Abstract
AIM: To evaluate the long-term natural history of the gastroduodenal lesions secondary to extrahepatic embolization with Ytrium 90 (90Y) spheres.
METHODS: From September 2003 to January 2012, 379 procedures of liver radioembolization (RE) using resin microspheres loaded with 90Y were performed in our center. We have retrospectively compiled the data from 379 RE procedures performed in our center. We report a comprehensive clinical, analytical, endoscopic and histologic long-term follow-up of a series of patients who developed gastroduodenal lesions after the treatment.
RESULTS: Six patients (1.5%) developed gastrointestinal symptoms and had gastrointestinal lesions as shown by upper endoscopy in the next 12 wk after RE. The mean time between RE and the appearance of symptoms was 5 wk. Only one patient required endoscopic and surgical treatment. The incidence of gastrointestinal ulcerations was 3.75% (3/80) when only planar images were used for the pre-treatment evaluation. It was reduced to 1% (3/299) when single-photon emission computed tomography (SPECT) images were also performed. The symptoms that lasted for a longer time were nausea and vomiting, until 25 mo after the treatment.
CONCLUSION: All patients were free from severe symptoms at the end of follow-up. The routine use of SPECT has decreased the incidence of gastrointestinal lesions due to unintended deployment of 90Y particles.
Keywords: Radioembolization, Liver neoplasms, Gastroduodenal ulcer, Single-photon emission computed tomography, Liver
INTRODUCTION
Radioembolization (RE) with microspheres embedded with Yttrium 90 (90Y) is a therapeutic option for primary and secondary liver tumors. Prior to treatment, an angiography is performed to identify the hepatic vasculature and to mimic the actual procedure by injecting 99Tc-radiolabeled macroaggregated albumin (99Tcm-MAA)[1]. During this procedure, guidelines recommend the embolization of the gastroduodenal artery, right gastric, and other extrahepatic arteries to isolate the hepatic circulation[2]. Unintended extrahepatic deployment of particles is a rare but well known complication of this procedure, that may result in cholescystitis[3], pancreatitis[4], radiation pneumonitis[5] and gastrointestinal ulcerations[6,7]. Radiation injury is likely to be the main pathogenical factor of these lesions[6]. However, although the small diameter of the particles (20-30 μm) does not induce a significant macroembolic effect, it has been shown in animal models that microspheres may sometimes aggregate and occlude small size vessels[8].
Radiation-induced gastrointestinal ulcerations may have a significant negative impact in quality of life due to decreased oral intake, pain or anemia, and the even need to perform gastric surgery has been described[9]. However, most case reports or series only describe the appearance and immediate consequences and very little is known about the long-term outcome of this complication. We describe for the first time a case series of six patients with gastrointestinal lesions secondary to RE and their clinical, endoscopic and histologic long-term follow-up.
MATERIALS AND METHODS
From September 2003 to January 2012, 379 procedures of liver RE using resin microspheres loaded with 90Y (SIR-Spheres, Sirtex Medical, Sidney, Australia) were performed in our center. Six patients (1.5%) developed gastrointestinal symptoms and had an upper endoscopy performed in the next 12 wk after the procedure that showed gastrointestinal lesions. Findings in the initial and the subsequent gastroduodenoscopies and their histopathologic studies were compiled retrospectively. Although the follow-up was not structured, at least two endoscopies were available from all but one patient. The maximum endoscopic follow-up time was 78 mo. We have gathered from the medical reports and thoroughly analyzed all clinical, analytical, endoscopic and histopathological findings.
RESULTS
Patient characteristics
The characteristics of the six patients included in the study are shown in Table 1. Mean age was 53.1 years and a range of primary tumors were treated including cholangiocarcinoma and liver metastases from different primary tumors. None of the patients had any significant gastrointestinal disease before RE and only two patients were receiving concomitant anticancer treatments, namely the combination of 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX, patient 1), and the combination of gemcitabine and oxaliplatin (GEMOX, patient 4).
Table 1.
Patient and treatment characteristics
| Patient | Age (yr) | Primary tumor | Concomitant treatment | Gastroduodenal artery embolization | Site of injection | Gastrointestinal activity on 99Tcm-MAA scan | Dose administrated | Pain during infusion |
| 1 | 60 | Colorectal | FOLFOX | Yes | Common hepatic artery | No | 75% | Yes |
| 2 | 39 | Pancreatic NET | No | No | Left hepatic artery | No | 100% | No |
| 3 | 53 | Ileal carcinoid | No | Yes | Proper hepatic artery | No | 100% | Yes |
| 4 | 65 | Cholangiocarcinoma | GEMOX | No | Right and left hepatic artery | No | 75% | Yes |
| 5 | 54 | Renal | No | Yes | Proper hepatic artery | No | 100% | Yes |
| 6 | 48 | Colorectal | No | Yes | Proper hepatic artery | No | 60% | Yes |
99Tcm-MAA scan: 99Tc-radiolabeled macroaggregated albumin; FOLFOX: Folinic acid, fluorouracil and oxaliplatin; GEMOX: Gemcitabine and oxaliplatine; NET: Neuroendocrine tumour.
RE procedure
From the day of RE and for the following 2 mo, all patients received pantoprazole (40 mg daily) and a tapered regimen of corticosteroids.
A selective embolization of any extrahepatic vessel that could be included in the treated volume was performed prior to microsphere injection. Regarding variant anatomies, patient 2 presented a complete occlusion of the celiac axis and reversed blood flow of the pancreatoduodenal artery and in patient 4 the right hepatic artery originated from the superior mesenteric artery. The mean activity of 90Y administered was 1.02 GBq. In 4 patients (66%) the spheres were infused in the common or proper hepatic artery. In one case a bilobar treatment was performed by injecting the microspheres into the left and right branches of the hepatic artery (patient 4). In this patient only half the prescribed dose could be injected into the left hepatic artery due to spasm, while the entire prescribed dose was injected into the right hepatic artery uneventfully. This was the only case in which premature occlusion of the vessel occurred. In the remaining case the site of injection was the left hepatic artery (lobar infusion, patient 2).
99Tcm-MAA scans obtained prior to the procedures consisted in planar scintigraphic images in 3 patients and planar plus single-photon emission computed tomography (SPECT) images in the 3 other patients. When considered relative to the number of RE procedures, the incidence of gastrointestinal ulcerations was 3.75% (3 out of 80 patients) in the early period when planar images were used, and only 1% (3 out of 299 patients) in the late period when SPECT was also performed. We have retrospectively reviewed the 99Tcm-MAA scans of these 6 patients and no activity was observed in the gastrointestinal area prior to RE.
Endoscopic and histological findings
Patients had their first upper endoscopy performed 1 to 12 wk after RE (Table 2) but none of them required an urgent procedure. At diagnosis, all of them showed ulcers and erosions in the stomach and/or duodenum (Figure 1). Gastrointestinal lesions were found only in the stomach in 3 cases, while the duodenum and the pyloric channel were also affected in 1 and 2 patients, respectively. Mucositis (gastritis or duodenitis) was present in 5 patients (83%) and a friable mucosa was described in 2 patients (33%). The mean endoscopic follow-up was 29.6 mo and the end of follow-up was usually due to clinical improvement (5 patients, 83%). One patient (case 6) died 2.5 mo after the procedure due to tumor progression. No patient showed neoangiogenesis, scars or antral deformities at diagnosis but these findings were lately seen in 4 patients (67%) after a mean period of 7.5 mo.
Table 2.
Endoscopic and histologic findings
| Patient | Time from RE to first endoscopy (wk) | Findings in first endoscopy (CTCAE 4.02 grading scale) | Histology | Endoscopic follow-up time (mo) | Total number of endoscopies | Endoscopic treatment |
| 1 | 6 | Ulcers in cisura angularis and gastric antrum (20 mm); Multiple erosions in duodenal bulb (2) | Microspheres in lamina propia;No H. pylori bacilli | No | 1 | No |
| 2 | 5 | Severe mucositis in gastric fundus, body and antrum, with mucosal friability and superficial ulcers (2) | Microspheres; No H. pylori bacilli | 15 | 3 | No |
| 3 | 8 | Severe gastritis (cisura angularis, antrum and pylorus) and duodenitis with ulcers (3) | Microspheres; No H. pylori bacilli | 53 | 8 | Argon plasma coagulation (after 13 mo of RE)1 |
| 4 | 7 | Mucositis in gastric body; Extense ulcer in antrum (2) | Microspheres; No H. pylori bacilli | 1 | 2 | No |
| 5 | 12 | Deep ulcer in pyloric channel and severe mucositis in gastric antrum; Superficial ulcer in duodenal bulb (2) | Microspheres in lamina propia; No H. pylori bacilli | 78 | 11 | No |
| 6 | 4 | Severe mucositis in gastric antrum, with mucosal friability;Ulcer in pyloric cannel (2) | Microspheres in lamina propia; No H. pylori bacilli | 1 | 2 | No |
A gastroenteroanastomosis was later performed (25 mo after the diagnosis of gastrointestinal ulcers). RE: Radioembolization; CTCAE: Common Terminology Criteria for Adverse Events.
Figure 1.
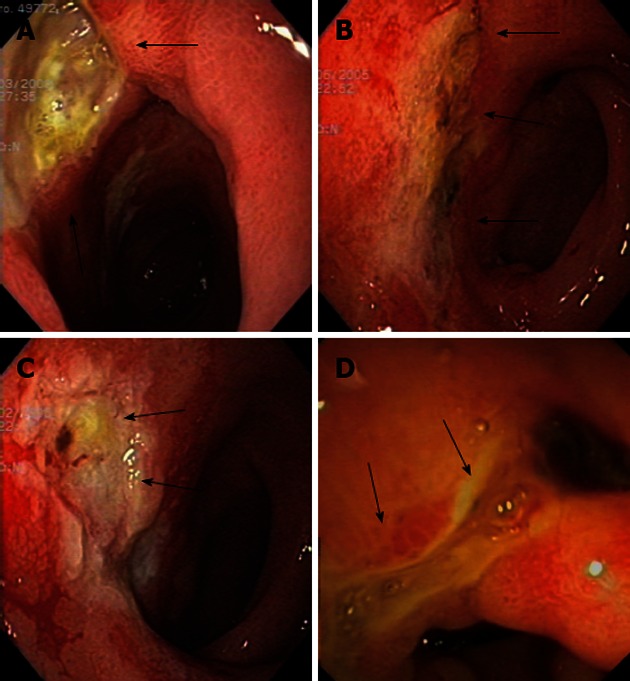
Figures of patient 5. A: A big and deep ulcer was seen in the pyloric channel and duodenal bulb at diagnosis (arrows); B: The same ulcer as in (A) in the same location seen 3 mo later, partially healed (arrows); C: Ulcer located in the duodenal bulb (arrows) with an irregular and friable mucosa after 11 mo; D: Endoscopical view of antral, pyloric and duodenal bulb deformity (arrows) seen with endoscopic ultrasound scope 78 mo after diagnosis.
Microspheres were detected in all the biopsy specimens, mainly located in the vessels of the lamina propria. Granulation tissue was frequently present next to the ulcers, along with a variable grade of linfoplasmocytic and eosinophilic inflammatory infiltrate. In some samples, focal atypia (patient 1) or anaplasia (patient 3) were present. None of the samples from the gastric mucosa showed bacilli suggestive of Helicobacter pylori (H. pylori) infection and none of the patients had a previous documented H. pylori infection.
Treatment
Only one patient (patient 3) required endoscopic treatment because of late anemia and duodenal stenosis. Thirteen mo after RE, argon plasma coagulation was successfully used to treat the antral and duodenal mucosa. He required this endoscopic treatment because of significant and progressive anemia (hemoglobin dropped from 13.7 g/dL at baseline to 8 g/dL prior to endoscopic treatment and the patient had already received transfusion of 6 units of packed red blood cells) and weight loss. Anemia resolved but his nutritional status worsened over the next mo due to a duodenal stenosis. Finally a gastroenteroanastomosis to the first jejunal loop was performed 25 mo after the diagnosis of gastrointestinal ulcers with antropyloric deformity.
All patients referred abdominal pain as the initial symptom, mainly located in the epigastric region (Table 3). Other symptoms were nausea and vomiting (5 patients, 83%) and anorexia (3 patients, 50%). The mean time between RE and the appearance of symptoms was 5 wk (range 2 to 12 wk). Patients were treated with multiple combinations of proton pump inhibitors, Sucralfate, Almagate, Domperidone, Misoprostole, Pentoxifylline, Cinitapride and Metoclopramide. A slow but progressive improvement was seen in all but one of the patients described above (83%). The majority of these symptoms were mild and graded 1 or 2 in the Common Terminology Criteria for Adverse Events grading scale. Abdominal pain had a maximum grade of 1 or 2, which means a mild pain that only in some patients limited activities of daily living. Nausea and vomiting was graded with a maximum of 1 in all but one patient. This correlates with one or two episodes of emesis a day in the worst period with symptoms during the follow-up.
Table 3.
Clinical follow-up and treatment
| Patient | Time from RE to symptoms (wk) | Clinical follow-up time (mo) |
Symptoms (CTCAE v4.02 grading scale) |
Treatment |
Weight loss1 (kg) | Hemoglobin2 (g/dL) | Reason for end of endoscopic follow up | ||||
| Pain | Nausea and vomiting | Anorexia | Treatment used | Time on treatment (mo) | Clinical response | ||||||
| 1 | 4 | 6 | Epigastric pain (1) | No | No | Pantoprazole, domperidone and almagate | 4 | Yes | 6 | -1.5 | Improvement |
| 2 | 4 | 29 | Epigastric pain (1) | Yes (1) | No | Esomeprazole, cinitapride sucralfate and ranitidine | 8 | Yes | 7 | -0.6 | Improvement |
| 3 | 4 | 88 | Epigastric pain (2) | Yes (1) | Yes (3) | Pantoprazole, metoclopramide, sucralfate and cinitapride | 10 | Yes | 17 | -6.9 | Improvement |
| 4 | 2 | 9 | Left subcostal pain (2) | Yes (1) | No | Pantoprazole, sucralfate and almagate | 5 | Yes | 4 | -3.1 | Improvement |
| 5 | 12 | 88 | Epigastric pain (1) | Yes (1) | Yes (2) | Pantoprazole, pentoxifylline, esomeprazole and almagate | 5 | Yes | 4 | -1.6 | Improvement; still on follow-up |
| 6 | 4 | 3 | Epigastric pain (2) | Yes (2) | Yes (2) | Esomeprazole, pentoxifylline and misoprostole | 1 | No | 4.1 | -2.4 | Exitus |
Maximal loss of weight over 4 mo;
Maximal change in hemoglobin over 4 mo. CTCAE: Common Terminology Criteria for Adverse Events; RE: Radioembolization.
DISCUSSION
RE is an accepted therapeutic technique for advanced primary and secondary liver tumors. It seems to be better tolerated than transarterial chemoembolization in terms of abdominal pain[10], length of hospital stay[11] and post-embolization symptoms[12]. Main complications do not result from the microembolic effect of the spheres, even in patients with portal vein occlusion, but rather from an excessive irradiation of non-target tissues, including the liver[13].
We describe in this case series an incidence of symptomatic gastroduodenal lesions after RE of 1.5% (6 out of 379 patients). Other authors have reported an incidence between 0% and 28%, with an average 4.8% calculated from the data previously published in 23 studies[7]. The two largest series of patients with secondary liver tumors from colorectal cancer or other malignancies reported rates of grade 3 gastrointestinal ulceration of 1% to 2%[14,15]. A higher incidence of 3.7% was found among 325 patients with hepatocellular carcinoma treated in 8 different European institutions[16].
A decrease in the incidence of gastroduodenal lesions was observed when SPECT images were used for evaluation the RE procedures compared with planar images. These data suggest that SPECT imaging is an important tool to minimize the risk of gastrointestinal adverse events secondary to RE treatment. Our results arise from a retrospective single center study. If they are further confirmed by other series, a possible change in the current guidelines could be considered in favor of the use of SPECT imaging.
In most patients (4 out of 6 patients, 66%) the spheres were injected from the common or proper hepatic artery. This site of injection leads to a greater chance of non-target embolization of the spheres in extrahepatic vessels. However, we have retrospectively reviewed all the angiographies and none of them showed collaterals or had extrahepatic deposits of MAA in the subsequent scans. In one of the patients treated by bilobar injection, a spasm in the left hepatic artery could have contributed to this complication.
In our case series, two patients were receiving combined concomitant treatment with the combination of oxaliplatin and 5-fluorouracil (FOLFOX) or gemcitabine (GEMOX). None of these drugs produce gastrointestinal lesions that could act as a confounding factor. In a literature search, we have found only one case of gastroduodenal ulceration with FOLFOX therapy and the patient was concurrently treated with Bevacizumab[17]. Saif et al[18] described a case of a patient treated with gemcitabine and warfarine who presented with gastrointestinal haemorrhage secondary to gastric and duodenal ulcers (with an international normalized ratio of 8.0). The lesions in our series are not likely to have been caused by these drugs, but we can not exclude a certain contribution.
The most common presenting symptoms in our series were abdominal pain, nausea/vomiting and anorexia. The intensity was usually mild and did not limit daily activities. Many drugs have been used, alone or in combination, demonstrating a slow but effective relieve of symptoms of this complication. However, more studies are needed to determine the best medical treatment. Only one patient required elective surgical treatment due to a late (> 1 year) duodenal stenosis. No urgent endoscopic or surgical treatment was performed. All but one patient are free of symptoms at the end of follow-up. All these data suggest that unintended gastrointestinal deployment of particles should be considered as a potential but transient cause of abdominal pain after the procedure. As shown in our series, the largest so far reported, a conservative approach must be considered as the primary treatment option. Nevertheless, the long-standing duration of symptoms (maximum 25 mo) in association with other factors (anemia, tumor progression and other comorbidities) certainly impacts the quality of life of affected patients. A common approach between oncologists, hepatologists and endoscopists would help improving the management and prognosis of the RE procedure and its complications.
COMMENTS
Background
Primary and secondary liver neoplasms are an important cause of morbidity and mortality worldwide. Radioembolization is a treatment indicated in those patients not eligible for curative resection or liver transplantation but still have their disease confined to the liver. It is considered a liver-directed therapy in which implanted radioactive microspheres are delivered into the arteries that feed the tumors. Secondary to this procedure some of the spheres can get into the arteries that feed some extrahepatic territories as the gastrointestinal tract.
Research frontiers
As the unintended extrahepatic deployment of Ytrium 90 spheres is a rare complication of radioembolization little is know about its management and long-term prognosis. The authors have reviewed the clinical, endoscopic, analytical and histologic long-term follow-up of patients treated with this liver-directed therapy. They also describe how the introduction of new image techniques has decreased the incidence of this complication.
Innovations and breakthroughs
In this retrospective single-center study, the authors have found an incidence of 1.5% of gastrointestinal lesions secondary to the inadvertent deployment of radioactive microspheres. The incidence of this complication decreased from 3.75% to 1% when single photon emission computed tomography images where included in the routine planification of the treatment. The most frequent symptoms referred at diagnosis where abdominal pain and nausea and vomiting.
Applications
The authors have shown the good long-term prognosis of this rare complication. It should be considered as a potential but transient cause of abdominal pain after the procedure. As it may be associated with long-standing symptoms the authors should take into account this complication to increase the patients’ quality of life. It seems like the inclusion of new image techniques have decreased the incidence of this complication, but more research is needed to confirm these promising results.
Terminology
Radioembolization: Treatment procedure in which radioactive microspheres loaded with Ytrium 90 are delivered into the hepatic artery.
Peer review
This paper is well written and adds to understanding of outcomes of patients with ulcers following radioembolization.
Footnotes
P- Reviewers Çil BE, Nosher J S- Editor Jiang L L- Editor A E- Editor Li JY
References
- 1.Bilbao JI, Reiser MF. Liver radioembolization with 90Y microspheres. Berlin: Springer Verlag; 2008. [Google Scholar]
- 2.Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol. 2012;35:91–99. doi: 10.1097/coc.0b013e3181f47583. [DOI] [PubMed] [Google Scholar]
- 3.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann RT, Jakobs TF, Kubisch CH, Stemmler HJ, Trumm C, Tatsch K, Helmberger TK, Reiser MF. Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease-Is it feasible? Eur J Radiol. 2010;74:199–205. doi: 10.1016/j.ejrad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, Metreweli C, Johnson PJ, Li AK. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995;33:919–924. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 6.Carretero C, Munoz-Navas M, Betes M, Angos R, Subtil JC, Fernandez-Urien I, De la Riva S, Sola J, Bilbao JI, de Luis E, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol. 2007;102:1216–1220. doi: 10.1111/j.1572-0241.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 7.Naymagon S, Warner RR, Patel K, Harpaz N, Machac J, Weintraub JL, Kim MK. Gastroduodenal ulceration associated with radioembolization for the treatment of hepatic tumors: an institutional experience and review of the literature. Dig Dis Sci. 2010;55:2450–2458. doi: 10.1007/s10620-010-1156-y. [DOI] [PubMed] [Google Scholar]
- 8.Bilbao JI, de Martino A, de Luis E, Díaz-Dorronsoro L, Alonso-Burgos A, Martínez de la Cuesta A, Sangro B, García de Jalón JA. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc Intervent Radiol. 2009;32:727–736. doi: 10.1007/s00270-009-9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjoquist KM, Goldstein D, Bester L. A serious complication of selected internal radiation therapy: case report and literature review. Oncologist. 2010;15:830–835. doi: 10.1634/theoncologist.2010-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 11.Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Goin JE, Dancey JE, Roberts CA, Sickles CJ, Leung DA, Soulen MC. Comparison of post-embolization syndrome in the treatment of patients with hepatocellular carcinoma: Trans-catheter arterial chemo-embolization versus yttrium-90 glass microspheres. World J Nucl Med. 2004;3:49. [Google Scholar]
- 13.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy AS, McNeillie P, Dezarn WA, Nutting C, Sangro B, Wertman D, Garafalo M, Liu D, Coldwell D, Savin M, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Sato KT, Lewandowski RJ, Mulcahy MF, Atassi B, Ryu RK, Gates VL, Nemcek AA, Barakat O, Benson A, Mandal R, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology. 2008;247:507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 16.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara K, Terakura M, Katsuragi K. [Severe gastric and duodenal ulcer after chemotherapy of mFOLFOX6 and bevacizumab] Gan To Kagaku Ryoho. 2011;38:457–459. [PubMed] [Google Scholar]
- 18.Saif MW. Interaction between Gemcitabine and Warfarin Causing Gastrointestinal Bleeding in a Patient with Pancreatic Cancer. J Appl Res. 2005;5:434–437. [PMC free article] [PubMed] [Google Scholar]


