A, Asquith DL, Batty GD, Burns H, Cavanagh J, Ford I, McLean JS, Packard CJ, et al. Soluble ST2 associates with diabetes but not established cardiovascular risk factors: a new inflammatory pathway of relevance to diabetes? PLoS One. 2012;7:e47830. doi: 10.1371/journal.pone.0047830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen LQ, de Lemos JA, Das SR, Ayers CR, Rohatgi A. Soluble ST2 is associated with all-cause and cardiovascular mortality in a population-based cohort: the Dallas Heart Study. Clin Chem. 2012;59:536–546. doi: 10.1373/clinchem.2012.191106. [DOI] [PubMed] [Google Scholar]
- 34.Caporali A, Meloni M, Miller AM, Vierlinger K, Cardinali A, Spinetti G, Nailor A, Faglia E, Losa S, Gotti A, et al. Soluble ST2 is regulated by p75 neurotrophin receptor and predicts mortality in diabetic patients with critical limb ischemia. Arterioscler Thromb Vasc Biol. 2012;32:e149–e160. doi: 10.1161/ATVBAHA.112.300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Goral V, Atayan Y, Kaplan A. The relation between pathogenesis of liver cirrhosis, hepatic encephalopathy and serum cytokine levels: what is the role of tumor necrosis factor α? Hepatogastroenterology. 2011;58:943–948. [PubMed] [Google Scholar]
- 37.Zhang X, Chen Y, Liu Y, Zhou X, Fan D. Local cytokines profile in gastric cancer lesions. Zhonghua Zhong Liu Za Zhi. 2002;24:14–16. [PubMed] [Google Scholar]
- 38.Ren Z, Pang G, Clancy R, Li LC, Lee CS, Batey R, Borody T, Dunkley M. Shift of the gastric T-cell response in gastric carcinoma. J Gastroenterol Hepatol. 2001;16:142–148. doi: 10.1046/j.1440-1746.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- 39.Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, Rodeck U. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol. 1999;155:537–547. doi: 10.1016/s0002-9440(10)65149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanazawa M, Yoshihara K, Abe H, Iwadate M, Watanabe K, Suzuki S, Endoh Y, Takita K, Sekikawa K, Takenoshita S, et al. Effects of PSK on T and dendritic cells differentiation in gastric or colorectal cancer patients. Anticancer Res. 2005;25:443–449. [PubMed] [Google Scholar]
- 41.Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, Miyagawa K, Nagura H, Yoshie O, Sasaki I. Selective infiltration of CCR5+CXCR3+ T lymphocytes in human colorectal carcinoma. Int J Cancer. 2005;116:949–956. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 42.Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, Fukuzawa M. Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol. 2002;34:416–420. doi: 10.1097/00004836-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Houghton-Trivino N, Salgado DM, Rodriguez JA, Bosch I, Castellanos JE. Levels of soluble ST2 in serum associated with severity of dengue due to tumour necrosis factor alpha stimulation. J Gen Virol. 2010;91:697–706. doi: 10.1099/vir.0.012971-0. [DOI] [PubMed] [Google Scholar]
- 44.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S, Tzimas MN, Griswold DE, Young PR. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun. 1997;235:474–478. doi: 10.1006/bbrc.1997.6810. [DOI] [PubMed] [Google Scholar]