Abstract
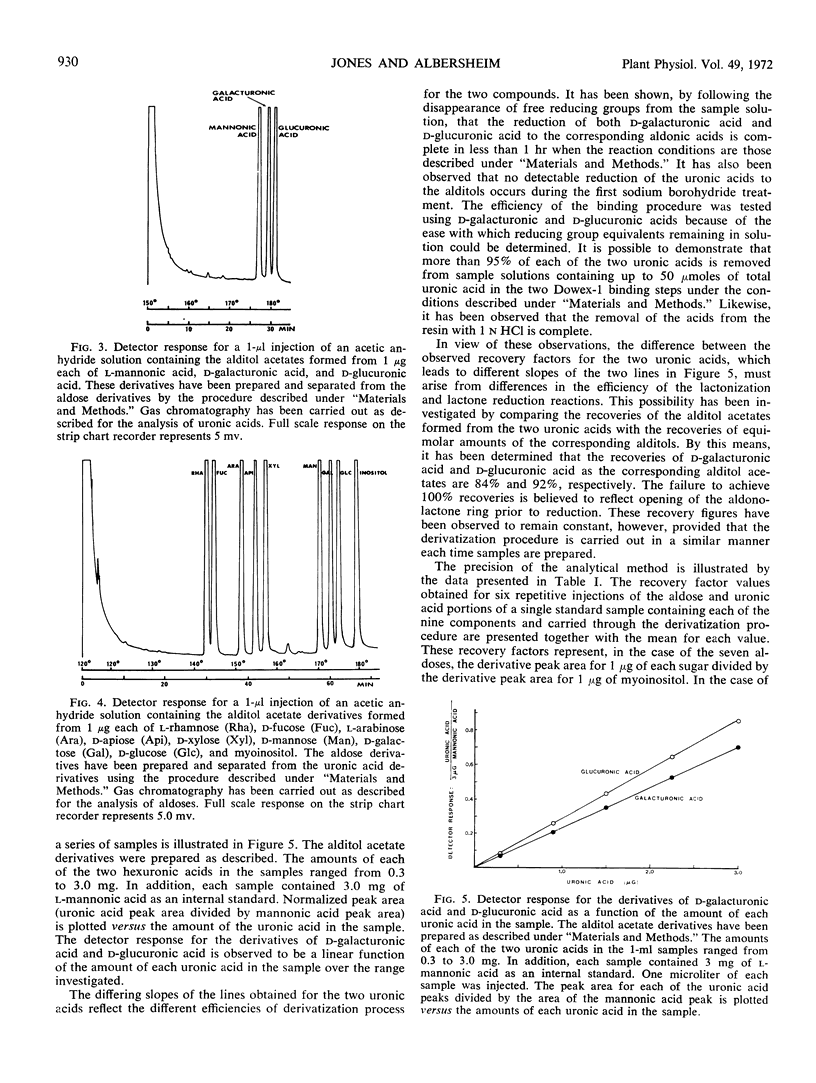
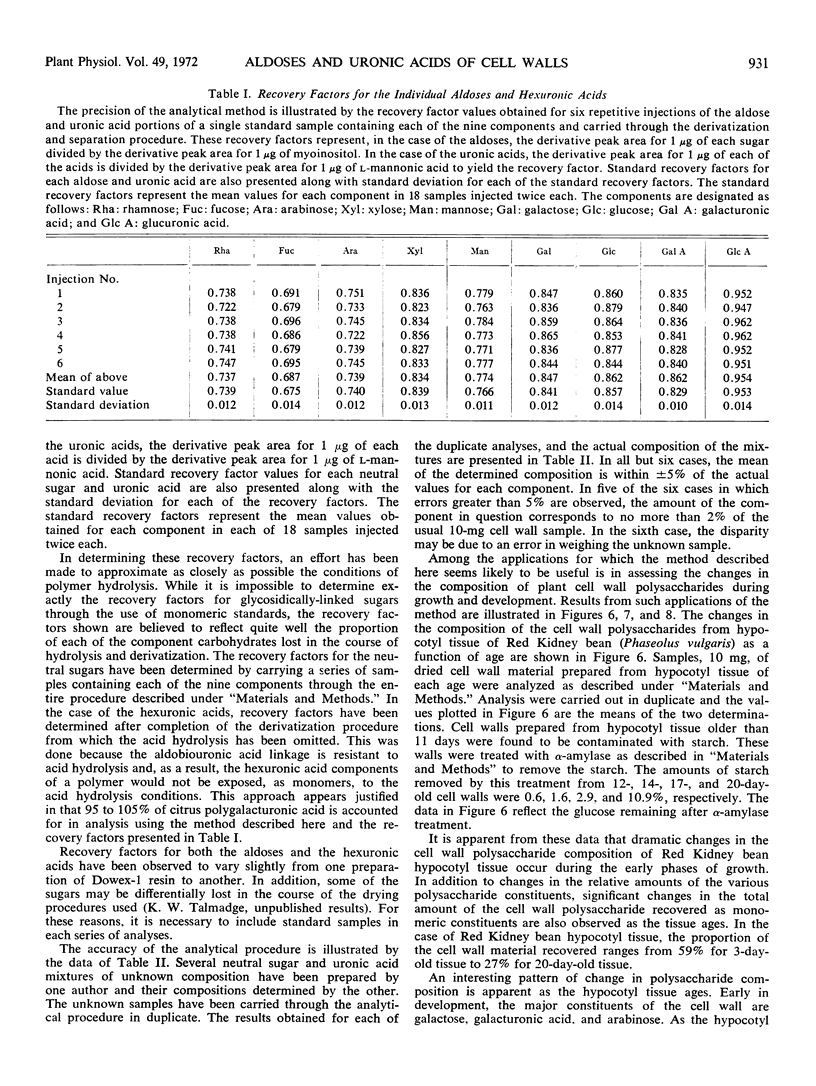
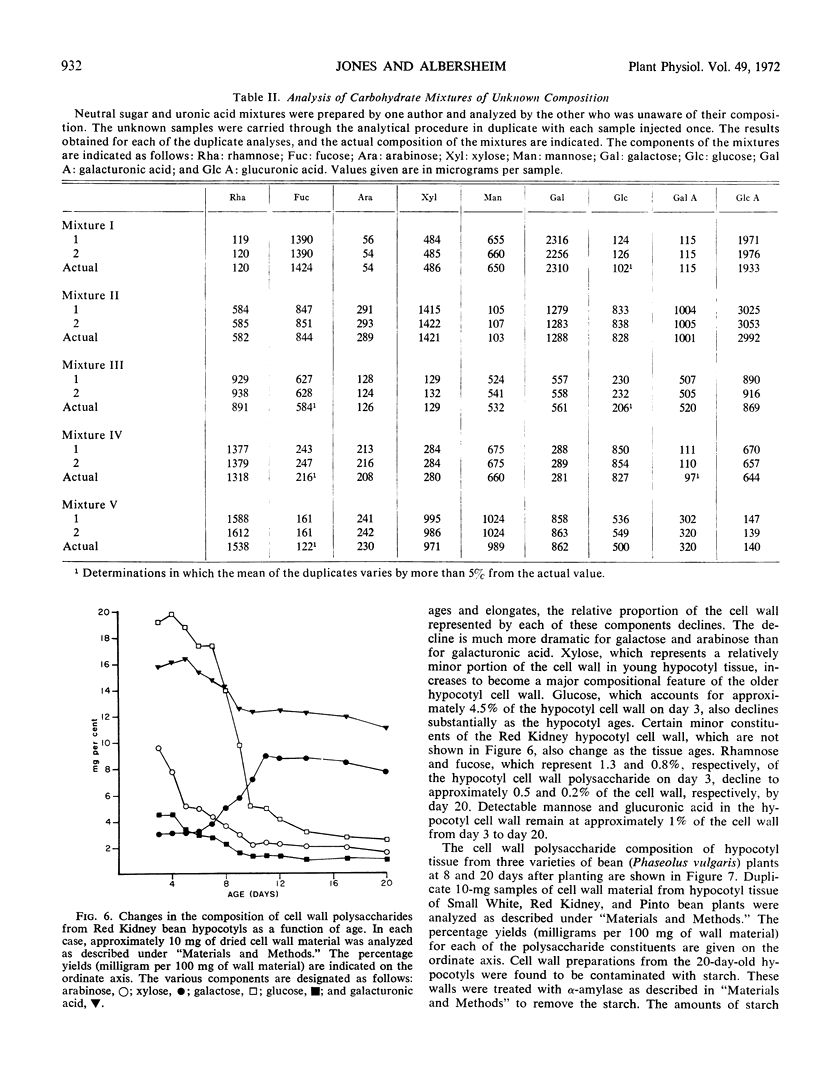
A major problem in determining the composition of plant cell wall polysaccharides has been the lack of a suitable method for accurately determining the amounts of galacturonic and glucuronic acids in such polymers. A gas chromatographic method for aldose analysis has been extended to include uronic acids. Cell wall polysaccharides are depolymerized by acid hydrolysis followed by treatment with a mixture of fungal polysaccharide-degrading enzymes. The aldoses and uronic acids released by this treatment are then reduced with NaBH4 to alditols and aldonic acids, respectively. The aldonic acids are separated from the alditols with Dowex-1 (acetate form) ion exchange resin, which binds the aldonic acids. The alditols, which do not bind, are washed from the resin and then acetylated with acetic anhydride to form the alditol acetate derivatives. The aldonic acids are eluted from the resin with HCl. After the resin has been removed, the HCl solution of the aldonic acids is evaporated to dryness, converting the aldonic acids to aldonolactones. The aldonolactones are reduced with NaBH4 to the corresponding alditols, dried and acetylated. The resulting alditol acetate mixtures produced from the aldoses and those from the uronic acids are analyzed separately by gas chromatography. This technique has been used to determine the changes in composition of Red Kidney bean (Phaseolus vulgaris) hypocotyl cell walls during growth, and to compare the cell wall polysaccharide compositions of several parts of bean plants. Galacturonic acid is found to be a major component of all the cell wall polysaccharides examined.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Abscission: role of cellulase. Plant Physiol. 1969 Mar;44(3):447–452. doi: 10.1104/pp.44.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATEMAN D. F., BEER S. V. SIMULTANEOUS PRODUCTION AND SYNERGISTIC ACTION OF OXALIC ACID AND POLYGALACTURONASE DURING PATHOGENESIS BY SCLEROTIUM ROLFSII. Phytopathology. 1965 Feb;55:204–211. [PubMed] [Google Scholar]
- BISHOP C. T. GAS-LIQUID CHROMATOGRAPHY OF CARBOHYDRATE DERIVATIVES. Adv Carbohydr Chem. 1964;19:95–147. doi: 10.1016/s0096-5332(08)60280-5. [DOI] [PubMed] [Google Scholar]
- Bateman D. F., Kosuge T., Kilgore W. W. Purification and properties of uronate dehydrogenase from Pseudomonas syringae. Arch Biochem Biophys. 1970 Jan;136(1):97–105. doi: 10.1016/0003-9861(70)90331-0. [DOI] [PubMed] [Google Scholar]
- BeMiller J. N. Acid-catalyzed hydrolysis of glycosides. Adv Carbohydr Chem Biochem. 1967;22:25–108. doi: 10.1016/s0096-5332(08)60151-4. [DOI] [PubMed] [Google Scholar]
- English P. D., Albersheim P. Host-Pathogen Interactions: I. A Correlation Between alpha-Galactosidase Production and Virulence. Plant Physiol. 1969 Feb;44(2):217–224. doi: 10.1104/pp.44.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English P. D., Jurale J. B., Albersheim P. Host-Pathogen Interactions: II. Parameters Affecting Polysaccharide-degrading Enzyme Secretion by Colletotrichum lindemuthianum Grown in Culture. Plant Physiol. 1971 Jan;47(1):1–6. doi: 10.1104/pp.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanari T., Tamura Z. Gas chromatography of glucuronides. Chem Pharm Bull (Tokyo) 1967 Nov;15(11):1677–1681. doi: 10.1248/cpb.15.1677. [DOI] [PubMed] [Google Scholar]
- Jaakonmaki P. I., Knox K. L., Horning E. C., Horning M. G. The characterization by gas-liquid chromatography of ethyll beta-D-glucosiduronic acid as a metabolite of ethanol in rat and man. Eur J Pharmacol. 1967 Jan;1(1):63–70. doi: 10.1016/0014-2999(67)90067-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehtonen A., Kärkkäinen J., Haahti E. Gas-chromatographic characterization of the electrophoretically separated fractions of acid mucopolysaccharides. J Chromatogr. 1966 Sep;24(1):179–182. doi: 10.1016/s0021-9673(01)98120-8. [DOI] [PubMed] [Google Scholar]
- Morre D. J. Cell wall dissolution and enzyme secretion during leaf abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1545–1559. [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M., Perry M. B. The analysis of neutral glycoses in biological materials by gas-liquid partition chromatography. Can J Biochem. 1966 Aug;44(8):1115–1126. doi: 10.1139/o66-129. [DOI] [PubMed] [Google Scholar]
- Nagel C. W., Hasegawa S. The enzymic determination of galacturonic acid. Anal Biochem. 1967 Dec;21(3):411–415. doi: 10.1016/0003-2697(67)90315-6. [DOI] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. Changes in cell wall polysaccharides associated with growth. Plant Physiol. 1968 Jun;43(6):914–922. doi: 10.1104/pp.43.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote D. H. The synthesis and metabolic control of polysaccharides and lignin during the differentiation of plant cells. Essays Biochem. 1969;5:89–137. [PubMed] [Google Scholar]
- Oades J. M. Gas-liquid chromatography of alditol acetates and its application to the analysis of sugars in complex hydrolysates. J Chromatogr. 1967 Jun;28(2):246–252. doi: 10.1016/s0021-9673(01)85963-x. [DOI] [PubMed] [Google Scholar]
- Ovodov Y. S., Evtushenko E. V. Analysis of sugar mixtures by gas-liquid chromatography. J Chromatogr. 1967 Dec;31(2):527–530. doi: 10.1016/s0021-9673(01)86105-7. [DOI] [PubMed] [Google Scholar]
- PERRY M. B., HULYALKAR R. K. THE ANALYSIS OF HEXURONIC ACIDS IN BIOLOGICAL MATERIALS BY GAS-LIQUID PARTITION CHROMATOGRAPHY. Can J Biochem. 1965 May;43:573–584. doi: 10.1139/o65-067. [DOI] [PubMed] [Google Scholar]
- Raunhardt O., Schmidt H. W., Neukom H. Gas-chromatographische Untersuchungen an Uronsäuren und Uronsäurederivaten. Helv Chim Acta. 1967 Jul 10;50(5):1267–1274. doi: 10.1002/hlca.19670500508. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem J. 1961 Dec;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Z., Imanari T. Gas chromatography of O-glucuronides. Chem Pharm Bull (Tokyo) 1964 Nov;12(11):1386–1388. doi: 10.1248/cpb.12.1386. [DOI] [PubMed] [Google Scholar]
- Tracey M. V. A manometric method for the estimation of milligram quantities of uronic acids. Biochem J. 1948;43(2):185–189. doi: 10.1042/bj0430185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- VANDENHEUVEL W. J., HORNING E. C. Gas chromatographic separations of sugars and related compounds as acetyl derivatives. Biochem Biophys Res Commun. 1961 Apr 28;4:399–403. doi: 10.1016/0006-291x(61)90297-2. [DOI] [PubMed] [Google Scholar]
- Van Etten H. D., Bateman D. F. Enzymatic degradation of galactan, galactomannan, and xylan by Sclerotium rolfsii. Phytopathology. 1969 Jul;59(7):968–972. [PubMed] [Google Scholar]


