Abstract
The identification of better regimens in currently available chemotherapeutic agents is crucial for treating patients with KRAS mutant metastatic colorectal cancer (mCRC). Records of mCRC patients who received first-line oxaliplatin-based or irinotecan-based regimens were reviewed retrospectively. Clinicopathologic features and treatment outcome of patients with first-line progression-free survival (PFS) and overall survival (OS) in association with KRAS mutation status were analyzed using the Cox proportional hazard model. Between 2007 and 2010, a total of 118 mCRC patients were enrolled. Among them, 67 were males and 51 were females. In patients who received first-line oxaliplatin-based regimens, the PFS was significantly longer in KRAS mutant patients (N = 32) than that in KRAS wild-type patients (N = 51). The median PFS was 8.5 months in KRAS mutant versus 5.8 months in KRAS wild-type patients (P = .008). In contrast, in patients who received first-line irinotecan-based regimens, the PFS was shorter in KRAS mutant patients (N = 15) than that in KRAS wild-type patients (N = 20). Median PFS was 3.9 months in KRAS mutant versus 6.0 months in KRAS wild-type patients (P = .23). Median OS between KRAS mutant and wild-type patients was not significantly different in both oxaliplatin-based and irinotecan-based regimens. In multivariate analyses, KRAS mutation remains an independent predictive factor for longer PFS in first-line oxaliplatin-based regimens. In conclusion, oxaliplatin-based chemotherapy in KRAS mutant mCRC might result in longer PFS than in KRAS wild-type mCRC.
Introduction
Mutation of the KRAS gene in metastatic colorectal cancer (mCRC) has been identified as a predictor of poor response to epidermal growth factor receptor (EGFR) monoclonal antibody [1]. Prospective randomized clinical trials have further confirmed the observation [2–4]. In patients who received first-line chemotherapy plus EGFR monoclonal antibodies, progression-free survival (PFS) in KRAS mutation group was shorter than that in KRAS wild-type group, with 7.6 versus 9.9 months in the CRYSTAL study [2], 5.5 versus 7.7 months in the OPUS study [3], and 7.3 versus 9.6 months in the PRIME [4] study. On the basis of the results of these studies, mCRC patients with mutant KRAS have no longer been suggested to use EGFR monoclonal antibody. Thereafter, KRAS mutant mCRC patients have fewer treatment options than KRAS wild-type patients. To identify better regimens from currently available systemic treatments or exploration of newer agents for the treatment of KRAS mutant mCRC patients is thus warranted.
If we primarily focus on chemotherapy alone group in the OPUS and PRIME studies, in which first-line oxaliplatin-based chemotherapy (FOLFOX, oxaliplatin/5-fluorouracil (5-FU)/leucovorin) was given in both studies, it is intriguing to find that the PFS in KRAS mutant group was longer than the PFS in KRAS wild-type group, with 8.6 versus 7.2 months in the OPUS study [3] and 8.8 versus 8.0 months in the PRIME study [4]. In contrast, in the CRYSTAL study [2], if we focused on chemotherapy alone group, in which first-line irinotecan-based chemotherapy (FOLFIRI, irinotecan/5-FU/leucovorin) was given, the median PFS was 7.7 months in KRAS mutant group and 8.4 months in the KRAS wild-type group.
According to these observations, we hypothesized that KRAS mutant mCRC patients might have longer PFS during first-line oxaliplatin based chemotherapy than patients with wild-type KRAS. We retrospectively collected clinical data from mCRC patients who had received first-line oxaliplatin-based or irinotecan-based regimens. We also determined the KRAS mutation status of these patients to compare differences in PFS during first-line chemotherapy and overall survival (OS) between the KRAS mutant and wild-type groups for both chemotherapy regimens. Univariate and multivariate analyses were performed in patients with first-line oxaliplatin-based or irinotecan-based regimens to identify potential biomarkers for PFS during first-line chemotherapy and OS.
Materials and Methods
Patient Eligibility
Between 2007 and 2010, lists of patients diagnosed with CRC of any stage were obtained from Medical Information Management Office and the Cancer Registry Office of National Taiwan University Hospital (NTUH). Study subjects were further identified by the following inclusion criteria. Patients were eligible if 1) their diseases had recurred to become metastatic after an initial diagnosis of stage I to III CRC; 2) they were initially diagnosed as stage IV diseases; 3) they were older than 18 years of age; 4) they had received first-line oxaliplatin-based regimens (oxaliplatin/5-FU/leucovorin or oxaliplatin/capecitabine) or irinotecan based regimens (irinotecan/5-FU/leucovorin or irinotecan/capecitabine); 5) they had adequate archival tumor samples for KRAS mutation analysis; 6) they had signed informed consent; 7) they had complete medical chart record and regular computed tomography (CT) scan follow-up reports. Patients were excluded if they 1) had resectable metastatic disease and immediately received complete resection of all tumors after the diagnosis (R0 resection); 2) they had received molecular targeted therapy (bevacizumab or cetuximab) in their first-line treatment; 3) they had multiple cancers; 4) they had active uncontrolled infection; 5) they had human immunodeficiency virus infection; 6) they had poorly controlled heart failure (New York Heart Association class IV). All patients were treated at NTUH, and the data for analysis were locked in December 2011. This study was approved by the Institutional Review Board of NTUH. Detailed information collected for analysis included 1) age at diagnosis, 2) sex, 3) pathology reports, 4) date that patients were diagnosed with CRC of any stage, 5) date of disease recurrence to become stage IV for those patients initially diagnosed as having stage I to III disease, 6) location of primary CRC, 7) sites of metastases, 8) initiation date of first-line oxaliplatin-based or irinotecan-based regimens, 9) date of first-line progression, and 10) date of death.
Diagnosis
Diagnosis of CRC was confirmed by reviewing the morphology of cancer cells and immunohistochemistry (CK20 or CDX2) of pathologic specimens by two independent pathologists. Disease extent was routinely determined by CT of the chest, abdomen, and pelvis as well as bone scans if bone metastasis was suspected. Positron emission tomography/CT scan was performed to identify potentially curable patients or to clarify suspected lesions to determine the clinical stage. The KRAS gene mutation test was performed by professionals at the pathology department in NTUH.
Treatment
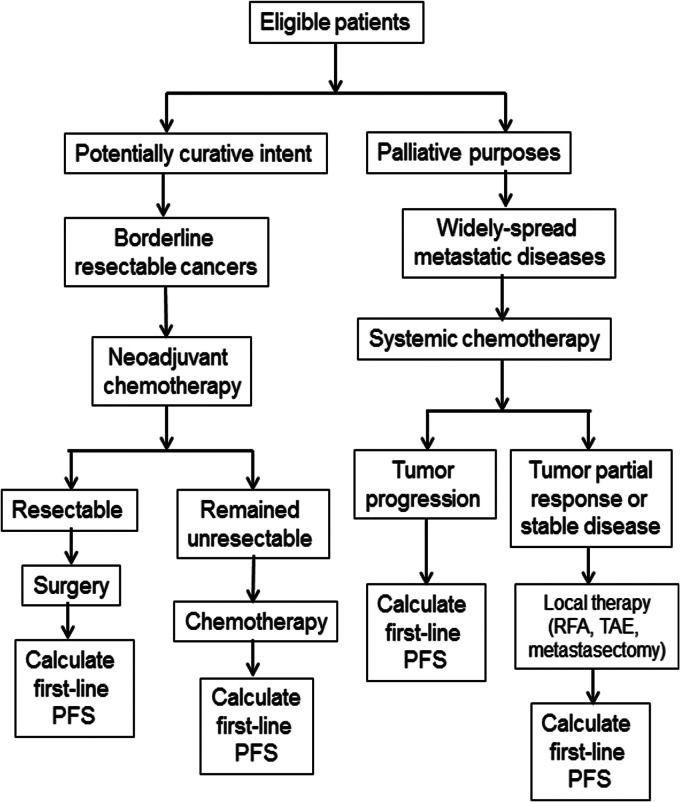
For patients who met all inclusion and exclusion criteria, their treatments were determined mainly by appropriate treatment goals, which were administered either with potentially curative intent or for palliative purposes. Figure 1 summarized all the multidisciplinary treatment that patients had received in the current study.
Figure 1.
Treatment flowchart of all the multidisciplinary treatment options that patients had received.
Patients, in whom the intent was potentially curative, with borderline resectable cancers, were treated with neoadjuvant chemotherapy, followed by surgery if applicable. In subsequent chemotherapy, either for patients whose tumors had been resected or for those whose tumors remained unresectable, the same regimens were continued until the disease progressed or toxicity levels became intolerable.
Patients with widely spread metastatic disease who were treated for palliative purposes received systemic chemotherapy until the disease progressed or became suitable for local therapy with either radiofrequency ablation, transarterial embolization, or metastasectomy. All systemic treatments were determined by treating physicians according to patients' performance status, age, and comorbidities.
Efficacy Assessment
The PFS during first-line chemotherapy and OS were compared between patients with KRAS mutation and those with KRAS wild type for both oxaliplatin-based and irinotecan-based regimens. The PFS during first-line chemotherapy was determined from the first day of first-line chemotherapy to the first-line progression as documented by either CT scan, positron emission tomography/CT scan, bone scan, or death. For those with borderline resectable cancers, whose tumors could be completely resected after neoadjuvant chemotherapy, the PFS during first-line chemotherapy was counted from the first day of chemotherapy to the date of surgery. These patients were recorded as censored at the date of surgery. For the rest of the patients with borderline resectable cancers, whose tumors remained unresectable after neoadjuvant chemotherapy, the PFS during first-line chemotherapy was counted from the first day of chemotherapy to the date of progression during first-line chemotherapy.
For patients with widely spread metastatic diseases, the PFS during first-line chemotherapy was counted from the first day of chemotherapy until the date of first-line chemotherapy progression or until the date of local therapy, including radiofrequency ablation, transarterial embolization, or metastasectomy, during which there was no evidence of progression within the period of first-line chemotherapy treatment. The patients were recorded as censored at the date of local therapy.
OS was calculated from the date diagnosed as stage IV CRC to the date of death or the last visit with censoring. Patients who had not died at the time of analysis were recorded as censored at the time they were last known to be alive. A final analysis of the OS was performed when 65% of the study patients died.
Assessment
Tumor assessments were routinely performed by CT scan of the chest/abdomen/pelvis and by bone scan if bone metastasis was suspected at the time of diagnosis (baseline), as well as every 3 months thereafter if disease status was under control. Once the disease progression was suspected, any diagnostic tools indicated for confirmation of the progression would be performed within the 3-month interval.
Assay to Detect Mutant KRAS
Mutation analysis of KRAS was performed by extraction of genomic DNA from formalin-fixed, paraffin-embedded tissue slides or sections. For KRAS analyses, the following primer sets for exon 2 were used: huKRAS2 ex2F, 5′GAATGGTCCTGCACCAGTAA3′; huKRAS2 ex2R, 5′GTGTGACATGTTCTAATATAGTCA3′. Genomic DNA was amplified using a primer pair; the length of the polymerase chain reaction (PCR) product was 220 bp. The PCR mixture contained 5 µl of 2x HotStarTaq Master Mix containing HotStarTaq DNA Polymerase (Qiagen, Hilden, Germany), PCR buffer, and deoxyribonucleotide triphosphates (dNTPs), 10 pmol of each primer, and 2 µl of genomic DNA (20 ng) in a final volume of 10 µl. Conditions for PCR carried out in the thermal cycler (Biometra TProfessional Basic) were given as follows: 1 cycle at 94°C for 15 minutes; 45 cycles at 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 30 seconds; followed by 1 cycle at 72°C for 7 minutes. Purification of the amplified product was then performed using the Geneaid Gel/PCR DNA Fragments Extraction Kit. The sequence of the purified amplification product (60 ng) was analyzed using an Applied Biosystems 3730 DNA Analyzer.
Statistical Analysis
We hypothesized that the first-line progression-free percentage in KRAS mutant and wild-type patients receiving oxaliplatin-based regimens at 12-month period would be 35% and 10%, respectively. A total of estimated 82 patients receiving oxaliplatin-based regimens would be needed to detect the potential of 25% difference, with a power of 80%, at the significance level of .05 (one-sided test). The Kaplan-Meier method was used to estimate the PFS during first-line chemotherapy and OS. The log-rank test was used for univariate comparisons, and the Cox proportional hazard model was used to identify potential biomarkers for PFS during first-line chemotherapy and OS. A P value < .05 was used to indicate statistical significance; all tests except sample size calculation were two-sided. These analyses were performed using SPSS version 16.0 for Windows.
Results
Patient Population
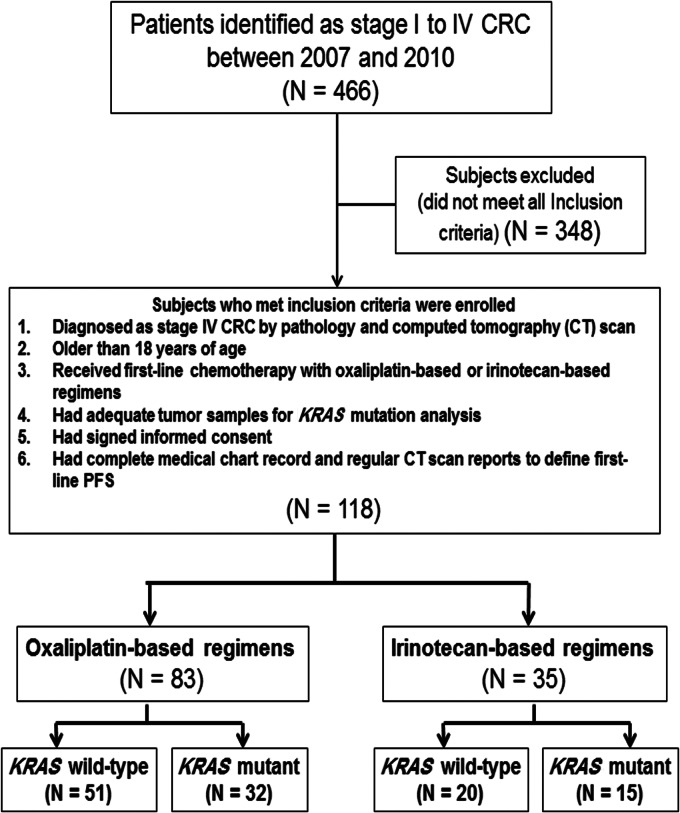
Between 2007 and 2010, 466 patients who received appropriate treatment were identified as stage I to IV CRC. Of these, 118 patients were eligible to analyze in the current study by inclusion and exclusion criteria. The selected patients (N = 118) were further stratified by oxaliplatin-based and irinotecan-based regimens as well as KRAS mutation status. A flowchart of the patients in each group is shown in Figure 2. Among those who received oxaliplatin-based regimens, four patients in KRAS mutant group and four patients in KRAS wild-type group had curative surgery after neoadjuvant chemotherapy. However, among those who received irinotecan-based regimens, none in KRAS mutant group while only one in KRAS wild-type group had curative surgery after neoadjuvant chemotherapy. Table 1 summarizes the baseline patient characteristics.
Figure 2.
Disposition of subjects at the time of data cutoff and analysis.
Table 1.
Patient and Disease Characteristics at Baseline.
| Characteristics | Overall (N = 118) | Oxaliplatin-Based (N = 83) | Irinotecan-Based (N = 35) | |||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Sex | ||||||
| Male | 67 | 57 | 45 | 54 | 22 | 63 |
| Female | 51 | 43 | 38 | 46 | 13 | 37 |
| Age, years | ||||||
| Median | 61 | 60 | 64 | |||
| Range | 15–86 | 15–86 | 25–81 | |||
| >50 | 96 | 81 | 66 | 80 | 30 | 86 |
| ≤50 | 22 | 19 | 17 | 20 | 5 | 14 |
| KRAS | ||||||
| Wild type | 72 | 61 | 52 | 63 | 20 | 57 |
| Mutant | 46 | 39 | 31 | 37 | 15 | 43 |
| Tumor site | ||||||
| Proximal* | 34 | 29 | 27 | 33 | 7 | 20 |
| Distal† | 84 | 71 | 56 | 67 | 28 | 80 |
| No. of metastatic sites | ||||||
| 1 | 67 | 57 | 50 | 60 | 17 | 49 |
| 2 | 41 | 35 | 27 | 33 | 14 | 40 |
| ≥3 | 10 | 8 | 6 | 7 | 4 | 11 |
| Initial stage | ||||||
| I–III | 38 | 32 | 19 | 23 | 19 | 54 |
| IV | 80 | 68 | 64 | 77 | 16 | 46 |
Proximal indicates cecum, ascending colon, hepatic flexure, and transverse colon.
Distal indicates splenic flexure, descending colon, sigmoid colon, and rectum.
Treatment Outcomes
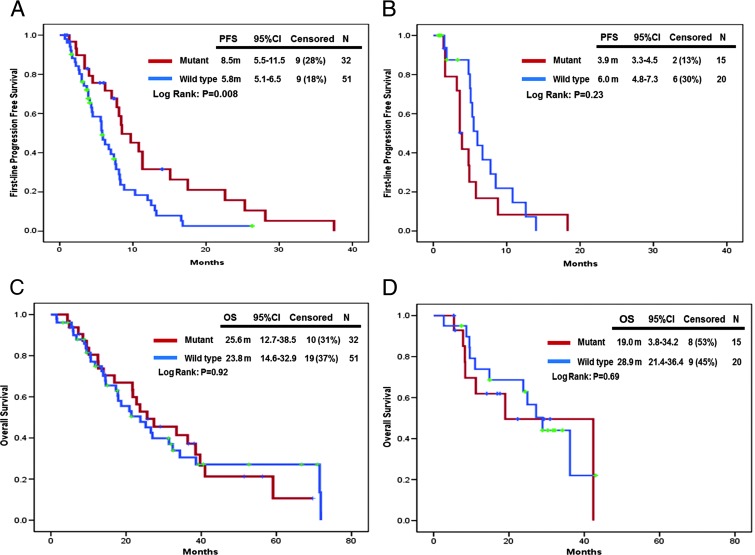
In patients who received first-line oxaliplatin-based regimens, the PFS in KRAS mutant group (N = 32) was significantly longer than that in KRAS wild-type group (N = 51). The PFS was 8.5 months [95% confidence interval (CI): 5.5–11.5] in KRAS mutant group and 5.8 months (95% CI: 5.1–6.5) in KRAS wild-type group (P = .008; Figure 3A); in contrast, for patients who received first-line irinotecan-based regimens, the PFS was 3.9 months (95% CI: 3.3–4.5) in KRAS mutant group (N = 15) and 6.0 months (95% CI: 4.8–7.3) in KRAS wild-type group (N = 20). The difference of PFS between KRAS mutant and wild type was not significantly different (P = .23; Figure 3B).
Figure 3.
(A) First-line PFS stratified by KRAS mutational status in patients with first-line oxaliplatin-based regimens. (B) First-line PFS stratified by KRAS mutational status in patients with first-line irinotecan-based regimens. (C) OS stratified by KRAS mutational status in patients with first-line oxaliplatin-based regimens. (D) OS stratified by KRAS mutational status in patients with first-line irinotecan-based regimens.
Comparison of the KRAS mutation and wild-type groups with respect to the outcome of OS revealed that the difference of OS in neither oxaliplatin-based nor irinotecan-based regimens was statistically significantly different between the groups. In patients who received first-line oxaliplatin-based regimens, the median OS was 25.6 months (95% CI: 12.7–38.5) in KRAS mutation group (N = 32) and 23.8 months (95% CI: 14.6–32.9) in KRAS wild-type group (N = 51, P = .92; Figure 3C). In addition, in patients who received first-line irinotecan-based regimens, OS was 19.0 months (95% CI: 3.8–34.2) in KRAS mutant group (N = 15) and 28.9 months (95% CI: 21.4–36.4) in KRAS wild-type (N = 20) group (P = .69; Figure 3D).
Furthermore, when we excluded patients who received curative surgery after neoadjuvant chemotherapy for OS analysis, we found that the difference of OS between KRAS mutant and wild-type groups in either oxaliplatin-based or irinotecan-based regimens remained not statistically significant. For patients who received oxaliplatin-based regimens, OS was 22.8 (95% CI: 18.2–27.4) months in KRAS mutant and 20.9 (95% CI: 14.1–27.7) months in KRAS wild-type patients (P = .9). For patients who received irinotecan-based regimens, OS was 19.0 (95% CI: 3.8–34.2) months in KRAS mutant patients and 27.2 (95% CI: 21.1–33.3) months in KRAS wild-type patients (P = .79).
Univariate and Multivariate Analyses
The Cox proportional hazard model was further used to test other potential confounding factors that might influence PFS during first-line chemotherapy or OS in patients with oxaliplatin-based or irinotecan-based regimens. The factors included were age (≤50 vs >50), sex (female vs male), initial stage of the disease (stages I to III vs stage IV), location of the tumor (distal vs proximal), and number of metastases (1 vs 2 vs ≥3). In summary, in patients who received first-line oxaliplatin-based regimens, the KRAS mutation was an independent predictive factor for longer PFS, which was demonstrated not only in univariate but also in multivariate analyses. Female gender and metastases in more than two sites were, in contrast, independent predictive and prognostic factors for shorter PFS and OS, respectively (Tables 2 and 3). In patients who received first-line irinotecan-based regimens, no factors were found to be independently predictive or prognostic for PFS during first-line chemotherapy or OS (Tables 2 and 3).
Table 2.
Cox Proportional Hazard Model for PFS in Patients Treated with Irinotecan-Based and Oxaliplatin-Based Regimens.
| Variable | Irinotecan-Based Regimens (N = 35) | Oxaliplatin-Based Regimens (N = 83) | ||||||||||
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR* | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.00 | .74 | .86 | .48 | ||||||||
| ≤50 | 1.00 | 1.00 | 1.00 | 0.51–1.75 | 1.00 | |||||||
| >50 | 1.00 | 0.37–2.74 | 1.22 | 0.38–3.97 | 0.95 | 0.78 | 0.39–1.55 | |||||
| Sex | .36 | .45 | .13 | .01 | ||||||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Male | 0.69 | 0.31–1.53 | 0.66 | 0.22–1.96 | 0.68 | 0.42–1.12 | 0.46 | 0.26–0.82 | ||||
| KRAS | .22 | .32 | .008 | .003 | ||||||||
| Mutant | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Wild type | 0.61 | 0.28–1.34 | 0.61 | 0.23–1.60 | 2.09 | 1.21–3.62 | 2.46 | 1.37–4.43 | ||||
| Initial diagnosis as stage IV | .30 | .44 | .23 | .13 | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 0.65 | 0.29–1.46 | 0.67 | 0.25–1.83 | 1.44 | 0.79–2.62 | 1.68 | 0.85–3.31 | ||||
| Tumor site | .72 | .92 | .94 | .33 | ||||||||
| Distal | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Proximal | 1.19 | 0.46–3.07 | 0.95 | 0.33–2.72 | 0.98 | 0.58–1.65 | 0.71 | 0.36–1.41 | ||||
| No. of metastatic sites | ||||||||||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 0.83 | 0.35–1.93 | .66 | 0.87 | 0.25–3.05 | .83 | 1.28 | 0.76–2.17 | .36 | 1.45 | 0.81–2.59 | .21 |
| ≥3 | 1.88 | 0.40–8.91 | .43 | 1.97 | 0.39–10.00 | .41 | 4.28 | 1.61–11.37 | .004 | 3.87 | 1.28–11.68 | .02 |
HR, hazard ratio.
Table 3.
Cox Proportional Hazard Model for OS in Patients Treated by Irinotecan-Based and Oxaliplatin-Based Regimens.
| Variable | Irinotecan-Based Regimens (N = 35) | Oxaliplatin-Based Regimens (N = 83) | ||||||||||
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR* | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | .91 | .91 | .21 | .83 | ||||||||
| ≤50 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| >50 | 0.92 | 0.21–4.06 | 0.91 | 0.17–4.78 | 0.66 | 0.35–1.27 | 0.92 | 0.43–1.98 | ||||
| Sex | .47 | .43 | .04 | .01 | ||||||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Male | 1.44 | 0.53–3.87 | 1.6 | 0.49–5.19 | 0.56 | 0.32–0.98 | 0.42 | 0.22–0.78 | ||||
| KRAS | .69 | .71 | .94 | .90 | ||||||||
| Mutant | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Wild type | 0.82 | 0.32–2.15 | 0.83 | 0.3–2.26 | 0.98 | 0.56–1.70 | 1.04 | 0.56–1.94 | ||||
| Initial diagnosis as stage IV | .49 | .45 | .14 | .16 | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 1.41 | 0.53–3.80 | 1.52 | 0.51–4.56 | 1.69 | 0.85–3.39 | 1.77 | 0.81–3.87 | ||||
| Tumor site | .66 | .87 | .45 | .40 | ||||||||
| Distal | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Proximal | 0.75 | 0.21–2.64 | 0.88 | 0.19–4.01 | 1.26 | 0.70–2.29 | 0.74 | 0.37–1.49 | ||||
| No. of metastatic sites | ||||||||||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 0.89 | 0.31–2.60 | .83 | 0.70 | 0.22–2.26 | .55 | 2.02 | 1.13–3.6 | .02 | 2.03 | 1.12–3.69 | .02 |
| ≥3 | 2.00 | 0.53–7.60 | .31 | 1.98 | 0.48–8.14 | .34 | 9.82 | 3.4–28.38 | <.001 | 8.22 | 2.45–27.6 | .001 |
HR, hazard ratio.
Discussion
Our study reveals that KRAS mutant mCRC patients might have longer PFS during first-line oxaliplatin-based chemotherapy than KRAS wild-type patients. This finding might be an important step toward personalized chemotherapy for mCRC.
In the era before targeted therapy, Tournigand et al. [5] published a pivotal article reporting that first-line chemotherapy with either irinotecan/5-FU/lecovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (FOLFOX6) in “nonselected” mCRC patients did not influence OS. Both regimens could thus be recommended as first-line treatment for mCRC. In the modern era of targeted therapy, current treatment for mCRC has been advanced to personalized therapy after mutant KRAS was identified as a predictor for unresponsiveness to EGFR monoclonal antibody. Currently, KRAS mutation status has been routinely checked in daily oncology practice to avoid inappropriate use of EGFR monoclonal antibody in KRAS mutant mCRC patients. As KRAS mutant mCRC patients has fewer treatments currently, to identify better regimens in current chemotherapy or newer treatment targets for the approximately 40% of KRAS mutant mCRC patients [6–9] is warranted.
Our study was initiated to validate better regimens in current chemotherapy. The hypothesis that KRAS mutant mCRC patients might have longer PFS during first-line oxaliplatin-based chemotherapy than KRAS wild-type patients was generated from subgroup analyses of randomized prospective clinical trials, PRIME [4] and OPUS [3] versus CRYSTAL [2]. Although our sample size is limited, our study demonstrated again that KRAS mutant mCRC patients might have longer PFS during first-line oxaliplatin-based chemotherapy than KRAS wild-type patients. Our finding is compatible to that in the subgroup analyses of the prospective, randomized PRIME and OPUS studies. Although this observation may warrant further confirmation by large prospective clinical trials, however, after entering the targeted therapy era, it is hard to conduct this kind of study without incorporating targeted therapy, bevacizumab or cetuximab, into the first-line setting. Our patient cohort was treated during 2007 and 2010. During that period of time, targeted agents, bevacizumab and cetuximab, had not been reimbursed in the first-line setting by the National Health Insurance System in Taiwan. Thus, this is a highly valuable data set to evaluate the impact of KRAS status on solely first-line chemotherapy without adding targeted agents. For this reason, it further pointed out the value of the current study toward personalized chemotherapy.
To further support our hypothesis that KRAS mutant mCRC patients might benefit more from oxaliplatin-based regimens, we recently completed an in vitro study [10] showing that KRAS mutation is a predictor of oxaliplatin sensitivity in colon cancer cells. In our in vitro study, KRAS gene was knocked down in KRAS mutant CRC cells (DLD-1G13D and SW480G12V) by small interfering RNA and overexpressed in KRAS wild-type CRC cells (COLO320DM) by KRAS mutant vectors to generate paired CRC cells for the experiments. We clearly demonstrated that KRAS mutant CRC cells are more sensitive to oxaliplatin than KRAS wild-type CRC cells by the mechanism of excision repair cross-complementation group 1 (ERCC1) downregulation. This in vitro finding further strengthened our results in the current study.
Personalized chemotherapy is important, if achievable, although we currently are in the era of molecular targeted therapy. Chemotherapy remains the backbone of the treatment for all mCRC patients. Every cancer patient may benefit from personalized chemotherapy if we can find robust and meaningful biomarkers. Our study demonstrated that determining the mutation status of the KRAS gene is not only essential for selecting the treatment of EGFR monoclonal antibody appropriately but also for personalizing current chemotherapeutic agents for patients with mCRC. Several biomarkers have been identified for the use in determining the efficacy of chemotherapy for colon cancers, including ERCC1 expression to predict resistance to oxaliplatin [11] and thymidylate synthase expression to determine the sensitivity of 5-FU [12]. However, tests for most of these biomarkers are not checked as extensively and routinely as the KRAS mutational status due to differences in the reliability of test results. ERCC1 and thymidylate synthase expression are determined using immunohistochemistry, the results of which are subjected to subjectively semi-quantitative interpretation. In contrast, the KRAS mutation status is determined by direct sequencing of tumor DNA, which yields more accurate results. KRAS mutant mCRC patients might have longer PFS during first-line oxaliplatin-based regimens than KRAS wild-type patients. Although the detailed mechanism underlying this observation remains elusive, cross talks between the mutated KRAS gene and DNA repair machinery pathways, which might be responsible for the effect of oxaliplatin-based treatment, have been investigated [13,14]. This novel finding might result in a potential paradigm shift in the current standard treatment for KRAS mutant mCRC.
Multivariate analysis in the present study revealed that female gender and metastases in more than two sites were independent predictive and prognostic factors for shorter PFS and OS in patients undergoing first-line oxaliplatin-based therapy. The fact that patients with more than two metastatic sites predicted worse outcomes may demonstrate the quality and accuracy of the data, but the reason that female gender is a biomarker of poorer treatment outcome remains elusive. Confirmation of the associations between gender and the KRAS mutation by further studies may be warranted.
Our study did have limitations. First, it was conducted retrospectively with a relatively small sample size. A further larger, prospective study is warranted to confirm our results. However, as we mentioned earlier, it is hard to conduct this kind of study without incorporating targeted therapy, bevacizumab or cetuximab, into the first-line setting, especially when bevacizumab has been reimbursed by the National Health Insurance System in Taiwan since June 2011. Second, although selection bias is hardly avoided in a retrospective study just like ours, we do not think there were major impacts of patient selection on OS in our study, because OS between first-line oxaliplatin-based and irinotecan based groups was not significantly different (P = .91) in our study, which was compatible to those reported by Tournigand et al. [5]. Third, our study was not powered to determine whether different KRAS mutant subtypes would give the same results as the current study. Different KRAS mutant subtypes may have different biologic behaviors with different treatment outcomes, as reported in several studies [15,16]. This question also warrants further clinical study with more patients to answer.
In conclusion, our data suggest that oxaliplatin-based chemotherapy might provide longer PFS in KRAS mutant mCRC than in KRAS wild-type mCRC.
Acknowledgments
The authors acknowledged the statistical assistance provided by the National Translational Medicine and Clinical Trial Resource Center (which is founded by the National Research Program for Biopharmaceuticals at the National Science Council of Taiwan; NSC101-2325-B-002-078) and the Department of Medical Research, National Taiwan University Hospital.
Footnotes
This study was supported by grants from the Department of Health, Executive Yuan, Taipei, Taiwan (DOH100-TD-C-111-001).
References
- 1.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, et al. Kras mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 3.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 5.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 6.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, Stephens P, Edkins S, Tsui WW, Chan AS, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451–6455. [PubMed] [Google Scholar]
- 7.Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 8.Boughdady IS, Kinsella AR, Haboubi NY, Schofield PF. K-ras gene mutations in adenomas and carcinomas of the colon. Surg Oncol. 1992;1:275–282. doi: 10.1016/0960-7404(92)90088-3. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein SD, Sayegh R, Christensen S, Swalsky PA. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlateswith k-ras-2 mutation type. Cancer. 1993;71:3827–3838. doi: 10.1002/1097-0142(19930615)71:12<3827::aid-cncr2820711207>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Lin YL, Liau JY, Yu SC, Ou DL, Lin LI, Tseng LH, Chang YL, Yeh KH, Cheng AL. KRAS mutation is a predictor of oxaliplatin sensitivity in colon cancer cells. PLoS One. 2012;7(11):e50701. doi: 10.1371/journal.pone.0050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994;94:703–708. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Triest B, Pinedo HM, van Hensbergen Y, Smid K, Telleman F, Schoenmakers PS, van der Wilt CL, van Laar JA, Noordhuis P, Jansen G, et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin Cancer Res. 1999;5:643–654. [PubMed] [Google Scholar]
- 13.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 14.Squatrito M, Holland EC. DNA damage response and growth factor signaling pathways in gliomagenesis and therapeutic resistance. Cancer Res. 2011;71:5945–5949. doi: 10.1158/0008-5472.CAN-11-1245. [DOI] [PubMed] [Google Scholar]
- 15.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer. 2001;85:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, et al. Association of kras p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]