Abstract
Mitochondria are partially autonomous organelles that depend on the import of certain proteins and lipids to maintain cell survival and membrane formation. Although phosphatidylglycerol, cardiolipin, and phosphatidylethanolamine are synthesized by mitochondrial enzymes, phosphatidylcholine, phosphatidylinositol, phosphatidylserine, and sterols need to be imported from other organelles. The origin of most lipids imported into mitochondria is the endoplasmic reticulum, which requires interaction of these two subcellular compartments. Recently, protein complexes that are involved in membrane contact between endoplasmic reticulum and mitochondria were identified, but their role in lipid transport is still unclear. In the present review, we describe components involved in lipid translocation between the endoplasmic reticulum and mitochondria and discuss functional as well as regulatory aspects that are important for lipid homeostasis.
Membrane contact plays an important role in intracellular lipid translocation. Protein complexes (e.g., ERMES), microtubules, and actin filaments may help bridge the membranes.
Biological membranes are major structural components of all cell types. They protect the cell from external influences, organize the interior in distinct compartments and allow balanced flux of components. Besides their specific proteome, organelles exhibit unique lipid compositions, which influence their shape, physical properties, and function. Major lipid classes found in biological membranes are phospholipids, sterols, and sphingolipids.
The major “lipid factory” within the cell is the endoplasmic reticulum (ER). It is able to synthesize the bulk of structural phospholipids, sterols, and storage lipids such as triacylglycerols and steryl esters (van Meer et al. 2008). Furthermore, initial steps of ceramide synthesis occur in the ER providing precursors for the formation of complex sphingolipids in other organelles (Futerman 2006). Besides the export of ceramides, the ER supplies a large portion of lipids to other organelles, which cannot produce their own lipids or have a limited capacity to do so. Organelle interaction and transport of lipids require specific carrier proteins, membrane contact sites, tethering complexes, and/or vesicle flux. These processes are highly important for the maintenance of cell structure and survival but are still a matter of dispute. Most prominent organelle interaction partners are the ER and mitochondria. A subfraction of the ER named mitochondria-associated membrane (MAM) (Vance 1990) was described to be involved in lipid translocation to mitochondria. MAM is part of the ER network, which was shown to be in contact or close proximity to the outer mitochondrial membrane (OMM). Contact sites between MAM and mitochondria were assumed to facilitate exchange of components between the two compartments. Interestingly, MAM harbor a number of lipid synthesizing enzymes (Gaigg et al. 1994). Recently, molecular components governing membrane contact between the two organelles were identified (Dolman et al. 2005; Csordás et al. 2006; de Brito and Scorrano 2008; Kornmann et al. 2009; Friedman et al. 2010; Lavieu et al. 2010), although the specific role of these components in lipid translocation is not yet clear.
MAJOR PHOSPHOLIPID CLASSES OF MITOCHONDRIA
Cardiolipin (CL) and/or phosphatidylglycerol (PG) are considered as mitochondria-specific phospholipids (Zinser et al. 1991). Both lipids are synthesized by mitochondria themselves (Davidson and Stanacev 1971). The importance of CL for ATP-production has been shown with different membrane types, e.g., bacteria, hydrogenosomes, and mitochondria (Mileykovskaya et al. 2005; Schlame 2008; Acehan et al. 2011). In mitochondria, the majority of CL is localized to the inner membrane (IMM) (Zinser et al. 1991), but substantial amounts were also detected in the OMM (Gebert et al. 2009). With its uncommon, dimeric structure (Fig. 1) CL together with phosphatidylethanolamine (PE) interacts with many mitochondrial proteins (Osman et al. 2011) and stabilizes their conformation (Joshi et al. 2012). Mutants lacking CL and PE biosynthesis are synthetic lethal in yeast and bacteria (Gohil et al. 2005). In bacteria, both lipids are organized in membrane clusters (Matsumoto et al. 2006). Specific interaction partners of CL are proteins of ATP production, mitochondrial transport systems (Bogdanov et al. 2008a; Schlame and Ren 2009), and proteins required for mitochondrial structure and fusion (Joshi et al. 2012).
Figure 1.
Major phospholipid classes of mitochondria. Chemical formulas and geometrical forms of the major mitochondrial phospholipids are shown. The shape-structure concept of lipids compares the area of the head group with the area of their acyl chains. If the cross-section area of the head group is similar to that of the acyl chains, lipids have an overall cylindrical shape and have a strong tendency of self-assembly into bilayer phases of biological membranes. A typical example for such geometry is PC. If the cross-section area of the head group is smaller than that of the acyl chains, lipids have a conical shape and form structures with negative curvature such as hexagonal phase. Examples of this type are PE and CL. While PC, PS, and PI exhibit cylindrical shape and self-organize into bilayers, PE and CL with their conical shape induce hexagonal phases and disturb bilayer arrangement.
Another prominent mitochondrial phospholipid is PE. Mitochondria are able to synthesize a large portion of PE by decarboxylation of phosphatidylserine (PS) (Vial et al. 1982; Trotter et al. 1993; Emoto et al. 1999; Birner et al. 2001; Nerlich et al. 2007). With its small hydrophilic head group and large hydrophobic tail, PE is a typical nonbilayer-forming phospholipid (see Fig. 1). This structure appears to be important for some peripheral and integral membrane proteins (van den Brink-van der Laan et al. 2004). Together with CL, PE plays a crucial role in maintaining mitochondrial morphology. Loss of both PE and CL is lethal probably due to defects in mitochondrial fusion (Joshi et al. 2012) and/or protein destabilizing (Bogdanov et al. 2008b; Osman et al. 2009b). Lack of PE synthesized in the IMM cannot be fully compensated by PE imported from extramitochondrial sites (Birner et al. 2001; Bürgermeister et al. 2004; Joshi et al. 2012). However, PE derived from exogenous lyso-PE can restore both intra- and extramitochondrial PE deficiencies by remodeling processes (Riekhof and Voelker 2006). Noteworthy, mitochondria are the major supplier of PE to other organelles (Voelker 1984).
Mitochondrial enzymes of CL and PG synthesis, as well as the major PS decarboxylase, are synthesized on cytosolic ribosomes, imported into mitochondria, and assembled into the IMM (Minskoff and Greenberg 1997; Jiang et al. 2000; Nowicki et al. 2005; Nebauer et al. 2007; Choi et al. 2012). Only recently were we able to demonstrate experimentally the involvement of components of the mitochondrial TOM complex and of mitochondrial proteases in import and processing of the mitochondrial PS decarboxylase Psd1p from the yeast (Horvath et al. 2012).
Phosphatidylcholine (PC) has a big hydrophilic head group and a long hydrophobic tail (see Fig. 1). Its cylindrical shape makes it a perfect component of bilayer membranes and its role as a structural component is essential (van Meer et al. 2008). Specific functions of PC in mitochondria are not well defined. Similar to PC, specific functions of phosphatidylinositol (PI) in mitochondria are not known, but its essential role in total cellular metabolism makes it directly or indirectly indispensible for the maintenance of mitochondria. The bulkhead group of PI makes this phospholipid a specific membrane bilayer component.
Phosphatidylserine (PS) has also a cylindrical shape that allows integration into bilayer membranes (see Fig. 1). Although PS is present in low concentrations in organelles of eukaryotic cells (Zinser and Daum 1995), it is important as a precursor for the two major phospholipids, PE and PC. Supply of PS to mitochondria is essential because in many cells the majority of PE is formed by decarboxylation of PS in mitochondria. The metabolic conversion of PS to PE upon import of PS into mitochondria provides a convenient biochemical method to study this import process (Voelker 1988, 1989b, 1991, 1992, 1993; Achleitner et al. 1995, 1999).
Typically, the lipid composition of mitochondria shows as major components 40%–44% PC, 27%–34% PE, 1%–3% PS, 5%–15% PI, and 13%–14% CL depending on the cell type (Zinser and Daum 1995; Daum and Vance 1997; van Meer 2008). OMM and IMM are strongly different with respect to their lipid equipment. Whereas the IMM is strongly enriched in proteins and contains only 20% lipids of total mass, the OMM is a lipid rich membrane (Zinser and Daum 1995; Dolis et al. 1996; Daum and Vance 1997). Accumulation of CL, PG, and also PE in the IMM appears to be related to functions mentioned above.
SYNTHESIS OF AMINOGLYCEROPHOSPHOLIPIDS INVOLVES INTERACTION OF ORGANELLES
The biosynthetic sequence of aminoglycerophospholipid formation starts with the synthesis of PS in the ER (Fig. 2). The highest concentration of PS synthesizing enzymes has been detected in the MAM (mitochondria-associated membrane) (Kuchler et al. 1986; Vance 1990). Interestingly, mammalian cells and yeast have different pathways to produce PS. In mammalian cells, two phosphatidylserine synthases (PSS-1 and PSS-2) produce PS by base exchange at the head group of PE or PC (Vance 2008) in a Ca2+-dependent reaction (Fig. 2B). This release of Ca2+ into the lumen of the ER is energy dependent. In yeast, formation of PS is catalyzed by the gene product of PSS1/CHO1 (Letts et al. 1983), which requires CDP-diacylglycerol (CDP-DAG) and serine (Nikawa and Yamashita 1981) as substrates and depends on Mg2+ or Mn2+ (Fig. 2A). Cellular energy is required for the formation of CDP-DAG. In plants, both pathways described above are active for PS production (Delhaize et al. 1999; Manoharan et al. 2000; Rontein et al. 2003). In all types of cells, PS synthesized in the ER is exported to other organelles, including mitochondria where it serves as a substrate for PE synthesis.
Figure 2.
Biosynthetic pathways of aminoglycerophospholipids. (A) Biosynthesis of aminoglycerophospholipids in yeast. Formation of PS is accomplished in the ER and catalyzed in a CDP-DAG-dependent reaction by the PS synthase, Pss1p. Decarboxylation of PS yielding PE occurs in mitochondria (via Psd1p) and a Golgi/vacuolar compartment (via Psd2p). Three-step methylation of PE in the ER catalyzed by Cho2p/Pem1p and Opi3p/Pem2p leads to formation of PC with S-adenosine methionine (SAM) as methyl donor. PC and PE can be formed also by the CDP-ethanolamine and CDP-choline branches of the so-called Kennedy pathway making use of exogenous or endogenous choline (Cho) and ethanolamine (Etn), respectively. SL, sphingolipids. (B) Aminoglycerophospholipid biosynthesis in mammalian cells. The major mechanism of PS production in mammals is base exchange with PC (PSS1) and PE (PSS2) as substrates. A major route of PE and PC production are the CDP-ethanolamine and CDP-choline pathways. In mammalian cells, PE can also be produced by decarboxylation of PS. In hepatocytes, a single methyltransferase catalyzes methylation of PE to PC. (C) The Land’s cycle describes a sequence of deacylation and reacylation. PE and PC are converted to lyso-PE and lyso-PC and vice versa.
PE is the second most abundant lipid of eukaryotic cells. It can be synthesized by four different pathways: (i) the CDP-ethanolamine pathway (also named Kennedy pathway) (Kanfer and Kennedy 1964), (ii) decarboxylation of PS to PE, (iii) base exchange between different phospholipids, and (iv) acylation of lyso-PE (see Fig. 2). Yeast has two PS decarboxylases (PSD) with overlapping functions (Trotter et al. 1993). Psd1p is localized to mitochondria (Trotter et al. 1993), and Psd2p has been found in a Golgi/vacuolar compartment (Trotter and Voelker 1995). Psd1p is a component of the IMM/intermembrane space and produces the majority of total cellular and mitochondrial PE (Trotter et al. 1993; Birner et al. 2001).
Yeast can also produce PE through the CDP-ethanolamine branch of the Kennedy pathway (Kennedy and Weiss 1956). The CDP-ethanolamine pathway incorporates externally added or endogenous ethanolamine through stepwise phosphorylation, activation with CTP, and attachment to diacylglycerol (DAG). In the final step, phosphoethanolamine is transferred from CDP-ethanolamine to a DAG acceptor forming PE. However, PE synthesized through this pathway cannot fully complement for the mitochondrial requirement of PE in cells deleted of both PSD1 and PSD2 (Trotter and Voelker 1995). Ethanolamine phosphate, which is an intermediate in this biosynthetic sequence, can also be derived from sphingolipid degradation providing a link between sphingolipid and PE metabolism (Mao et al. 1997; Saba et al. 1997). Finally, yeast harbors enzymes that catalyze acylation of lyso-PE (Riekhof and Voelker 2006; Riekhof et al. 2007b; Deng et al. 2010). This type of reaction named Land’s cycle includes deacylation/reacylation of phospholipids (Lands 1958) and appears to be important for remodeling processes. The reason for the existence of overlapping pathways is still a matter of dispute, although evidence for distinct pools of PE has been presented (Bürgermeister et al. 2004).
Mammalian cells have the capacity to synthesize their entire PE through the PSD pathway in mitochondria (Voelker and Frazier 1986), but in the presence of ethanolamine the CDP-ethanolamine pathway can fulfill most of the extramitochondrial requirements for this lipid (Vance 2008). The Land’s cycle is important for remodeling phospholipids that have been formed by other pathways. As an example, formation of PE from lyso-PE can be accomplished by LPEAT2 (acyl-CoA:lysophosphatidylethanolamine acyltransferase 2) in the brain cells. Ethanolamine phospholipids are major constituents of the myelin sheath increasing the signal transmission speed along the axons (Cao et al. 2008). In plants, remodeling of PE to lyso-PE was reported to be involved in plant growth promotion and leaf senescence (Cowan 2009; Hong et al. 2009). In the parasites, Trypanosoma brucei and Plasmodium berghei, PE is exclusively produced through the CDP-ethanolamine branch of the Kennedy pathway (Serricchio and Bütikofer 2011).
PC is the most abundant phospholipid in eukaryotic cells (van Meer et al. 2008). Because mitochondria lack PC synthesizing enzymes, this phospholipid has to be imported from the ER. PC can be produced via three pathways: (i) the CDP-choline branch of the Kennedy pathway (Gibellini and Smith 2010), (ii) methylation of PE (Sundler and Akesson 1975; Li et al. 2005), and (iii) the Land’s cycle, in which LPCAT (lysophosphocholine acyltransferase) produces PC from lyso-PC and fatty acids (Riekhof et al. 2007a; Hishikawa et al. 2008; Shindou et al. 2009) (see Fig. 2). In most mammalian cells, the majority of PC is formed through the CDP-choline pathway (Kennedy 1956; Gibellini and Smith 2010; Hermansson et al. 2011), and only in hepatocytes PE methylation is the predominant pathway (Sundler and Akesson 1975; Li et al. 2005). Reactions of the CDP-choline pathway are similar to the CDP-ethanolamine pathway and catalyzed by choline kinase, phosphocholine cytidyltransferase, and choline phosphotransferase. In the final step, phosphocholine is transferred from CDP-choline to DAG forming PC. In yeast (Boumann et al. 2004) as well as in mammals (Henneberry and McMaster 1999), enzymes of the both branches of the Kennedy pathway have overlapping substrate specificities.
The second pathway of PC production in mammalian cells (Van Pilsum and Carlson 1970) and yeast (Kodaki and Yamashita 1987) is PE methylation. In this sequence, S-adenosylmethionine (SAM) serves as methyl donor for three steps of methyltransferase reactions. In the first step, PE is methylated to monomethyl-PE and then further converted to dimethyl-PE in the second step. The biosynthetic sequence is completed by a third step of methylation yielding the final product PC. In yeast, the gene products of CHO2/PEM1 and OPI3/PEM2 are involved (Kodaki and Yamashita 1987), whereas hepatocytes harbor only one N-methyltransferase, which catalyzes all three steps of PE methylation (Sundler and Akesson 1975; Ridgway et al. 1989). The lower eukaryote, Trypanosoma brucei, lacks genes coding for PE N-methyltransferases (Gibellini et al. 2009).
CARDIOLIPIN AND PHOSPHATIDYLGLYCEROL SYNTHESIS IN MITOCHONDRIA
Synthesis of PG and CL in mitochondria occurs by a multistep reaction sequence (Schlame et al. 1993). First, PA is converted to CDP-DAG by Cds1p, either in the ER/MAM or in mitochondria. Then, conversion of CDP-DAG to phosphatidylglycerol phosphate (PGP) catalyzed by Pgs1p occurs (Chang et al. 1998a). PGP is dephosphorylated to PG in a reaction that is accomplished by the PGP phosphatase Gep4p (Osman et al. 2010). In the final step of CL synthesis, a phosphatidate (PA) moiety is transferred from CDP-DAG to the hydroxyl group in the head group of PG. The cleavage of the pyrophosphate group provides the chemical energy for the latter reaction. In yeast (Jiang et al. 1997; Chang et al. 1998b; Tuller et al. 1998), Arabidopsis (Katayama et al. 2004; Nowicki et al. 2005), and human cells (Chen et al. 2006; Houtkooper et al. 2006; Lu et al. 2006), CL synthase was localized to mitochondria. Importantly, CL undergoes remodeling processes (Joshi et al. 2009; Schlame and Ren 2009) with so-called tafazzins (Malhotra et al. 2009) involved. Tafazzins are phospholipid transacylases that transfer acyl groups from phospholipids, preferentially PC, to monolysocardiolipin (MLCL). The reverse reaction of this modification is the transfer of an acyl group from CL to lyso-PC. The reaction does not require activation of fatty acids but occurs in a lysophospholipid–phospholipid complex by deprotonation and nucleophilic attack on the ester bond of the acyl donor (Xu et al. 2006).
PA and CDP-DAG are important intermediates not only for PG/CL synthesis but also for the formation of PI and PS (Athenstaedt and Daum 1999). In the yeast, PA is synthesized from glycerol-3-phosphate and/or dihydroxyacetone phosphate (DHAP) in the ER and lipid droplets by two steps of acylation. The first acetylation reaction with DHAP as a substrate leads to acyl-DHAP, which is then reduced by the 1-acyl-DHAP reductase Ayr1p in an NADPH-dependent reaction to lyso-PA. Alternatively, acylation of glycerol-3-phosphate leads to formation of lyso-PA. In a final acylation step, lyso-PA is converted to PA (Athenstaedt et al. 1999a; Sorger and Daum 2003). PA can also be generated by phospholipase D (Osman et al. 2011) in mitochondria. In plants, PA synthesis occurs in plastids, mitochondria, and microsomes. Dephosphorylation of PA is catalyzed by mammalian lipin and the yeast ortholog Pah1p (Carman and Han 2006, 2009; Brindley et al. 2009; Harris and Finck 2011), which were recently shown to be pacemakers and switch points in lipid metabolism. Dephosphorylation of PA is a crucial step, because the product of the dephosphorylation reaction, DAG, becomes substrate for triacylglycerol synthases and thus promotes biogenesis of lipid droplets (Adeyo et al. 2011). PA, which is not dephosphorylated, is preferentially converted to CDP-DAG, which is further used for the synthesis of phospholipids. CDP-DAG synthase activity in yeast, as well as in mammals, was localized to the ER and mitochondria (Kuchler et al. 1986; Kelley and Carman 1987; Chen et al. 2006).
PHOSPHATIDYLINOSITOL SYNTHESIS
PI is produced in the ER and has to be imported into mitochondria. PI is synthesized from CDP-DAG and inositol (Gardocki et al. 2005). In the yeast, deletion of the only phosphatidylinositol synthase gene PIS1 is lethal (Nikawa et al. 1987). Furthermore, PI serves as precursor for cell signaling molecules such as phosphatidylinositol phosphates (PIP), phosphatidylinositol bisphosphates (PIP2) and phosphatidylinositol triphosphates (PIP3) and for the biosynthesis of GPI anchors (Serricchio and Bütikofer 2011).
TRANSPORT OF PHOSPHOLIPIDS BETWEEN THE ENDOPLASMIC RETICULUM AND MITOCHONDRIA
As the biosynthetic sequence of PS-PE-PC synthesis occurs in different subcellular compartments (see above), the amounts of intermediates and products are indication and measure for transport between organelles. Therefore, interorganelle translocation of aminoglycerophospholipids can be investigated by following the incorporation of serine into PS in the ER, decarboxylation of PS to PE in the mitochondria, and methylation of PE to PC upon the return of PE to the ER without isolation of organelles. Making use of this experimental strategy, phospholipid transport studies with intact cells, isolated organelles, and permeabilized cells have been performed (Butler and Thompson 1975; Voelker 1985, 1989a,b, 1990, 1991; Vance 1990; Achleitner et al. 1995; Shiao et al. 1995; Emoto et al. 1999; Kuge et al. 2001; Schumacher et al. 2002; Wu and Voelker 2004; Carrasco et al. 2006; Riekhof and Voelker 2006; Kornmann et al. 2009; Nguyen et al. 2012; Padilla-López et al. 2012; Tamura et al. 2012). These studies showed that in mammalian cells PS transport from MAM to the OMM requires ATP. Further import of PS to the IMM yields PE (Voelker and Frazier 1986; Voelker 1990, 1991; Shiao et al. 1995).
Experiments with isolated organelles provided more detailed information about the process of phospholipid import into mitochondria. Reconstitution of transport systems using isolated mitochondria and microsomes/MAM (Kuchler et al. 1986; Simbeni et al. 1990; Vance 1990; Achleitner et al. 1999; Emoto et al. 1999) revealed that uptake of PS by mitochondria depended on the PS concentration in the donor membranes (ER, Golgi) (Wu and Voelker 2004). Although ATP was required for transport of PS in intact and permeabilized cells (see below), no such observation was made in the reconstituted in vitro system. It was argued that close apposition of donor (ER/MAM) and acceptor (mitochondria) membranes was sufficient for transport, but that ATP was probably required to provide appropriate conditions for transport of this phospholipid in intact cells. Experiments making use of the coisolation of MAM with mitochondria suggested that the contact of these two cellular fractions is fairly tight (Vance 1990; Achleitner et al. 1999). Altogether, it was concluded that membrane contact between ER and mitochondria is important for lipid translocation between these two organelles.
Experiments performed with permeabilized cells provided a useful alternative to the experiments described above (Lim et al. 1986; Voelker 1992; Achleitner et al. 1995; Kuge et al. 2001; Wu and Voelker 2001). Permeabilized cells are obtained by mild chemical or mechanical treatment of whole cells, resulting in the internal organelle structures remaining largely intact, while access to the interior is possible for compounds that cannot enter an intact cell. In permeabilized yeast cells (Achleitner et al. 1999) [3H]serine is incorporated into PS in the ER. Transport of [3H]PS to mitochondria yields [3H]PE formed by mitochondrial Psd1p. Use of psd2Δ mutants and depletion of ethanolamine from the medium allows attribution of the formed [3H]PE to transport of [3H]PS from the ER. Transport of [3H]PE from mitochondria to the ER results in formation of [3H]PC as a measure for this transport route (Achleitner et al. 1995, 1999; Shiao et al. 1998).
The advantage of permeabilized cells is that reactions can be manipulated by addition of chemicals such as divalent ions, energy blockers, or cytoskeleton inhibiting reagents (Eilers et al. 1989) or by removing the cytosol. This feature allowed additional characterization of lipid transport between ER and mitochondria with mammalian (Voelker 1990) and yeast cells (Achleitner et al. 1995). Transport of PS to mitochondria was shown to be dependent on ATP in mammalian cells (Voelker 1989a) but not in yeast (Achleitner et al. 1999). However, a certain amount of ATP must be present in both cell types (Shiao et al. 1995) because upstream reactions of lipid precursor formation are energy consuming. It was also shown that ongoing synthesis of PS is not required for translocation to mitochondria because preformed PS was efficiently used as a substrate for mitochondrial PS decarboxylation. Interestingly, when exogenous mitochondria were added to permeabilized cells, they failed to serve as acceptors for PS import (Voelker 1993). This result suggests that pre-existing, stable associations between mitochondria and ER/MAM play an important role in the transport process.
In a yeast genetic screen, Voelker and coworkers searched for components that influence the translocation of PS from ER to the sites of decarboxylation in mitochondria (via Psd1p) (Schumacher et al. 2002) and Golgi/vacuoles (via Psd2p) (Trotter et al. 1998; Wu and Voelker 2001, 2004). The first gene product identified was Met30p, which influenced the import of PS into mitochondria. MET30 encodes a subunit of a multicomponent E3 ubiquitin ligase (Aghajan et al. 2010; Ouni et al. 2010) and affects substrate specificity of the ubiquitin ligase (Chandrasekaran et al. 2006; Yan and Xiong 2010). One substrate for the E3 ubiquitin ligase is the transcription factor Met4p, which is inactivated upon ubiquitination. It was suggested that Met30p mediates ubiquitination of certain target proteins and may play a role either in ER-mitochondria recognition, in the inhibition of this docking process, or in regulation of transcription of a factor involved in lipid transport. The second component identified in the screen was the phosphatidylinositol 4-kinase Stt4p, which affects transport of PS to the site of Psd2p-driven conversion to PE (Trotter et al. 1998). The mode of action of Stt4p on organelle interaction and/or PS translocation remains to be elucidated. It was suggested that the C2 domain of Psd2p, which binds Ca2+, interacts with proteins or lipids and recognizes anionic lipids such as PS and polyphosphoinositides (Choi et al. 2005) and may be the bridge for PS translocation. Finally, the gene product of PDR17/SFH4 was found to affect PS transport to Psd2p. Reconstitution assays with permeabilized cells and isolated organelles showed that Pdr17p must be present on the acceptor membrane for transfer of PS to Psd2p (van den Hazel et al. 1999; Wu et al. 2000). The mechanism of action of Pdr17p is still unknown.
Reconstitution of PS synthesis and transport in permeabilized mammalian cells identified two other components that affect PS transport from the ER/MAM to mitochondria (Emoto et al. 1999; Kuge et al. 2001). One of these proteins is S100B (NP_006236), an EF-hand domain-Ca2+-binding protein. It is not known whether this protein participates in transport or promotes stability and/or assembly of interactions of ER/MAM and mitochondria. The second component was only indirectly identified in the mammalian cell line R41, which has a defect in PS transport between the OMM and IMM.
Studies with isolated organelles treated with protease (Shiao et al. 1998; Achleitner et al. 1999) or dinitrophenol, which alters the distance between the ER and mitochondria (Hovius et al. 1992), suggested the participation of organelle surface proteins in PS transport. Electron microscopy supported this view by demonstrating close contact zones between ER/MAM and mitochondria, which might serve as bridges for lipid translocation (Csordás et al. 2006). Furthermore, the involvement of acetylated microtubules in ER-mitochondria dynamics was demonstrated, a mechanism by which membrane contact can be established and/or maintained (Friedman et al. 2010). Moreover, mitochondrial division sites produced by Dnm1p and Drp1p were found to be physically connected with the ER (Friedman et al. 2011).
To shed more light on the role of ER-mitochondria contact, genetic and synthetic genetic interactions analysis mainly with the yeast, Saccharomyces cerevisiae, were performed (Birner et al. 2003; Kornmann et al. 2009; Osman et al. 2009a). Such genetic screens were designed in a way that defects in two or more interacting gene products become lethal. Recently, Kornmann et al. (2009) identified components of ER-mitochondria contact in yeast at the molecular level. They showed that in wild-type cells, a protein complex tethers the ER/MAM and mitochondria. Mutations in this protein complex caused slow growth or were lethal for the cells, but could be suppressed by an artificial tethering construct called ChiMERA, which carried a GFP molecule. When staining the mitochondria with mito-tracker and expressing the ChiMERA, association was visualized confirming that ChiMERA acted as a bridge between the ER and mitochondria. The authentic complex was named ERMES (ER mitochondria encountered structures) and contains the OMM proteins, Mdm10p, Mdm12p, and Mdm34p, which interact with the ER membrane anchored protein Mmm1p. In the absence of ERMES, the CL level and the PS/PC conversion rates were decreased, suggesting the involvement of ERMES in phospholipids transport between ER and mitochondria (Fig. 3). Moreover, genetic interactions between PSD1, GEM1, and the ERMES complex were shown (Kornmann et al. 2009). The Ca2+ Miro (mitochondria rho-like) GTPase Gem1p is a regulatory component of ERMES (Kornmann et al. 2011; Stroud et al. 2011), maintains mitochondrial morphology and inheritance (Frederick et al. 2004, 2008) and most likely connects ERMES to the actin cytoskeleton (Kornmann et al. 2011; Michel and Kornmann 2012). It was assumed that Gem1p shuttles between a free and ERMES bound form (see Fig. 3), although the molecular mechanism of Gem1p remains unknown. Physical interaction of Psd1p with ERMES or Gem1p was not demonstrated. Nevertheless, it was reported that a gem1Δpsd2Δdpl1Δ yeast mutant grown on a nonfermentable carbon source was defective in its PC synthesis (Kornmann et al. 2011), most likely by impaired PE and PS transport. A similar growth phenotype was reported before for the gem1Δ single deletion strain grown on synthetic glycerol media (Frederick et al. 2008). GEM1 also shows strong synthetic lethality with GEP4, encoding a PGP phosphatase (Kornmann et al. 2011). Gem1p is well conserved in the eukaryotic kingdom, suggesting that Miro GTPases are general components of ER-mitochondria connections. Miro-1, the mammalian homolog of Gem1p, interacts with mitofusin 1 and 2, the human homologs of yeast Fzo1p. Mfn-2 was suggested to tether mitochondria to ER and being involved in mitochondrial movement along axons (de Brito and Scorrano 2008; Misko et al. 2010). Most recently, however, the role of ERMES and Gem1p in the import of PS into mitochondria and its conversion to PE was challenged (Nguyen et al. 2012). It was argued that, despite their genetic and physical interaction, ERMES and Gem1p function in distinct pathways, and the absence of ERMES and Gem1p had only little effect on PS import into mitochondria and conversion to PE. The effect on PC formation as described by Kornmann et al. (2009) was regarded as minor. Nguyen et al. (2012) argued that changes in the lipid profile of cells lacking ERMES were side effects of defects in mitochondrial morphology.
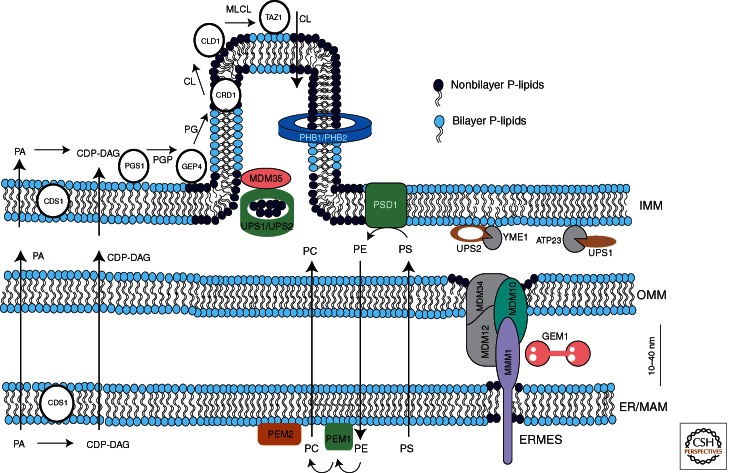
Figure 3.
Components involved in translocation of phospholipids to and within mitochondria of yeast. The directions of lipid translocation are indicated by arrows. While PS and PC are imported into mitochondria, a large portion PE is exported. Upon import the majority of PS is converted to PE by Psd1p. Decarboxylation of PS to PE in mitochondria and methylation of PE to PC in the ER are sufficient for supplying cells with PE and PC even in the absence of external ethanolamine or choline. Similar to PS and PC, PA and CDP-DAG also have to be imported into mitochondria where they are required for CL synthesis. The pathway of CL synthesis in the IMM starts with CDP-DAG and is completed during several steps by a multienzyme cascade. A small portion of CL is exported to the OMM. The ERMES complex containing gene products of MDM10, MDM34, MDM12, and MMM1 tethers ER and mitochondria. In mammalian cells, Mfn1/2 seem to have a similar function. ERMES associates with the GTPase GEM1, cycling between ERMES bound and free form. UPS1/UPS2 interact with Mdm35p and regulate the mitochondrial levels of PE and CL. UPS1 and UPS2 are degraded by ATP23 and YEM1, respectively. Prohibitin ringlike structures made from two proteins PHB1/2 contribute to the formation of PE and CL clusters in the IMM. For a detailed description of biosynthetic and translocation processes and components involved, see text.
Recently, another mitochondrial complex named MitOS, MICOS, or MINOS was identified (Harner et al. 2011; Hoppins et al. 2011; von der Malsburg et al. 2011). This IMM-associated complex functions in cristae formation and morphology (Rabl et al. 2009). It was proposed (van der Laan et al. 2012) that MINOS forms the central core of a large and complex organizing system named ERMIONE, which includes the ERMES complex, the prohibitin ringlike structures, the TOM and TIM complexes, and Mdm31/32 proteins required for mtDNA maintenance. MINOS was also found to interact with the protein VDAC and with the fusion protein Ugo1 (van der Laan et al. 2012). Both ERMES and MINOS are genetically linked to the prohibitin ring complexes of the IMM that are integrated into the lipid network metabolism (Psd1, Ups1/Ups2) (Birner et al. 2003; Gohil et al. 2005; Kornmann et al. 2009; Osman et al. 2009a; Tamura et al. 2009, 2012; Potting et al. 2010). However, direct involvement of MINOS in lipid transport and/or assembly into mitochondrial membrane has not been demonstrated.
LINK BETWEEN CARDIOLIPIN FORMATION AND INTRAMITOCHONDRIAL TRANSPORT OF AMINOGLYCEROPHOSPHOLIPIDS
A link between CL and PE assembly in mitochondrial membranes, PE synthesis by Psd1p, and the role of prohibitins was uncovered in synthetic lethal screens with the yeast (Birner et al. 2003; Gohil et al. 2005). It was shown that a phb1Δphb2Δpsd1Δ triple mutation was lethal due to reduced PE levels and loss of mtDNA. Osman et al. (2009a) demonstrated that ringlike prohibitin complexes organize CL and PE in clusters in the IMM and become essential in strains with low levels of PE and CL. This finding supported the view of physical similarities of CL and PE and their importance for mitochondria morphology (Osman et al. 2011; Joshi et al. 2012). In subsequent work, 35 genetic interactions of prohibitins (GEP) were identified, which were required for cell survival in the absence of prohibitins. Among the genes detected, UPS1, GEP1/UPS2, PSD1, MDM35, MMM1, GEP4, and CRD1 were prominent (Osman et al. 2009b) (see Fig. 3). Prohibitins seem to control IMM organization and integrity by acting as lipid scaffolds for PE and CL (Osman et al. 2009a,b). Ups1p and Ups2p antagonistically regulate the CL (Tamura et al. 2009) level, and Ups2p also regulates the PE level (Potting et al. 2010) in the IMM. Most recently, it was shown that Ups1p promotes conversion of PE to PC, whereas Ups2p suppresses this process (Tamura et al. 2012). It was concluded that UPS proteins affect export of PE from the IMM, although the mechanism of this regulation is unknown. The authors showed that loss of Ups1, the ERMES complex, and Mdm31p caused similar defects in mitochondria, especially in CL and PE homeostasis in the IMM. Yeast Ups1p and Ups2p are per se unstable proteins and degraded by the proteases Yme1p and Atp23p in the IMM (Potting et al. 2010). The newly identified protein Mdm35p protects them from proteolytic degradation (Tamura et al. 2009, 2012; Potting et al. 2010).
IMPORT OF PHOSPHATIDYLCHOLINE AND PHOSPHATIDYLINOSITOL INTO MITOCHONDRIA
Although PC and PI are major phospholipids of mitochondrial membranes, little is known about their import into this organelle. Both phospholipids are synthesized in the ER from where they are translocated to mitochondria and assembled into membranes. Lampl et al. (1994) designed an in vitro assay to study the import of [3H]-labeled PI and PC from unilamellar donor vesicles to isolated yeast mitochondria. Both phospholipids ended up in the IMM. During import, they were detected in contact sites between OMM and IMM, supporting the notion that these sites are involved in intramitochondrial phospholipid transport. The uncoupler CCCP, the antibiotic adriamycin, and energy depletion did not inhibit this process. Janssen et al. (1999) described transbilayer movement of PC in isolated OMM vesicles from yeast. They showed that this translocation was rapid and bidirectional. Pretreatment of the OMM with proteinase K or sulfhydryl reagents had no effect on PC transport.
STEROLS AND SPHINGOLIPIDS OF MITOCHONDRIA
Sterols and sphingolipids are minor lipid components of mitochondria. Both lipid classes are synthesized in extramitochondrial compartments and need to be imported and assembled into mitochondrial membranes. Sterol biosynthesis is accomplished by a complex sequence of reactions that are localized to the ER and peroxisomes of mammalian cells (Ikonen 2008; Miller and Auchus 2011; Miller and Bose 2011). In mammalian cells, cholesterol is the end product of this biosynthetic pathway (Soccio and Breslow 2004; Brown and Goldstein 2009), whereas in yeast ergosterol is the major sterol (Wagner and Daum 2005; Jacquier and Schneiter 2012). In yeast, the ER and lipid droplets contribute to sterol biosynthesis (Leber et al. 1994; Athenstaedt et al. 1999b). Plants produce a variety of sterols such as phytosterols (Suzuki and Muranaka 2007). In mammalian mitochondria the majority of cholesterol is localized to the OMM (Daum 1985), whereas in yeast (Sperka-Gottlieb et al. 1988; Wriessnegger et al. 2009) most of the mitochondrial sterol was found in the IMM.
Biosynthesis of sphingolipids occurs in the ER and in organelles along the protein secretory pathway, mainly the Golgi (Perry and Ridgway 2005; Breslow and Weissman 2010; Levy and Futerman 2010; Mencarelli et al. 2010). However, recent work showed the presence of ceramide metabolic enzymes in mitochondria from mammals and yeast (Kitagaki et al. 2007; Novgorodov et al. 2011). The majority of this lipid class is located to the plasma membrane, whereas amounts in mitochondria are minor (Breslow and Weissman 2010; Stiban et al. 2010; van Meer and Hoetzl 2010). In the OMM, the concentration of ceramide is threefold higher than in the IMM (Mencarelli et al. 2010).
IMPORT OF STEROLS INTO MITOCHONDRIA
Sterols in mitochondria may serve as structural components of membranes and/or as precursors for hormone synthesis. For both purposes, they have to be imported into mitochondria. Transport of sterols from the ER to mitochondria appears to occur by machinery, which is different from that described for phospholipids. A nonvesicular and a vesicular pathway have been proposed for cholesterol translocation to mitochondria (Maxfield and Wüstner 2002).
A number of components appear to contribute to sterol transport to mitochondria (Fig. 4). The best studied protein of this kind is the so-called steroidogenic acute regulatory protein (StAR) (Clark et al. 1994). StAR is almost exclusively expressed in steroid-producing cells and regulated by cAMP (Strauss et al. 1999; Benmessahel et al. 2002). StAR requires ATP and an electrochemical gradient for maximal activity (King and Stocco 1996). Import of StAR into mitochondria results in cleavage of an amino-terminal mitochondrial targeting sequence (Arakane et al. 1998). The carboxyl-terminus of StAR displays cholesterol transferring capacity (Strauss et al. 1999; Stocco 2000). Ligand binding to StAR changes its secondary structure; the protein itself alters the domain structure of the OMM to facilitate rapid sterol transfer (Petrescu et al. 2001). StAR interacts with the cAMP-dependent protein kinase (PKA) and a peripheral-type benzodiazepine receptor (PBR) (Hauet et al. 2002), which was later named TSPO (translocator protein) (Mukhin et al. 1989; Krueger and Papadopoulos 1990). A protein named PAP7 was identified as a regulator of PBR and PKA. PBR/TSPO (translocator protein) was shown to support import and processing of StAR in the mitochondria (Hauet et al. 2005). VDAC (voltage-dependent anion channel) and ANT (adenine nucleotide transporter) were identified as interaction partners of PBR/TSPO (McEnery et al. 1992; Rone et al. 2009). VDAC binds cholesterol and influences its mitochondrial distribution (Campbell and Chan 2007). It appears that TSPO and VDAC together with TOM (translocase of the OMM) function in StAR assembly into mitochondria. Also members of the ACBD family, ACBD1 and ACBD3/ PAP7, seem to participate in the function of the transduceosome (Midzak et al. 2011). Finally, a protein named MLN64 (metastatic lymph node protein 64) with a carboxy-terminal START domain for cholesterol binding was identified as a mediator for cholesterol transport from late endosomes to the plasma membrane and also to mitochondria (Zhang et al. 2002; Charman et al. 2010; Liapis et al. 2012). It was shown that in Niemann-Pick Type C (NPC) mutated cells, MLN64 supplies cholesterol from endosomes to mitochondria. In analogy to StAR, MLN64 needs direct contact with mitochondria to transfer cholesterol, indicating that direct contact between endosomes and mitochondria may occur (Charman et al. 2010).
Figure 4.
Import of sterols into mitochondria for steroidogenesis. (A) Upon binding of cholesterol the StAR precursor protein changes its conformation. StAR expression is regulated by cAMP. (B) StAR comes in contact with PKA, PAP7/ACBD3, and ACBD1 and is imported into the mitochondria with sterols bound. Upon interaction with TSPO, StAR induces changes in the OMM and a transduceosome is formed. This structure, which includes TSPO/VDAC of the OMM and ANT in the IMM, facilitates imports and maturation of StAR. (C) Upon arrival in the mitochondrial matrix, the mature StAR protein delivers cholesterol to CPY11A1 where it is converted to pregnenolone.
Once cholesterol has been imported into mitochondria, it is further converted to steroids (Miller and Auchus 2011) (see Fig. 4). The first and rate-limiting step in steroidogenesis is the conversion of cholesterol to pregnenolone by a cytochrome P450. Further conversion requires the action of hydroxysteroid dehydrogenases. Steroidogenic cells in humans are localized to a variety of tissues, most prominently to adrenal glands and gonads. In the adrenal glands, mainly the conversion of cholesterol to aldosterol, dehydroepiandrosterone (DHEA), and cortisol-like is catalyzed. In the gonal glands, cholesterol is converted to steroid hormones, namely estradiol in the ovarian granulose cells and to testosterone by the testicular leyding cells (Miller and Auchus 2011).
Evidence about import of sterols in nonsteroidogenic cells is rare. Import of radiolabeled ergosterol and cholesterol from unilamellar vesicles into isolated mitochondria has been studied in Saccharomyces cerevisiae (Tuller and Daum 1995). Supply of ergosterol to the mitochondrial surface was enhanced by a cytosolic fraction, whereas no such additive was required for cholesterol transport. Both sterols reached the IMM in an energy-independent process but accumulated to some extent in contact sites between OMM and IMM, supporting the idea that these zones are sites of intramitochondrial lipid translocation.
SPHINGOLIPID INCORPORATION INTO MITOCHONDRIA
Little is known about import and function of sphingolipids in mitochondria. There is evidence that sphingolipid metabolism affects the mitochondrial pathway of apoptosis (Chipuk et al. 2012). Ceramides with their short and long acyl-chains could form large protein-permeable channels in the OMM (Siskind and Colombini 2000), and such channels may contribute to the release of proapoptotic proteins from mitochondria (Futerman 2006). It was proposed that ceramide is produced by hydrolysis of sphingomyelin by a neutral sphingomyelinase in MAM (Chipuk et al. 2012; Mullen et al. 2012) and then transferred to mitochondria where it is converted to sphingosine-1-phosphate and hexadecenal. It has also been proposed that in liver mitochondria, thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA (Novgorodov and Gudz 2009; Novgorodov et al. 2011). In vitro, ceramide synthase, or reverse ceramidase activity was detected in mitochondria (Bionda et al. 2004). In yeast, Isc1p has been suggested to generate ceramides in mitochondria (Kitagaki et al. 2007, 2009).
Evidence for the existence of two ceramide transporting proteins has been presented: the Goodpasture antigen-binding protein (GPBP) and its splice variant, the ceramide transporter (CERT) (Mencarelli et al. 2010). It was proposed that these proteins might be involved in mitochondrial lipid homeostasis, although this view is still a matter of debate.
CONCLUSIONS
Among the different subcellular compartments, mitochondria are one of the most unique when considering lipid composition. Presence of PG and CL, as well as the large amount of PE, is characteristic for mitochondrial membranes. Recent studies have shown that especially these nonbilayer forming lipids are important for mitochondrial function (Birner et al. 2003; Gohil et al. 2005; Nebauer et al. 2007; Osman et al. 2009a; Acehan et al. 2011; Joshi et al. 2012; Tamura et al. 2012). However, low abundant lipids in mitochondria such as sterols or sphingolipids must not be ignored, although at present ascribing specific functions to these components in mitochondria is difficult.
Large amounts of lipids have to be imported into mitochondria. Different mechanisms of intracellular lipid transport have been discussed for several decades (Daum and Vance 1997; Holthuis and Levine 2005; Voelker 2005; Schulz and Prinz 2007; Osman et al. 2011). The role of lipid transfer proteins, which were enthusiastically studied in the beginning of these investigations, has been scrutinized more recently. Such transfer proteins may be more relevant for lipid sorting or sensing than bulk lipid transport. Vesicle flux was recognized as a more potent mechanism of lipid transport because it allows rapid, robust, and efficient translocation to target membranes. It appears that at least significant portions of lipids are cotransported to the cell periphery by the classical pathway of protein secretion (Salama and Schekman 1995; Kohlwein et al. 1996). However, other types of vesicles may also be relevant although experimental evidence for such processes is missing. Last but not least, membrane contact has become an attractive alternative for intracellular lipid translocation (Levine and Loewen 2006; Lebiedzinska et al. 2009; Voelker 2009; de Brito and Scorrano 2010; Elbaz and Schuldiner 2011; Osman et al. 2011). Contact of the ER with mitochondria through the MAM fraction, association of the ER with the plasma membrane or nuclear–vacuolar junctions became prominent examples of this kind. Finally, transmembrane movements of lipids by translocases and flippases contribute to appropriate lipid distribution in organelles (Holthuis and Levine 2005).
Close proximity of ER and mitochondria as shown by electron microscopy (Csordás et al. 2006; Friedman et al. 2011) supports the idea of lipid transport by membrane contact. The distance is sufficient for a protein complex to bridge the gap between organelles and to connect membranes without causing fusion (Achleitner et al. 1999). A hemistalk structure caused by high amounts of mitochondrial PE and CL, which provide negative membrane curvature, may support attachment and point fusion events (Chernomordik and Kozlov 2005, 2008; Chernomordik et al. 2006). Protein bridges like ERMES stabilize the energy-unfavorable state (Kozlov et al. 2010). Microtubules and actin filaments may help by bridging ER and mitochondria and establishing or maintaining protein contact between membranes (Friedman et al. 2010, 2011). Although ERMES and associated proteins were shown to create or maintain contact between ER and mitochondria (Kornmann et al. 2009; Stroud et al. 2011), direct functions of these components in lipid transport were scrutinized (Nguyen et al. 2012). It was argued that ERMES facilitates membrane contact, but other components may be more directly involved in lipid translocation. MAM-OMM contact sites appear to be further linked to complexes of the IMM (de Brito and Scorrano 2008; Kornmann et al. 2009). Transport of lipids from OMM to IMM may occur either by membrane contact or by translocation through the intermembrane space. Despite its role in IMM organization the cristae-forming MINOS complex (Harner et al. 2011; Hoppins et al. 2011; van der Laan et al. 2012) is most likely not involved in this process. The role of Gem1p and Ups-proteins in OMM-IMM lipid translocation has to be taken into account (Tamura et al. 2012). Interaction of prohibitins with the mitochondrial lipid synthesizing machinery also appears to be important for lipid homeostasis in this organelle (Birner et al. 2003; Gohil et al. 2005; Gohil and Greenberg 2009; Osman et al. 2009a,b).
An intriguing although unsolved question is regulation of lipid transport to/from and within mitochondria. Fluidity of the membrane or membrane curvature affected by PE and CL are parameters that may influence biophysical properties of the membrane environment. Also the acyl chain length of specific phospholipid species could have an effect (Dolis et al. 1996; Heikinheimo and Somerharju 1998; Kevala and Kim 2001). A balanced concentration of phospholipids, sterols, and sphingolipids appears to be important for membrane fusion or fission. In that respect, the low-abundant lipids in mitochondria such as sterols or sphingolipids may be relevant. Regulation of the mitochondrial lipid assembly by Ups1p and Ups2p, which are in turn subject to proteolytic degradation/protection by Yme1p, Atp23p, and Mdm35p (Potting et al. 2010; Tamura et al. 2012) are examples for the widespread network of lipid assembly and homeostasis in mitochondrial membranes, which we are just beginning to understand in more detail at the molecular level.
ACKNOWLEDGMENTS
We thank Susanne Horvath and Edina Harsay for critically reading this manuscript. This work is financially supported by the FWF (DK Molecular Enzymology W901-B05 and project P21429 to G.D.).
Footnotes
Editors: Susan Ferro-Novick, Tom A. Rapoport, and Randy Schekman
Additional Perspectives on The Endoplasmic Reticulum available at www.cshperspectives.org
REFERENCES
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M 2011. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J 100: 2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achleitner G, Zweytick D, Trotter PJ, Voelker DR, Daum G 1995. Synthesis and intracellular transport of aminoglycerophospholipids in permeabilized cells of the yeast, Saccharomyces cerevisiae. J Biol Chem 270: 29836–29842 [DOI] [PubMed] [Google Scholar]
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G 1999. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem 264: 545–553 [DOI] [PubMed] [Google Scholar]
- Adeyo O, Horn PJ, Lee S, Binns DD, Chandrahas A, Chapman KD, Goodman JM 2011. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol 192: 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajan M, Jonai N, Flick K, Fu F, Luo M, Cai X, Ouni I, Pierce N, Tang X, Lomenick B, et al. 2010. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotechnol 28: 738–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane F, Kallen CB, Watari H, Stayrook SE, Lewis M, Strauss JF 3rd 1998. Steroidogenic acute regulatory protein (StAR) acts on the outside of mitochondria to stimulate steroidogenesis. Endocr Res 24: 463–468 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K, Daum G 1999. Phosphatidic acid, a key intermediate in lipid metabolism. Eur J Biochem 266: 1–16 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K, Weys S, Paltauf F, Daum G 1999a. Redundant systems of phosphatidic acid biosynthesis via acylation of glycerol-3-phosphate or dihydroxyacetone phosphate in the yeast Saccharomyces cerevisiae. J Bacteriol 181: 1458–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt K, Zweytick D, Jandrositz A, Kohlwein SD, Daum G 1999b. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J Bacteriol 181: 6441–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmessahel Y, Guennoun R, Cadepond F, Baulieu EE, Schumacher M, Groyer G 2002. Expression of steroidogenic acute regulatory protein in cultured Schwann cells and its regulation by cAMP. Ann NY Acad Sci 973: 83–87 [DOI] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D 2004. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner R, Bürgermeister M, Schneiter R, Daum G 2001. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol Biol Cell 12: 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner R, Nebauer R, Schneiter R, Daum G 2003. Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine biosynthetic machinery with the prohibitin complex of Saccharomyces cerevisiae. Mol Biol Cell 14: 370–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M, Mileykovskaya E, Dowhan W 2008a. Lipids in the assembly of membrane proteins and organization of protein supercomplexes: Implications for lipid-linked disorders. Subcell Biochem 49: 197–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M, Xie J, Heacock P, Dowhan W 2008b. To flip or not to flip: Lipid-protein charge interactions are a determinant of final membrane protein topology. J Cell Biol 182: 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumann HA, de Kruijff B, Heck AJR, de Kroon AIPM 2004. The selective utilization of substrates in vivo by the phosphatidylethanolamine and phosphatidylcholine biosynthetic enzymes Ept1p and Cpt1p in yeast. FEBS Lett 569: 173–177 [DOI] [PubMed] [Google Scholar]
- Breslow DK, Weissman JS 2010. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol Cell 40: 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Pilquil C, Sariahmetoglu M, Reue K 2009. Phosphatidate degradation: Phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim Biophys Acta 1791: 956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 2009. Cholesterol feedback: From Schoenheimer’s bottle to Scap’s MELADL. J Lipid Res 50 (Suppl): S15–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgermeister M, Birner-Grünberger R, Nebauer R, Daum G 2004. Contribution of different pathways to the supply of phosphatidylethanolamine and phosphatidylcholine to mitochondrial membranes of the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1686: 161–168 [DOI] [PubMed] [Google Scholar]
- Butler MM, Thompson W 1975. Transfer of phosphatidylserine from liposomes or microsomes to mitochondria. Stimulation by a cell supernatant factor. Biochim Biophys Acta 388: 52–57 [DOI] [PubMed] [Google Scholar]
- Campbell AM, Chan SHP 2007. The voltage dependent anion channel affects mitochondrial cholesterol distribution and function. Arch Biochem Biophys 466: 203–210 [DOI] [PubMed] [Google Scholar]
- Cao J, Shan D, Revett T, Li D, Wu L, Liu W, Tobin JF, Gimeno RE 2008. Molecular identification of a novel mammalian brain isoform of acyl-CoA:lysophospholipid acyltransferase with prominent ethanolamine lysophospholipid acylating activity, LPEAT2. J Biol Chem 283: 19049–19057 [DOI] [PubMed] [Google Scholar]
- Carman GM, Han G-S 2006. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci 31: 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman GM, Han G-S 2009. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J Biol Chem 284: 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MP, Jiménez-López JM, Martinez-Dueñas L, Ubiña S, Segovia JL, Marco C 2006. Ethanol specifically alters the synthesis, acylation and transbilayer movement of aminophospholipids in rat-liver microsomes. Life Sci 78: 2781–2786 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S, Deffenbaugh AE, Ford DA, Bailly E, Mathias N, Skowyra D 2006. Destabilization of binding to cofactors and SCFMet30 is the rate-limiting regulatory step in degradation of polyubiquitinated Met4. Mol Cell 24: 689–699 [DOI] [PubMed] [Google Scholar]
- Chang SC, Heacock PN, Clancey CJ, Dowhan W 1998a. The PEL1 gene (renamed PGS1) encodes the phosphatidylglycerophosphate synthase of Saccharomyces cerevisiae. J Biol Chem 273: 9829–9836 [DOI] [PubMed] [Google Scholar]
- Chang SC, Heacock PN, Mileykovskaya E, Voelker DR, Dowhan W 1998b. Isolation and characterization of the gene (CLS1) encoding cardiolipin synthase in Saccharomyces cerevisiae. J Biol Chem 273: 14933–14941 [DOI] [PubMed] [Google Scholar]
- Charman M, Kennedy BE, Osborne N, Karten B 2010. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein. J Lipid Res 51: 1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhang X-Y, Shi Y 2006. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem J 398: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM 2005. Membrane hemifusion: Crossing a chasm in two leaps. Cell 123: 375–382 [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM 2008. Mechanics of membrane fusion. Nat Struct Mol Biol 15: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Zimmerberg J, Kozlov MM 2006. Membranes of the world unite! J Cell Biol 175: 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR 2012. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148: 988–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-Y, Wu W-I, Voelker DR 2005. Phosphatidylserine decarboxylases as genetic and biochemical tools for studying phospholipid traffic. Anal Biochem 347: 165–175 [DOI] [PubMed] [Google Scholar]
- Choi J-Y, Augagneur Y, Ben MC, Voelker DR 2012. Identification of gene encoding Plasmodium knowlesi phosphatidylserine decarboxylase by genetic complementation in yeast and characterization of in vitro maturation of encoded enzyme. J Biol Chem 287: 222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269: 28314–28322 [PubMed] [Google Scholar]
- Cowan AK 2009. Plant growth promotion by 18:0-lyso-phosphatidylethanolamine involves senescence delay. Plant Signal Behav 4: 324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Renken C, Várnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnóczky G 2006. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G 1985. Lipids of mitochondria. Biochim Biophys Acta 822: 1–42 [DOI] [PubMed] [Google Scholar]
- Daum G, Vance JE 1997. Import of lipids into mitochondria. Prog Lipid Res 36: 103–130 [DOI] [PubMed] [Google Scholar]
- Davidson JB, Stanacev NZ 1971. Biosynthesis of cardiolipin in mitochondria isolated from guinea pig liver. Biochem Biophys Res Commun 42: 1191–1199 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L 2010. An intimate liaison: Spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J 29: 2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Hebb DM, Richards KD, Lin JM, Ryan PR, Gardner RC 1999. Cloning and expression of a wheat (Triticum aestivum L.) phosphatidylserine synthase cDNA. Overexpression in plants alters the composition of phospholipids. J Biol Chem 274: 7082–7088 [DOI] [PubMed] [Google Scholar]
- Deng L, Fukuda R, Kakihara T, Narita K, Ohta A 2010. Incorporation and remodeling of phosphatidylethanolamine containing short acyl residues in yeast. Biochim Biophys Acta 1801: 635–645 [DOI] [PubMed] [Google Scholar]
- Dolis D, de Kroon AI, de Kruijff B 1996. Transmembrane movement of phosphatidylcholine in mitochondrial outer membrane vesicles. J Biol Chem 271: 11879–11883 [DOI] [PubMed] [Google Scholar]
- Dolman NJ, Gerasimenko JV, Gerasimenko OV, Voronina SG, Petersen OH, Tepikin AV 2005. Stable Golgi-mitochondria complexes and formation of Golgi Ca2+ gradients in pancreatic acinar cells. J Biol Chem 280: 15794–15799 [DOI] [PubMed] [Google Scholar]
- Eilers M, Endo T, Schatz G 1989. Adriamycin, a drug interacting with acidic phospholipids, blocks import of precursor proteins by isolated yeast mitochondria. J Biol Chem 264: 2945–2950 [PubMed] [Google Scholar]
- Elbaz Y, Schuldiner M 2011. Staying in touch: The molecular era of organelle contact sites. Trends Biochem Sci 36: 616–623 [DOI] [PubMed] [Google Scholar]
- Emoto K, Kuge O, Nishijima M, Umeda M 1999. Isolation of a Chinese hamster ovary cell mutant defective in intramitochondrial transport of phosphatidylserine. Proc Natl Acad Sci 96: 12400–12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM 2004. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J Cell Biol 167: 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick RL, Okamoto K, Shaw JM 2008. Multiple pathways influence mitochondrial inheritance in budding yeast. Genetics 178: 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK 2010. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol 190: 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK 2011. ER tubules mark sites of mitochondrial division. Science 334: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH 2006. Intracellular trafficking of sphingolipids: Relationship to biosynthesis. Biochim Biophys Acta 1758: 1885–1892 [DOI] [PubMed] [Google Scholar]
- Gaigg B, Simbeni R, Hrastnik C, Paltauf F, Daum G 1994. Characterization of a microsomal subfraction associated with mitochondria of the yeast, Saccharomyces cerevisiae. Involvement in synthesis and import of phospholipids into mitochondria. Biochim Biophys Acta 1234: 214–220 [DOI] [PubMed] [Google Scholar]
- Gardocki ME, Jani N, Lopes JM 2005. Phosphatidylinositol biosynthesis: Biochemistry and regulation. Biochim Biophys Acta 1735: 89–100 [DOI] [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, et al. 2009. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: Implications for Barth syndrome. Curr Biol 19: 2133–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini F, Smith TK 2010. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62: 414–428 [DOI] [PubMed] [Google Scholar]
- Gibellini F, Hunter WN, Smith TK 2009. The ethanolamine branch of the Kennedy pathway is essential in the bloodstream form of Trypanosoma brucei. Mol Microbiol 73: 826–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil VM, Greenberg ML 2009. Mitochondrial membrane biogenesis: Phospholipids and proteins go hand in hand. J Cell Biol 184: 469–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil VM, Thompson MN, Greenberg ML 2005. Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine and cardiolipin biosynthetic pathways in Saccharomyces cerevisiae. J Biol Chem 280: 35410–35416 [DOI] [PubMed] [Google Scholar]
- Gulshan K, Schmidt JA, Shahi P, Moye-Rowley WS 2008. Evidence for the bifunctional nature of mitochondrial phosphatidylserine decarboxylase: Role in Pdr3-dependent retrograde regulation of PDR5 expression. Mol Cell Biol 28: 5851–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner M, Körner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F, Neupert W 2011. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J 30: 4356–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TE, Finck BN 2011. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab 22: 226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauet T, Liu J, Li H, Gazouli M, Culty M, Papadopoulos V 2002. PBR, StAR, and PKA: Partners in cholesterol transport in steroidogenic cells. Endocr Res 28: 395–401 [DOI] [PubMed] [Google Scholar]
- Hauet T, Yao Z-X, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V 2005. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol 19: 540–554 [DOI] [PubMed] [Google Scholar]
- Heikinheimo L, Somerharju P 1998. Preferential decarboxylation of hydrophilic phosphatidylserine species in cultured cells. Implications on the mechanism of transport to mitochondria and cellular aminophospholipid species compositions. J Biol Chem 273: 3327–3335 [DOI] [PubMed] [Google Scholar]
- Henneberry AL, McMaster CR 1999. Cloning and expression of a human choline/ethanolaminephosphotransferase: Synthesis of phosphatidylcholine and phosphatidylethanolamine. Biochem J 339(Pt 2): 291–298 [PMC free article] [PubMed] [Google Scholar]
- Hermansson M, Hokynar K, Somerharju P 2011. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog Lipid Res 50: 240–257 [DOI] [PubMed] [Google Scholar]
- Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T 2008. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc Natl Acad Sci 105: 2830–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Levine TP 2005. Lipid traffic: Floppy drives and a superhighway. Nat Rev Mol Cell Biol 6: 209–220 [DOI] [PubMed] [Google Scholar]
- Hong JH, Chung G, Cowan AK 2009. Delayed leaf senescence by exogenous lyso-phosphatidylethanolamine: Towards a mechanism of action. Plant Physiol Biochem 47: 526–534 [DOI] [PubMed] [Google Scholar]
- Hoppins S, Collins SR, Cassidy-Stone A, Hummel E, Devay RM, Lackner LL, Westermann B, Schuldiner M, Weissman JS, Nunnari J 2011. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J Cell Biol 195: 323–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath SE, Böttinger L, Vögtle F-N, Wiedeman N, Meisinger C, Becker T, Daum G 2012. Processing and topology of the yeast mitochondrial phosphatidylserine decarboxylase 1. J Biol Chem 287: 36744–36755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Akbari H, van Lenthe H, Kulik W, Wanders RJA, Frentzen M, Vaz FM 2006. Identification and characterization of human cardiolipin synthase. FEBS Lett 580: 3059–3064 [DOI] [PubMed] [Google Scholar]
- Hovius R, Faber B, Brigot B, Nicolay K, de Kruijff B 1992. On the mechanism of the mitochondrial decarboxylation of phosphatidylserine. J Biol Chem 267: 16790–16795 [PubMed] [Google Scholar]
- Ikonen E 2008. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 9: 125–138 [DOI] [PubMed] [Google Scholar]
- Jacquier N, Schneiter R 2012. Mechanisms of sterol uptake and transport in yeast. J Steroid Biochem Mol Biol 129: 70–78 [DOI] [PubMed] [Google Scholar]
- Janssen MJ, Koorengevel MC, de Kruijff B, de Kroon AI 1999. Transbilayer movement of phosphatidylcholine in the mitochondrial outer membrane of Saccharomyces cerevisiae is rapid and bidirectional. Biochim Biophys Acta 1421: 64–76 [DOI] [PubMed] [Google Scholar]
- Jiang F, Rizavi HS, Greenberg ML 1997. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol Microbiol 26: 481–491 [DOI] [PubMed] [Google Scholar]
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML 2000. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem 275: 22387–22394 [DOI] [PubMed] [Google Scholar]
- Joshi AS, Zhou J, Gohil VM, Chen S, Greenberg ML 2009. Cellular functions of cardiolipin in yeast. Biochim Biophys Acta 1793: 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AS, Thompson MN, Fei N, Hüttemann M, Greenberg ML 2012. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J Biol Chem 287: 17589–17597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer J, Kennedy EP 1964. Metabolism and function of bacterial lipids. II. Biosynthesis of phospholipids in escherichia coli. J Biol Chem 239: 1720–1726 [PubMed] [Google Scholar]
- Katayama K, Sakurai I, Wada H 2004. Identification of an Arabidopsis thaliana gene for cardiolipin synthase located in mitochondria. FEBS Lett 577: 193–198 [DOI] [PubMed] [Google Scholar]
- Kelley MJ, Carman GM 1987. Purification and characterization of CDP-diacylglycerol synthase from Saccharomyces cerevisiae. J Biol Chem 262: 14563–14570 [PubMed] [Google Scholar]
- Kennedy EP 1956. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J Biol Chem 222: 185–191 [PubMed] [Google Scholar]
- Kennedy EP, Weiss SB 1956. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem 222: 193–214 [PubMed] [Google Scholar]
- Kevala JH, Kim HY 2001. Determination of substrate preference in phosphatidylserine decarboxylation by liquid chromatography-electrospray ionization mass spectrometry. Anal Biochem 292: 130–138 [DOI] [PubMed] [Google Scholar]
- King SR, Stocco DM 1996. ATP and a mitochondrial electrochemical gradient are required for functional activity of the steroidogenic acute regulatory (StAR) protein in isolated mitochondria. Endocr Res 22: 505–514 [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Cowart LA, Matmati N, Vaena de Avalos S, Novgorodov SA, Zeidan YH, Bielawski J, Obeid LM, Hannun YA 2007. Isc1 regulates sphingolipid metabolism in yeast mitochondria. Biochim Biophys Acta 1768: 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagaki H, Cowart LA, Matmati N, Montefusco D, Gandy J, de Avalos SV, Novgorodov SA, Zheng J, Obeid LM, Hannun YA 2009. ISC1-dependent metabolic adaptation reveals an indispensable role for mitochondria in induction of nuclear genes during the diauxic shift in Saccharomyces cerevisiae. J Biol Chem 284: 10818–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaki T, Yamashita S 1987. Yeast phosphatidylethanolamine methylation pathway. Cloning and characterization of two distinct methyltransferase genes. J Biol Chem 262: 15428–15435 [PubMed] [Google Scholar]
- Kohlwein SD, Daum G, Schneiter R, Paltauf F 1996. Phospholipids: Synthesis, sorting, subcellular traffic—the yeast approach. Trends Cell Biol 6: 260–266 [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P 2009. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Osman C, Walter P 2011. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci 108: 14151–14156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, McMahon HT, Chernomordik LV 2010. Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci 35: 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KE, Papadopoulos V 1990. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem 265: 15015–15022 [PubMed] [Google Scholar]
- Kuchler K, Daum G, Paltauf F 1986. Subcellular and submitochondrial localization of phospholipid-synthesizing enzymes in Saccharomyces cerevisiae. J Bacteriol 165: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O, Yamakawa Y, Nishijima M 2001. Enhancement of transport-dependent decarboxylation of phosphatidylserine by S100B protein in permeabilized Chinese hamster ovary cells. J Biol Chem 276: 23700–23706 [DOI] [PubMed] [Google Scholar]
- Lampl M, Leber A, Paltauf F, Daum G 1994. Import of phosphatidylinositol and phosphatidylcholine into mitochondria of the yeast, Saccharomyces cerevisiae. FEBS Lett 356: 1–4 [DOI] [PubMed] [Google Scholar]
- Lands WE 1958. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J Biol Chem 231: 883–888 [PubMed] [Google Scholar]
- Lavieu G, Orci L, Shi L, Geiling M, Ravazzola M, Wieland F, Cosson P, Rothman JE 2010. Induction of cortical endoplasmic reticulum by dimerization of a coatomer-binding peptide anchored to endoplasmic reticulum membranes. Proc Natl Acad Sci 107: 6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber R, Zinser E, Zellnig G, Paltauf F, Daum G 1994. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast 10: 1421–1428 [DOI] [PubMed] [Google Scholar]
- Lebiedzinska M, Szabadkai G, Jones AWE, Duszynski J, Wieckowski MR 2009. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol 41: 1805–1816 [DOI] [PubMed] [Google Scholar]
- Letts VA, Klig LS, Bae-Lee M, Carman GM, Henry SA 1983. Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc Natl Acad Sci 80: 7279–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T, Loewen C 2006. Inter-organelle membrane contact sites: Through a glass, darkly. Curr Opin Cell Biol 18: 371–378 [DOI] [PubMed] [Google Scholar]
- Levy M, Futerman AH 2010. Mammalian ceramide synthases. IUBMB Life 62: 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Agellon LB, Vance DE 2005. Phosphatidylcholine homeostasis and liver failure. J Biol Chem 280: 37798–37802 [DOI] [PubMed] [Google Scholar]
- Liapis A, Chen FW, Davies JP, Wang R, Ioannou YA 2012. MLN64 transport to the late endosome is regulated by binding to 14-3-3 via a non-canonical binding site. PLoS ONE 7: e34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P, Cornell R, Vance DE 1986. The supply of both CDP-choline and diacylglycerol can regulate the rate of phosphatidylcholine synthesis in HeLa cells. Biochem Cell Biol 64: 692–698 [DOI] [PubMed] [Google Scholar]
- Lu B, Xu FY, Jiang YJ, Choy PC, Hatch GM, Grunfeld C, Feingold KR 2006. Cloning and characterization of a cDNA encoding human cardiolipin synthase (hCLS1). J Lipid Res 47: 1140–1145 [DOI] [PubMed] [Google Scholar]
- Malhotra A, Xu Y, Ren M, Schlame M 2009. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim Biophys Acta 1791: 314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan K, Chae HS, Cha JM, Cho SH, Shin SH, Cho BH, Lee WS 2000. Synthesis of phosphatidylserine in carrot cells cultured under carbon-source starvation. Plant Cell Physiol 41: 1143–1148 [DOI] [PubMed] [Google Scholar]
- Mao C, Wadleigh M, Jenkins GM, Hannun YA, Obeid LM 1997. Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J Biol Chem 272: 28690–28694 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kusaka J, Nishibori A, Hara H 2006. Lipid domains in bacterial membranes. Mol Microbiol 61: 1110–1117 [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Wüstner D 2002. Intracellular cholesterol transport. J Clin Invest 110: 891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH 1992. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci 89: 3170–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli C, Losen M, Hammels C, De Vry J, Hesselink MKC, Steinbusch HWM, De Baets MH, Martínez-Martínez P 2010. The ceramide transporter and the Goodpasture antigen binding protein: One protein–one function? J Neurochem 113: 1369–1386 [DOI] [PubMed] [Google Scholar]
- Michel AH, Kornmann B 2012. The ERMES complex and ER-mitochondria connections. Biochem Soc Trans 40: 445–450 [DOI] [PubMed] [Google Scholar]
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V 2011. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol 336: 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Zhang M, Dowhan W 2005. Cardiolipin in energy transducing membranes. Biochemistry Mosc 70: 154–158 [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32: 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Bose HS 2011. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J Lipid Res 52: 2111–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minskoff SA, Greenberg ML 1997. Phosphatidylglycerophosphate synthase from yeast. Biochim Biophys Acta 1348: 187–191 [DOI] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH 2010. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30: 4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Papadopoulos V, Costa E, Krueger KE 1989. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci 86: 9813–9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen TD, Hannun YA, Obeid LM 2012. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J 441: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebauer R, Schuiki I, Kulterer B, Trajanoski Z, Daum G 2007. The phosphatidylethanolamine level of yeast mitochondria is affected by the mitochondrial components Oxa1p and Yme1p. FEBS J 274: 6180–6190 [DOI] [PubMed] [Google Scholar]
- Nerlich A, von Orlow M, Rontein D, Hanson AD, Dörmann P 2007. Deficiency in phosphatidylserine decarboxylase activity in the psd1 psd2 psd3 triple mutant of Arabidopsis affects phosphatidylethanolamine accumulation in mitochondria. Plant Physiol 144: 904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Lewandowska A, Choi J-Y, Markgraf DF, Junker M, Bilgin M, Ejsing CS, Voelker DR, Rapoport TA, Shaw JM 2012. Gem1 and ERMES do not directly affect phosphatidylserine transport from ER to mitochondria or mitochondrial inheritance. Traffic 13: 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa JI, Yamashita S 1981. Characterization of phosphatidylserine synthase from Saccharomyces cerevisiae and a mutant defective in the enzyme. Biochim Biophys Acta 665: 420–426 [DOI] [PubMed] [Google Scholar]
- Nikawa J, Kodaki T, Yamashita S 1987. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem 262: 4876–4881 [PubMed] [Google Scholar]
- Novgorodov SA, Gudz TI 2009. Ceramide and mitochondria in ischemia/reperfusion. J Cardiovasc Pharmacol 53: 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM 2011. Novel pathway of ceramide production in mitochondria: Thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem 286: 25352–25362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki M, Müller F, Frentzen M 2005. Cardiolipin synthase of Arabidopsis thaliana. FEBS Lett 579: 2161–2165 [DOI] [PubMed] [Google Scholar]
- Osman C, Haag M, Potting C, Rodenfels J, Dip PV, Wieland FT, Brügger B, Westermann B, Langer T 2009a. The genetic interactome of prohibitins: Coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol 184: 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Merkwirth C, Langer T 2009b. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 122: 3823–3830 [DOI] [PubMed] [Google Scholar]
- Osman C, Haag M, Wieland FT, Brügger B, Langer T 2010. A mitochondrial phosphatase required for cardiolipin biosynthesis: The PGP phosphatase Gep4. EMBO J 29: 1976–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Voelker DR, Langer T 2011. Making heads or tails of phospholipids in mitochondria. J Cell Biol 192: 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouni I, Flick K, Kaiser P 2010. A transcriptional activator is part of an SCF ubiquitin ligase to control degradation of its cofactors. Mol Cell 40: 954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-López S, Langager D, Chan C-H, Pearce DA 2012. BTN1, the Saccharomyces cerevisiae homolog to the human Batten disease gene, is involved in phospholipid distribution. Dis Model Mech 5: 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Ridgway ND 2005. Molecular mechanisms and regulation of ceramide transport. Biochim Biophys Acta 1734: 220–234 [DOI] [PubMed] [Google Scholar]
- Petrescu AD, Gallegos AM, Okamura Y, Strauss JF 3rd, Schroeder F 2001. Steroidogenic acute regulatory protein binds cholesterol and modulates mitochondrial membrane sterol domain dynamics. J Biol Chem 276: 36970–36982 [DOI] [PubMed] [Google Scholar]
- Potting C, Wilmes C, Engmann T, Osman C, Langer T 2010. Regulation of mitochondrial phospholipids by Ups1/PRELI-like proteins depends on proteolysis and Mdm35. EMBO J 29: 2888–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl R, Soubannier V, Scholz R, Vogel F, Mendl N, Vasiljev-Neumeyer A, Körner C, Jagasia R, Keil T, Baumeister W, et al. 2009. Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J Cell Biol 185: 1047–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway ND, Yao Z, Vance DE 1989. Phosphatidylethanolamine levels and regulation of phosphatidylethanolamine N-methyltransferase. J Biol Chem 264: 1203–1207 [PubMed] [Google Scholar]
- Riekhof WR, Voelker DR 2006. Uptake and utilization of lyso-phosphatidylethanolamine by Saccharomyces cerevisiae. J Biol Chem 281: 36588–36596 [DOI] [PubMed] [Google Scholar]
- Riekhof WR, Wu J, Gijón MA, Zarini S, Murphy RC, Voelker DR 2007a. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: The role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J Biol Chem 282: 36853–36861 [DOI] [PubMed] [Google Scholar]
- Riekhof WR, Wu J, Jones JL, Voelker DR 2007b. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J Biol Chem 282: 28344–28352 [DOI] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V 2009. Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states. Biochim Biophys Acta 1791: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rontein D, Wu W-I, Voelker DR, Hanson AD 2003. Mitochondrial phosphatidylserine decarboxylase from higher plants. Functional complementation in yeast, localization in plants, and overexpression in Arabidopsis. Plant Physiol 132: 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba JD, Nara F, Bielawska A, Garrett S, Hannun YA 1997. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J Biol Chem 272: 26087–26090 [DOI] [PubMed] [Google Scholar]
- Salama NR, Schekman RW 1995. The role of coat proteins in the biosynthesis of secretory proteins. Curr Opin Cell Biol 7: 536–543 [DOI] [PubMed] [Google Scholar]
- Schlame M 2008. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res 49: 1607–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Ren M 2009. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta 1788: 2080–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Brody S, Hostetler KY 1993. Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular acyl species. Eur J Biochem 212: 727–735 [DOI] [PubMed] [Google Scholar]
- Schulz TA, Prinz WA 2007. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta 1771: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MM, Choi J-Y, Voelker DR 2002. Phosphatidylserine transport to the mitochondria is regulated by ubiquitination. J Biol Chem 277: 51033–51042 [DOI] [PubMed] [Google Scholar]
- Serricchio M, Bütikofer P 2011. Trypanosoma brucei: A model microorganism to study eukaryotic phospholipid biosynthesis. FEBS J 278: 1035–1046 [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Lupo G, Vance JE 1995. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem 270: 11190–11198 [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Balcerzak B, Vance JE 1998. A mitochondrial membrane protein is required for translocation of phosphatidylserine from mitochondria-associated membranes to mitochondria. Biochem J 331: 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou H, Hishikawa D, Harayama T, Yuki K, Shimizu T 2009. Recent progress on acyl CoA: Lysophospholipid acyltransferase research. J Lipid Res 50 (Suppl): S46–S51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbeni R, Paltauf F, Daum G 1990. Intramitochondrial transfer of phospholipids in the yeast, Saccharomyces cerevisiae. J Biol Chem 265: 281–285 [PubMed] [Google Scholar]
- Siskind LJ, Colombini M 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem 275: 38640–38644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio RE, Breslow JL 2004. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol 24: 1150–1160 [DOI] [PubMed] [Google Scholar]
- Sorger D, Daum G 2003. Triacylglycerol biosynthesis in yeast. Appl Microbiol Biotechnol 61: 289–299 [DOI] [PubMed] [Google Scholar]
- Sperka-Gottlieb CD, Hermetter A, Paltauf F, Daum G 1988. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 946: 227–234 [DOI] [PubMed] [Google Scholar]
- Stiban J, Tidhar R, Futerman AH 2010. Ceramide synthases: Roles in cell physiology and signaling. Adv Exp Med Biol 688: 60–71 [DOI] [PubMed] [Google Scholar]
- Stocco DM 2000. Intramitochondrial cholesterol transfer. Biochim Biophys Acta 1486: 184–197 [DOI] [PubMed] [Google Scholar]
- Strauss JF 3rd, Kallen CB, Christenson LK, Watari H, Devoto L, Arakane F, Kiriakidou M, Sugawara T 1999. The steroidogenic acute regulatory protein (StAR): A window into the complexities of intracellular cholesterol trafficking. Recent Prog Horm Res 54: 394–395 [PubMed] [Google Scholar]
- Stroud DA, Oeljeklaus S, Wiese S, Bohnert M, Lewandrowski U, Sickmann A, Guiard B, van der Laan M, Warscheid B, Wiedemann N 2011. Composition and topology of the endoplasmic reticulum-mitochondria encounter structure. J Mol Biol 413: 743–750 [DOI] [PubMed] [Google Scholar]
- Sundler R, Akesson B 1975. Regulation of phospholipid biosynthesis in isolated rat hepatocytes. Effect of different substrates. J Biol Chem 250: 3359–3367 [PubMed] [Google Scholar]
- Suzuki M, Muranaka T 2007. Molecular genetics of plant sterol backbone synthesis. Lipids 42: 47–54 [DOI] [PubMed] [Google Scholar]
- Tamura Y, Endo T, Iijima M, Sesaki H 2009. Ups1p and Ups2p antagonistically regulate cardiolipin metabolism in mitochondria. J Cell Biol 185: 1029–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Onguka O, Hobbs AEA, Jensen RE, Iijima M, Claypool SM, Sesaki H 2012. Role for two conserved intermembrane space proteins, Ups1p and Up2p, in intra-mitochondrial phospholipid trafficking. J Biol Chem 287: 15205–15218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter PJ, Voelker DR 1995. Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J Biol Chem 270: 6062–6070 [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Pedretti J, Voelker DR 1993. Phosphatidylserine decarboxylase from Saccharomyces cerevisiae. Isolation of mutants, cloning of the gene, and creation of a null allele. J Biol Chem 268: 21416–21424 [PubMed] [Google Scholar]
- Trotter PJ, Pedretti J, Yates R, Voelker DR 1995. Phosphatidylserine decarboxylase 2 of Saccharomyces cerevisiáe. Cloning and mapping of the gene, heterologous expression, and creation of the null allele. J Biol Chem 270: 6071–6080 [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Wu WI, Pedretti J, Yates R, Voelker DR 1998. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, STT4p, as an essential component in phosphatidylserine metabolism. J Biol Chem 273: 13189–13196 [DOI] [PubMed] [Google Scholar]
- Tuller G, Daum G 1995. Import of sterols into mitochondria of the yeast Saccharomyces cerevisiae. FEBS Lett 372: 29–32 [DOI] [PubMed] [Google Scholar]
- Tuller G, Hrastnik C, Achleitner G, Schiefthaler U, Klein F, Daum G 1998. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS Lett 421: 15–18 [DOI] [PubMed] [Google Scholar]
- Vance JE 1990. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265: 7248–7256 [PubMed] [Google Scholar]
- Vance JE 2008. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J Lipid Res 49: 1377–1387 [DOI] [PubMed] [Google Scholar]
- van den Brink-van der Laan E, Killian JA, de Kruijff B 2004. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666: 275–288 [DOI] [PubMed] [Google Scholar]
- van den Hazel HB, Pichler H, do Valle Matta MA, Leitner E, Goffeau A, Daum G 1999. PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J Biol Chem 274: 1934–1941 [DOI] [PubMed] [Google Scholar]
- van der Laan M, Bohnert M, Wiedemann N, Pfanner N 2012. Role of MINOS in mitochondrial membrane architecture and biogenesis. Trends Cell Biol 22: 185–192 [DOI] [PubMed] [Google Scholar]
- van der Veen JN, Lingrell S, Vance DE 2012. The membrane lipid, phosphatidylcholine, is an unexpected source of triacylglycerol in the liver. J Biol Chem 287: 23418–23426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Hoetzl S 2010. Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS Lett 584: 1800–1805 [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW 2008. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial HJ, Thuet MJ, Philippot JR 1982. Phospholipid biosynthesis in synchronous Plasmodium falciparum cultures. J Protozool 29: 258–263 [DOI] [PubMed] [Google Scholar]
- Voelker DR 1984. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci 81: 2669–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker DR 1985. Disruption of phosphatidylserine translocation to the mitochondria in baby hamster kidney cells. J Biol Chem 260: 14671–14676 [PubMed] [Google Scholar]
- Voelker DR 1988. Phosphatidylserine translocation in animal cells. Prog Clin Biol Res 282: 153–164 [PubMed] [Google Scholar]
- Voelker DR 1989a. Phosphatidylserine translocation to the mitochondrion is an ATP-dependent process in permeabilized animal cells. Proc Natl Acad Sci 86: 9921–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker DR 1989b. Reconstitution of phosphatidylserine import into rat liver mitochondria. J Biol Chem 264: 8019–8025 [PubMed] [Google Scholar]
- Voelker DR 1990. Characterization of phosphatidylserine synthesis and translocation in permeabilized animal cells. J Biol Chem 265: 14340–14346 [PubMed] [Google Scholar]
- Voelker DR 1991. Adriamycin disrupts phosphatidylserine import into the mitochondria of permeabilized CHO-K1 cells. J Biol Chem 266: 12185–12188 [PubMed] [Google Scholar]
- Voelker DR 1992. Phosphatidylserine synthesis and transport to mitochondria in permeabilized animal cells. Methods Enzymol 209: 530–534 [DOI] [PubMed] [Google Scholar]
- Voelker DR 1993. The ATP-dependent translocation of phosphatidylserine to the mitochondria is a process that is restricted to the autologous organelle. J Biol Chem 268: 7069–7074 [PubMed] [Google Scholar]
- Voelker DR 2005. Bridging gaps in phospholipid transport. Trends Biochem Sci 30: 396–404 [DOI] [PubMed] [Google Scholar]
- Voelker DR 2009. Genetic and biochemical analysis of non-vesicular lipid traffic. Annu Rev Biochem 78: 827–856 [DOI] [PubMed] [Google Scholar]
- Voelker DR, Frazier JL 1986. Isolation and characterization of a Chinese hamster ovary cell line requiring ethanolamine or phosphatidylserine for growth and exhibiting defective phosphatidylserine synthase activity. J Biol Chem 261: 1002–1008 [PubMed] [Google Scholar]
- von der Malsburg K, Müller JM, Bohnert M, Oeljeklaus S, Kwiatkowska P, Becker T, Loniewska-Lwowska A, Wiese S, Rao S, Milenkovic D, et al. 2011. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev Cell 21: 694–707 [DOI] [PubMed] [Google Scholar]
- Wagner A, Daum G 2005. Formation and mobilization of neutral lipids in the yeast Saccharomyces cerevisiae. Biochem Soc Trans 33: 1174–1177 [DOI] [PubMed] [Google Scholar]
- Wriessnegger T, Leitner E, Belegratis MR, Ingolic E, Daum G 2009. Lipid analysis of mitochondrial membranes from the yeast Pichia pastoris. Biochim Biophys Acta 1791: 166–172 [DOI] [PubMed] [Google Scholar]
- Wu WI, Voelker DR 2001. Characterization of phosphatidylserine transport to the locus of phosphatidylserine decarboxylase 2 in permeabilized yeast. J Biol Chem 276: 7114–7121 [DOI] [PubMed] [Google Scholar]
- Wu W-I, Voelker DR 2004. Reconstitution of phosphatidylserine transport from chemically defined donor membranes to phosphatidylserine decarboxylase 2 implicates specific lipid domains in the process. J Biol Chem 279: 6635–6642 [DOI] [PubMed] [Google Scholar]
- Wu WI, Routt S, Bankaitis VA, Voelker DR 2000. A new gene involved in the transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/phosphatidylcholine transfer protein, SEC14. J Biol Chem 275: 14446–14456 [DOI] [PubMed] [Google Scholar]
- Xu Y, Malhotra A, Ren M, Schlame M 2006. The enzymatic function of tafazzin. J Biol Chem 281: 39217–39224 [DOI] [PubMed] [Google Scholar]
- Yan J, Xiong Y 2010. Targeted ubiquitylation: The prey becomes predator. Mol Cell 40: 853–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu P, Dwyer NK, Christenson LK, Fujimoto T, Martinez F, Comly M, Hanover JA, Blanchette-Mackie EJ, Strauss JF 3rd 2002. MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J Biol Chem 277: 33300–33310 [DOI] [PubMed] [Google Scholar]
- Zinser E, Daum G 1995. Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast 11: 493–536 [DOI] [PubMed] [Google Scholar]
- Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G 1991. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173: 2026–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]