Abstract
AIM: To investigate the effect of Danzhijiangtang capsule (DJC) on monocyte chemoattractant protein-1 (MCP-1) mRNA expression in newly diagnosed type 2 diabetes mellitus (T2DM) subclinical vascular lesions.
METHODS: Sixty-two patients with newly diagnosed T2DM subclinical vascular lesions were randomly divided into a control group and treatment group of 31 cases each. Oral antidiabetic therapy with routine western medicine was conducted in both groups, and the treatment group was additionally treated with DJCs. The treatment course for both groups was 12 wk. Before and after treatment, the total efficiency and traditional Chinese medicine (TCM) syndrome score were calculated. The fasting plasma glucose (FPG), 2-h plasma glucose (2hPG), fasting insulin (FINS), insulin resistance index (IRI), hemoglobin (Hb)A1c, blood lipids, and hemorheology indices were determined. In addition, the levels of vascular endothelial growth factors including thrombomodulin (TM), von Willebrand factor (vWF), P-selectin and MCP-1 mRNA were determined.
RESULTS: After 12 wk of treatment, the TCM syndrome score was significantly decreased compared to before treatment in both groups. After treatment, FPG, 2hPG, HbA1c, FINS, IRI, total cholesterol, triglycerides, low-density lipoprotein, high-density lipoprotein, whole blood low shear specific viscosity, plasma specific viscosity, TM, vWF, P-selectin and MCP-1 mRNA were significantly improved compared to before treatment in both groups. After treatment, the total efficiency and TCM syndrome score in the treatment group were better than in the control group. FINS, IRI, whole blood high shear specific viscosity, plasma specific viscosity, TM, vWF, P-selectin and MCP-1 mRNA level in the treatment group were significantly reduced after treatment compared with control group.
CONCLUSION: DJCs are efficacious in supplementing qi, nourishing yin and invigorating blood circulation, and upregulate MCP-1 mRNA expression in patients with T2DM subclinical vascular lesions.
Keywords: Danzhijiangtang capsule, Type 2 diabetes mellitus, Subclinical vascular lesions, Monocyte chemoattractant protein-1
Core tip: The occurrence and development of type 2 diabetes mellitus (T2DM) is accompanied by an inflammatory response. Monocyte chemoattractant protein (MCP)-1 is a member of the CC chemokine subfamily, and is a key mediator of inflammation. MCP-1 also belongs to the chemokine superfamily. T2DM subclinical vascular lesions are a type of mild inflammation. Endothelial cell MCP-1 can be increased markedly. Danzhijiangtang capsule is a traditional Chinese medicine compound preparation. The technique can restrain mononuclear cell chemotactic activity by supplementing qi, nourishing yin and invigorating blood circulation, thereby preventing and treating subclinical vascular lesions.
INTRODUCTION
The occurrence and development of type 2 diabetes mellitus (T2DM) is accompanied by an inflammatory response, which then promotes the process of T2DM. Monocyte chemoattractant protein (MCP)-1 is a member of the CC chemokine subfamily, and is a key cytokine mediator of the inflammatory response. MCP-1 also belongs to the chemokine superfamily[1]. As shown in a large number of experimental studies, MCP-1 is a risk factor for the occurrence and development of insulin resistance (IR), T2DM and related vascular complications[2].
Danzhijiangtang capsule (DJC) is a traditional Chinese medicine (TCM) compound preparation, and is efficacious in supplementing qi, nourishing yin and invigorating blood circulation. In this study, we investigated the effects of DJCs on general indexes, including fasting plasma glucose (FPG), 2-h postprandial plasma glucose (2hPG), fasting insulin (FINS), insulin resistance index (IRI), hemoglobin (Hb)A1c, blood lipids, and hemorheology indicators in newly diagnosed T2DM subclinical vascular lesions, and the effects on expression of vascular endothelial growth factors including thrombomodulin (TM), von Willebrand factor (vWF), P-selectin and MCP-1 mRNA. The objective was to investigate preliminarily the mechanism of TCM on delaying vascular lesions. This study has importance for achieving a comprehensive understanding of the efficacy of DJCs and illuminating the multichannel and multitarget regulating effects of TCM on newly diagnosed T2DM subclinical vascular lesions.
MATERIALS AND METHODS
General data
A total of 62 outpatients and inpatients with T2DM in the Department of Metabolism and Endocrinology, First Affiliated Hospital of Anhui College of Traditional Chinese Medicine were enrolled in this study from 2009 to 2011. All patients conformed to the diagnostic criteria of diabetes according to World Health Organization (1999), without clinical manifestations of heart, brain or kidney vascular lesions. Color Doppler ultrasound showed that the intima-media thickness of the common carotid, femoral and iliac arteries was > 0.6 cm. The exclusion criteria were as follows: patients with diabetes duration > 1 year; pregnant or lactating women; patients with metabolic disorders (e.g., ketoacidosis) and complicated severe infection in the preceding month; patients complicated with a vascular lesion of the heart, brain, kidney or other region, or other severe primary diseases; patients taking anticoagulant, antiplatelet or antifibrinolytic drugs during the preceding month.
Patients were randomly divided into a control group (31 cases, 7 male and 14 female, mean age 53.67 ± 9.32 years) and treatment group (31 cases, 16 male and 15 female, mean age 51.90 ± 10.13 years). There was no significant differences in age, sex, FPG, 2hPG, HbA1c, FINS, body mass index and IRI between the two groups (P > 0.05).
Experimental methods
Basic treatments including diabetes education, alimentary control, and regular exercise were conducted in both groups. According to actual conditions, patients were treated with one or more antidiabetic drugs, with uniform administration in both groups. The antidiabetic drugs were as follows: metformin hydrochloride tablets (Glucophage; 0.5 g; Shanghai Squibb Pharmaceutical Co. Ltd., China), Acarbose (Glucoba; 50 mg; Bayer Pharmaceutical Co. Ltd., Leverkusen, Germany), pioglitazone hydrochloride (Kasiping; 15 mg; East China Pharmaceutical Group, Zhejiang, China), gliclazide sustained release tablets (Gliclazide tablets; 30 mg; Servier Pharmaceutical Company, Paris, France), and repaglinide tablets (NovoNorm; 1 mg; Novo Nordisk Pharmaceutical Industries, Bagsvaerd, Denmark). In the treatment group, in addition to the oral administration with the above western medicines, the patients were treated with DJCs (provided by the Pharmaceutical Formulations Centre, First Affiliated Hospital of Anhui College of Traditional Chinese Medicine; 1.8 g of effective extract in each capsule) at a dose of five capsules, three times daily (a total daily dose of 15 capsules).
Observational indexes
Blood glucose and blood lipid: Blood glucose was determined by the glucose hexokinase method. Total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) with fasting for > 12 h were determined using a Hitachi-7600 fully automatic biochemical analyzer.
Hemorheology indices: High shear specific viscosity and low shear specific viscosity of whole blood, plasma specific viscosity, hematocrit and fibrinogen were determined using a rotary whole blood viscometer and plasma viscometer in the Experimental Centre, First Affiliated Hospital of Anhui College of Traditional Chinese Medicine.
Vascular endothelial growth factors: Fasting venous blood was obtained during the early morning. After anticoagulation with ethylenediaminetetraacetic acid and centrifugation (4 °C, 3000 g, 10 min), the plasma was separated and stored at -70 °C. TM was quantitatively determined using an enzyme-linked immunosorbent assay (ELISA) kit (ADL Company, CA, United States). vWF was quantitatively determined by the double antibody sandwich ELISA method (Shanghai Sun Biotechnology). P-selectin was quantitatively determined by the ABC-ELISA double antibody sandwich method (R and D Systems, Minneapolis, MN, United States).
MCP-1 mRNA: Expression of serum MCP-1 mRNA was determined using the reverse transcriptase polymerase chain reaction (Promega, Madison, WI, United States) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 statistical software. Non-normally distributed data were logarithmically transformed to normally distributed data. Normally distributed measurement data are expressed as mean ± SD. Analysis of variance was performed for comparisons of multiple means. Student’s t tests were used to analyze the differences between two samples. χ2 tests were conducted to compare the enumerated data. P < 0.05 or P < 0.01 was considered to be statistically significant.
RESULTS
Comparison of blood glucose and blood lipid levels
Before treatment, there was no significant difference in FPG, 2hPG, HbA1c, FINS, IRI, TC, TG, LDL and HDL level between the two groups (P > 0.05 for all comparisons). After treatment with western medicines, the above indexes were significantly improved in both groups (P < 0.05 or P < 0.01). In addition, after treatment, FINS and IRI levels in the treatment group were significantly lower than in the control group (P < 0.05), and HDL in the treatment group was significantly higher than in the control group (P < 0.05) (Table 1).
Table 1.
Comparison of fasting plasma glucose, 2-h plasma glucose, haemoglobin A1c, fasting insulin, insulin resistance index, total cholesterol, triglycerides, low density lipoprotein, high density lipoprotein, haemorheology indices, thrombomodulin, von Willebrand factor, P-selectin levels and monocyte chemoattractant protein-1 mRNA levels
| Index |
Control group |
Treatment group |
||
| Before treatment (n = 31) | After treatment (n = 29) | Before treatment (n = 31) | After treatment (n = 30) | |
| FPG (mmol/L) | 8.74 ± 1.42 | 8.01 ± 1.09b | 9.02 ± 1.14 | 7.94 ± 0.91b |
| 2hPG (mmol/L) | 12.42 ± 1.76 | 10.03 ± 1.39b | 11.96 ± 1.53 | 9.81 ± 1.34b |
| HbA1c (%) | 7.98 ± 0.71 | 7.11 ± 0.69b | 8.03 ± 0.68 | 6.98 ± 0.65b |
| FINS (uIU mL-1) | 12.05 ± 2.93 | 8.68 ± 1.83b | 11.93 ± 2.45 | 7.58 ± 1.82bc |
| IRI | 1.81 ± 0.24 | 1.60 ± 0.19b | 1.79 ± 0.23 | 1.48 ± 0.19bc |
| TC (mmol/L) | 5.36 ± 0.76 | 4.55 ± 0.59b | 5.27 ± 0.69 | 4.32 ± 0.49b |
| TG (mmol/L) | 2.01 ± 0.38 | 1.78 ± 0.36a | 1.99 ± 0.42 | 1.76 ± 0.31a |
| LDL (mmol/L) | 3.15 ± 0.31 | 3.06 ± 0.29a | 3.24 ± 0.28 | 3.07 ± 0.28a |
| HDL (mmol/L) | 1.41 ± 0.19 | 1.66 ± 0.17b | 1.37 ± 0.21 | 1.77 ± 0.15bc |
| High shear specific viscosity (mPa.s) | 5.37 ± 0.78 | 5.10 ± 0.71 | 5.43 ± 0.65 | 4.73 ± 0.54bc |
| Low shear specific viscosity (mPa.s) | 12.65 ± 0.89 | 11.91 ± 1.02 | 12.51 ± 0.92 | 11.84 ± 0.94b |
| Hematocrit (%) | 42.95 ± 3.69 | 42.89 ± 3.51 | 43.15 ± 4.31 | 41.98 ± 3.01 |
| Plasma specific viscosity (mPa.s) | 1.93 ± 0.35 | 1.72 ± 0.37 | 2.01 ± 0.31 | 1.54 ± 0.24bc |
| Fibrinogen (mg/L) | 4.52 ± 0.48 | 4.36 ± 0.37 | 4.61 ± 0.38 | 4.39 ± 0.35 |
| TM (μg/L) | 25.68 ± 6.54 | 23.27 ± 4.06 | 25.41 ± 6.28 | 19.32 ± 4.29ac |
| vWF (U/L) | 1540.17 ± 74.70 | 1480.66 ± 72.32 | 1551.92 ± 82.23 | 1146.70 ± 94.45b,d |
| P-selectin (ng/mL) | 25.03 ± 6.06 | 22.57 ± 5.56 | 25.39 ± 6.81 | 17.08 ± 5.82ac |
| MCP-1 mRNA (%) | 0. 537 ± 0. 136 | 0.473 ± 0.134a | 0. 541 ± 0. 148 | 0.421 ± 0.132bc |
P < 0.05,
P < 0.01 vs before treatment;
P < 0.05 vs control group. FPG: Fasting plasma glucose; 2hPG: 2-h plasma glucose; HbA1c: Haemoglobin A1c; FINS: Fasting insulin; IRI: Insulin resistance index; TC: Total cholesterol; TG: Triglycerides; LDL: Low density lipoprotein; HDL: High density lipoprotein; TM: Thrombomodulin; vWF: von Willebrand factor; MCP-1: Monocyte chemoattractant protein-1.
Comparison of hemorheology indices
Before treatment, there was no significant difference in high shear specific viscosity and low shear specific viscosity of whole blood, plasma specific viscosity, hematocrit, and fibrinogen level between the two groups (P > 0.05 for all comparisons). After treatment, the whole blood high shear specific viscosity, plasma specific viscosity and hematocrit in the treatment group were significantly lower than in the control group (P < 0.05) (Table 1).
Comparison of TM, vWF and P-selectin levels
In the control group, after treatment, TM, vWF and P-selectin levels decreased, but the differences compared with baseline values before treatment were not significant (P > 0.05). In the treatment group, the above indexes were significantly reduced after treatment (P < 0.05 or P < 0.01) and compared with the control group (P < 0.05 or P < 0.01) (Table 1).
Comparison of MCP-1 mRNA levels
Before treatment, there was no significant difference in MCP-1 mRNA levels between the two groups (P > 0.05). After treatment, the MCP-1 mRNA level in the treatment group was significantly lower than before (P < 0.01) and compared with control group (P < 0.05) (Figure 1 and Table 1).
Figure 1.
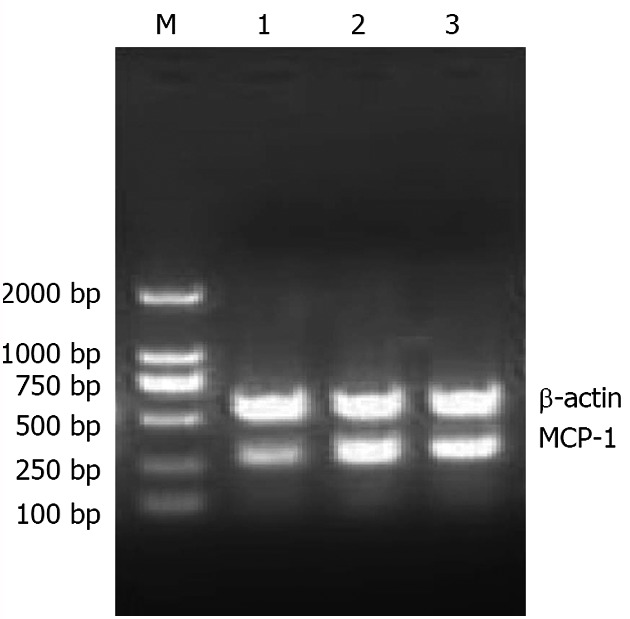
Electropherogram of monocyte chemoattractant protein-1 mRNA obtained by reverse transcription-polymerase chain reaction. MCP-1: Monocyte chemoattractant protein-1; M: Marker; 1: Normal group; 2: Control group; 3: Treatment group.
DISCUSSION
The disease course of newly diagnosed T2DM is < 1 year. There are different degrees of vascular lesions in 50% of patients with newly diagnosed T2DM. Subclinical vascular lesions exist in some patients with newly diagnosed T2DM, but the clinical symptoms are atypical[3-5]. They are the pathological basis of diabetic complications, with an extensive scope. Once the subclinical vascular lesions occur, they develop rapidly and both large and small vessels, arteries, capillaries and veins may be involved, with complicated lesions in many organs, such as angiocarpy, brain, kidney, eyeground, nerve and skin. T2DM subclinical vascular lesions are caused by many factors including abnormal glucose metabolism, IR, lipid metabolism disorders, inflammatory factors, and oxidative stress[6,7]. Vascular endothelial injury is the initiating step of T2DM subclinical vascular lesions, and the anatomical endothelial layer is also the first involved site. During T2DM, continued hyperglycemia and high capillary pressure can cause vascular endothelial cell injury and dysfunction, changing angiostasis and hemodynamics, activate the coagulation system and platelets, and increase the vascular permeability, thus leading to a series of pathological changes[8]. Therefore, studying the change in endothelial cell function is beneficial for further preventing the occurrence and development of T2DM subclinical vascular lesions.
In recent years, TM has been confirmed as a molecular marker of endothelial cell injury[9,10]. In addition, it can be used as a marker for judging the severity of atherosclerosis of peripheral arteries and coronaries, and a sensitive detection index for the occurrence of vascular complications[11]. As found in prospective studies, vWF is involved in blood coagulation, and can promote platelet adhesion[12]. It is an important marker for predicting early subclinical endothelial cell injury in T2DM and development of urinary albumin excretion rate[13]. In addition, it is an important risk factor for new microvascular lesions. P-selectin has been a research hotspot for vascular lesions in recent years. It is an important marker of endothelial cell injury and platelet activation, and is considered to be an important factor in the initial pathological process of atherosclerosis[14].
MCP-1 is one member of the CC chemokine subfamily, and is a key cytokine mediator of the inflammatory response[15]. In T2DM, high-level blood glucose, non-enzymatic glycated protein, angiotensin, oxidative stress, interleukin-1, and tumor necrosis factor-α can all upregulate MCP-1 expression[16-19]. MCP-1 can specifically induce blood monocytes to enter the endothelium of the lesion. This is an important mechanism in the occurrence and development of T2DM vascular lesions[20]. MCP-1 mainly attracts monocytes and T lymphocytes, and induces monocytes and endothelial cells to express adhesion molecules. It can promote various inflammatory cells, especially monocytes, to aggregate in the lesion sites, and respond to stimulation of the inflammatory cytokines[21]. In addition, MCP-1 can promote peripheral blood mononuclear cells to adhere, chemoattract and migrate to the intima, and then phagocytose lipids and transform into foam cells[22]. The activated leukocytes and vascular wall cells can release a variety of growth factors, which promote vascular smooth muscle cell proliferation, stimulate inflammation[23], and participate in the occurrence and development of atherosclerosis. It has been found that MCP-1 plays an important role in atherosclerosis in diabetes, and can be used as a useful index for predicting T2DM subclinical atherosclerosis[24]. At the same time, MCP-1 is considered to be a potential biochemical marker for reflecting early atherosclerotic changes and prognosis[25].
Therefore, MCP-1 is closely related to the occurrence and development of T2DM vascular lesions. Further study of MCP-1 will enrich the theoretical basis for pathogenesis of T2DM vascular lesions. Analysis of the chemokine dynamic change, regulatory mechanism and interactions, and treatment aimed at chemokines and receptors is a new method for therapy of T2DM vascular complications. The deepening awareness of the complexity of the chemokine network has provided new methods and means for prevention and treatment of T2DM vascular lesions.
TCM believes that the prethrombotic status of T2DM is similar to the blood stasis syndrome of emaciation-thirst disease. The pathogenesis of emaciation-thirst disease is that the dryness-heat, impairment of yin, and body fluid scorching will cause blood viscosity and blood stagnation. The long-term yin deficiency injures the healthy qi, leading to qi and yin deficiency and weakness, which aggravate the blood stasis. Deficiency of yin affects yang, and insufficiency of yang brings about cold syndrome. The cold coagulation causes blood stasis. The blood stasis is present throughout the disease course, and is a common pathological product and pathogenic factor of emaciation-thirst disease. In the treatment of this disease, supplementing qi, nourishing yin, invigorating blood circulation and dissolving stagnant blood should be carried out, and the treatment emphasis should be focused on regulating qi-blood circulation and improving blood circulation. DJCs are composed of Radix Pseudostellariae, Radix Rehmanniae, Cortex Moutan, Dodder, Alisma, leech and others. Radix Pseudostellariae can invigorate the qi of the spleen and kidney. Radix Rehmanniae can nourish the yin of the spleen and kidney. Dodder can tonify the kidney to arrest spontaneous emission. Cortex Moutan and leech can promote qi circulation, invigorate blood circulation, dissolve stagnant blood, and resolve kidney collateral obstruction. Alisma can clear heat and purge phlegm. The whole prescription can supplement qi and nourish yin, invigorate blood circulation and dissolve stagnant blood, thus achieving the efficacy of balancing yin and yang, dispelling blood stasis, and making the kidney pulse unobstructed. Modern pharmacological research shows that Radix Pseudostellariae, Radix Rehmanniae and Alisma have certain antidiabetic and antihypertensive effects. Moutan bark can inhibit a cyclo-oxygenase reaction, thus inhibiting platelet aggregation. The hirudin contained in leech can inhibit the effect of thrombin on fibrinogen, impede blood coagulation, and prevent thrombus formation. In addition, Leech also secretes a histamine-like substance, which can directly expand the blood vessels, activate plasmin, and inhibit collagen synthesis, thus reducing the blood viscosity and improving the hemorheology.
Results of this study have shown that, after treatment with DJCs, FINS and IRI levels are significantly lower than those obtained from basic treatment, with an increase in HDL level. In addition, after treatment with DJCs, the whole blood high shear specific viscosity, plasma specific viscosity and hematocrit, as well as TM, vWF and P-selectin expression levels are significantly reduced, with downregulated peripheral blood MCP-1 mRNA expression. This indicates that DJCs can alleviate lipid metabolism disorders, downregulate expression of TM, vWF, P-selectin and MCP-1 mRNA, lower whole blood viscosity and plasma specific viscosity, reduce hematocrit, antagonize platelet activation, inhibit platelet aggregation, and protect vascular endothelial cells, thus intervening in the newly diagnosed T2DM subclinical vascular lesions. It is believed that this therapy, by supplementing qi, nourishing yin and invigorating blood circulation, can enhance the anticoagulant and fibrinolytic activity, intervene in the hypercoagulable state, and improve the function of impaired endothelial cells. This has important significance in delaying vascular complications associated with T2DM.
COMMENTS
Background
The occurrence and development of type 2 diabetes mellitus (T2DM) is accompanied by an inflammatory response, which in turn promotes the process of T2DM. Monocyte chemoattractant protein (MCP)-1 is a member of the CC chemokine subfamily, and is a key cytokine mediatior of the inflammatory response. MCP-1 also belongs to the chemokine superfamily. As shown in a large number of experimental studies, MCP-1 is a risk factor for the occurrence and development of insulin resistance, T2DM and related vascular complications.
Research frontiers
The disease course of newly diagnosed T2DM is < 1 year. There are different degrees of vascular lesions in 50% of patients with newly diagnosed T2DM. Subclinical vascular lesions exist in some patients with newly diagnosed T2DM, but the clinical symptoms are atypical. They are the pathological basis of diabetic complications, with an extensive scope.
Innovations and breakthroughs
This study has important significance for comprehensive understanding of the efficacy of Danzhijiangtang capsules (DJCs) and illuminating the multichannel and multitarget regulating effects of traditional Chinese medicine on newly diagnosed T2DM subclinical vascular lesions.
Applications
DJCs are efficacious in supplementing qi, nourishing yin and invigorating blood circulation, and can upregulate MCP-1 mRNA expression in patients with T2DM subclinical vascular lesions.
Terminology
In recent years, thrombomodulin has been confirmed as a molecular marker of endothelial cell injury. In addition, it can be used as a marker for judging the atherosclerosis severity of peripheral arteries and coronaries, and a sensitive detection index for the occurrence of vascular complications.
Peer review
This manuscript describes a traditional Chinese medicine DJCs for treatment of T2DM subclinical vascular lesions, and it has significant research interest.
Footnotes
P- Reviewers Assy N, Ismail-Beigi F S- Editor Song XX L- Editor Kerr C E- Editor Li JY
References
- 1.He Q, Luo HM, Tang XH, Zhu BS, Chen AB, Chen JY. The effects of blood glucose control on levels of monocyte chemoattractant protein-1 (MCP-1) activity in peripheral blood of patients with hyperglycemic crisis. Zhongguo Tangniaobing Zazhi. 2012;3:216–218. [Google Scholar]
- 2.Sakallioğlu EE, Ayas B, Lütfioğlu M, Keleş GC, Açikgöz G, Firatli E. Gingival levels of monocyte chemoattractant protein-1 (MCP-1) in diabetes mellitus and periodontitis: an experimental study in rats. Clin Oral Investig. 2008;12:83–89. doi: 10.1007/s00784-007-0148-6. [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki Y, Kodama M, Nishizawa H, Sakamoto K, Matsuhisa M, Kajimoto Y, Kosugi K, Shimizu Y, Kawamori R, Hori M. Carotid intima-media thickness in Japanese type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care. 2000;23:1310–1315. doi: 10.2337/diacare.23.9.1310. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, Sera Y, Nakamura H, Ueki Y, Miyake S. Correlation between common carotid arterial wall thickness and ischemic stroke in patients with type 2 diabetes mellitus. Metabolism. 2002;51:244–247. doi: 10.1053/meta.2002.28971. [DOI] [PubMed] [Google Scholar]
- 5.Taniwaki H, Shoji T, Emoto M, Kawagishi T, Ishimura E, Inaba M, Okuno Y, Nishizawa Y. Femoral artery wall thickness and stiffness in evaluation of peripheral vascular disease in type 2 diabetes mellitus. Atherosclerosis. 2001;158:207–214. doi: 10.1016/s0021-9150(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 6.Pechánová O, Simko F. The role of nitric oxide in the maintenance of vasoactive balance. Physiol Res. 2007;56 Suppl 2:S7–S16. doi: 10.33549/physiolres.931392. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HF, Zhao ZG. Relations of adipocyte factors with type 2 diabetes and macrovascular lesions. Zhongguo Lingchuang Baojian Zazhi. 2007;1:96–99. [Google Scholar]
- 8.Lorenzi M. Glucose toxicity in the vascular complications of diabetes: the cellular perspective. Diabetes Metab Rev. 1992;8:85–103. doi: 10.1002/dmr.5610080202. [DOI] [PubMed] [Google Scholar]
- 9.Szegedi N, May Z, Ováry C, Skopál J, Nagy Z. Molecular markers of endothelial dysfunction in acute ischemic stroke. Ideggyogy Sz. 2002;55:102–108. [PubMed] [Google Scholar]
- 10.Borawski J, Myśliwiec M. Soluble thrombomodulin: a marker of endothelial injury, an antithrombotic agent, or both? Med Sci Monit. 2001;7:785–786. [PubMed] [Google Scholar]
- 11.Kobayashi H, Sadakata H, Suzuki K, She MY, Shibata S, Terao T. Thrombomodulin release from umbilical endothelial cells initiated by preeclampsia plasma-induced neutrophil activation. Obstet Gynecol. 1998;92:425–430. doi: 10.1016/s0029-7844(98)00217-8. [DOI] [PubMed] [Google Scholar]
- 12.Blann AD, Lip GY. Hypothesis: is soluble P-selectin a new marker of platelet activation? Atherosclerosis. 1997;128:135–138. doi: 10.1016/s0021-9150(96)05980-1. [DOI] [PubMed] [Google Scholar]
- 13.Lip GY, Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–265. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 14.Yao W, Yang L, Chen TD, Li PJ, Ma YH. Study of P-selectin and cardiovascular risk. Zhongguo Jijiu Yixue. 2002;22:18–20. [Google Scholar]
- 15.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 16.Lee SK, Kim BS, Yang WS, Kim SB, Park SK, Park JS. High glucose induces MCP-1 expression partly via tyrosine kinase-AP-1 pathway in peritoneal mesothelial cells. Kidney Int. 2001;60:55–64. doi: 10.1046/j.1523-1755.2001.00770.x. [DOI] [PubMed] [Google Scholar]
- 17.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25 Suppl 2:S9–12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- 20.Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63:209–216. doi: 10.1046/j.1523-1755.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou XJ, Jiang LZ, Yang GM, Gong Y, Liu Y, Wang JZ, Zhu D, Qi G. The association of MCP-1 with type 2 diabetes and insulin resistance. Zhongguo Tangniaobing Zazhi. 2008;3:152–153. [Google Scholar]
- 22.Han KH, Ryu J, Hong KH, Ko J, Pak YK, Kim JB, Park SW, Kim JJ. HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated monocyte recruitment in vivo. Circulation. 2005;111:1439–1447. doi: 10.1161/01.CIR.0000158484.18024.1F. [DOI] [PubMed] [Google Scholar]
- 23.He DC, Lin H, Cheng YH, Ding Xj. Effect of Chinese medicine compound on the expression of blood NF-κB and mRNA of MCP-1 in rats with diabetes. Xiandai Zhongxiyijiehe Zazhi. 2009;6:1856–1858. [Google Scholar]
- 24.Huang TT, Chen XW, Zhang P, He YB. Correlation between levels of serum MCP-1, IL-18 and carotid intima-media thickness in patients with type 2 diabetes. Linchuang Huicui. 2010;8:654–657. [Google Scholar]
- 25.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]


