Abstract
Protein-protein interactions lie at the heart of cellular signaling pathways and the deregulation of which has frequently led to diseases. In contrast to inhibitors that bind to distinctive enzyme active sites, molecules targeting protein surface topologies have been underexploited in drug development. The challenges in developing protein surface antagonists or agonists originate from the relatively large and flat surface areas that lack well-defined cavities required for sufficient binding affinity. In the past decade, our understanding of protein recognition has served as solid basis for the design of synthetic mimetics to modulate these protein-protein interactions. Herein, we summarize recent successes in the development of synthetic α-helix mimetics, proteomimetics, and biologics with the therapeutic potentials of inhibiting tumourgenesis or cancer-related viral infections.
Introduction
Rapid advancements in cancer and virological research have led to the discovery of numerous key processes in tumourgenesis and viral infections with great potential for therapeutic intervention. The rationale behind traditional drug designs was to target known and well-defined active or allosteric sites within the biomolecules. In many instances, small-molecule drugs are inefficient at disrupting the association of large interface between the protein-protein interactions, because they may lack the dimension and valency needed for the projection of functionalities to complement such domains on the protein surfaces. Moreover, the inhibitory effects of these small-molecule drugs could be overcome by a single mutation of the amino acid residue within the target. Synthetic topological mimetics that bind to the protein surface or protein-protein interacting domain offer the possibility of achieving large domain recognition within the targets that were previously deemed “undruggable” by the traditional approach.
In this brief review, we first describe recent developments of synthetic mimetics of α-helices, proteomimetics, and biologics as novel strategies to selectively modulate protein-protein interactions through protein surface recognition that have been difficult to target with small molecules. We then present the molecular basis for the rationale behind the design and application of these mimetics (Figure 1) in de novo drug development with the focus on the inhibition of tumourgenesis, as well as viral infections that are tightly linked with cancer morbidity, such as the human immunodeficiency virus 1 (HIV-1) and hepatitis C virus (HCV). Finally, we provide updates on the preclinical and clinical studies of the compounds in this review.
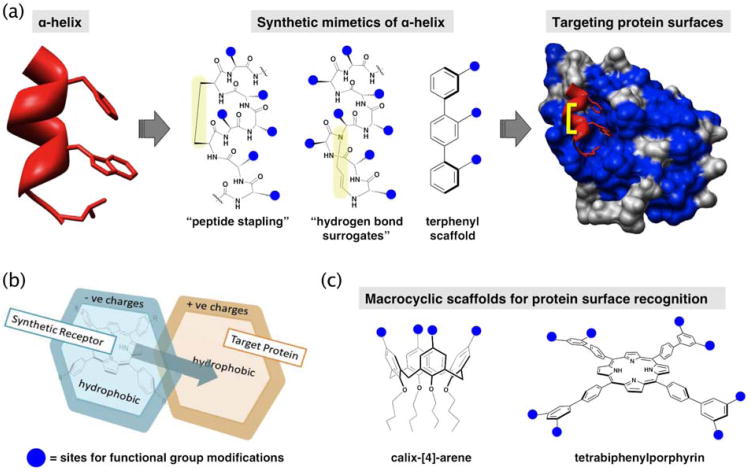
Figure 1.
Strategies and molecular scaffolds of synthetic mimetics for targeting protein surfaces discussed in the text. (a) α-helix mimetic can be synthesized to install peptide “staples” or “hydrogen bond surrogate” for stabilizing and enhancing the rigidity of the mimetic, as well as project functional groups towards the recognition sites. Terphenyl scaffold as synthetic mimetic of the i, i+4, and i+7 positions of an α-helix. (b) Strategy for targeting large protein surface by complementary recognitions of the surface residues. (c) Macrocyclic molecular scaffolds used for antagonists design to disrupt protein-protein interactions.
Synthetic mimetics that target the RTK pathways
Deregulation of receptor tyrosine kinase (RTK) signaling is one of the major hallmarks for cancer cell proliferation. Constitutive propagation of intracellular RTK and overstimulation of Ras signaling are responsible for various cancers. Ras activation is catalyzed by the Ras-specific guanine nucleotide exchange factor Sos. The insertion of αH and αI helices from Sos into the switch regions of Ras is essential for destabilizing the nucleotide-bound state of Ras [1]. At the Ras-Sos interface, αH helix makes direct contact with Ras. Bar-Sagi and coworkers have recently reported the synthesis and evaluation of a cell-permeable orthosteric α-helical mimetic of αH that selectively disrupts the Ras-Sos interaction [2]. Using the hydrogen bond surrogate (HBS) approach [3,4], the optimized α-helix mimetic HBS 3 encodes a ring-closing metathesis reaction to install a covalent carbon-carbon bond to replace a backbone intramolecular hydrogen bond (Figure 1a). This main chain modification allows the preorganization of the scaffold and projects multiple interacting residues, such as Phe929, Glu942, and Asn944 at the Ras-Sos interface. With strong evidence from the Heteronuclear Single Quantum Coherence (HSQC) nuclear magnetic resonance (NMR) experiment, it is shown that HBS 3 act as an αH mimetic by binding to the shallow cleft region of Ras where the native Sos αH helix binds. Moreover, HBS 3 significantly reduces the EGF triggered activation and the levels of downstream ERK phosphorylation. Even though it is still early to judge whether HBS 3 or its related compounds have therapeutic potential, this proof of concept study has firmly provided new chemical design with a innovative molecular scaffold.
Angiogenesis is the main process for sustaining tumor growth and is triggered by several key growth factors (GFs) that are secreted by the tumors [5]. Association of polypeptide GFs such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) with the extracellular domain of RTKs activate intracellular tyrosine kinase and cross phosphorylation of the receptors, ultimately leading to the formation of new blood vessels within the microenvironment of the tumors [5]. In particular, VEGF plays an essential role in the initiation of formation of new blood vessels, while PDGF is involved in maintaining vessel integrity.
As a novel molecular design and building on the first generation of molecules that target the surface topology of PDGF (Figure 1b) [6], Sebti and coworkers have reported a focused library of calix-[4]-arene based inhibitors (Figure 1c) for blocking the bindings of PDGF and VEGF to their respective cell surface receptors [7]. The macrocyclic scaffold contains alkylether modification at the lower rim for maintaining a cone conformation to project the functionalities at the upper rim of the antagonist for targeting the surface residues of the GFs that are important for binding to their receptor. Among them, GFB-204 is the first dual inhibitor that antagonizes both PDGF- and VEGF-stimulated receptor tyrosine phosphorylation with IC50 values of 190nM and 480nM, respectively. GFB-204 selectively disrupts PDGF and VEGF but not EGF, bFGF or IGF-1 stimulation of Erk1, Erk2, Akt and STAT3 phosphorylation [7]. Moreover, GFB-204 inhibits capillary network formation in vitro and its antiangiogenic effect can markedly suppress the growth of several types of human tumors in nude mice at the daily dosage of 5mg/kg. Currently, GFB-204 is in the preclinical development stage by Tigris pharmaceutical.
Synthetic mimetics as modulators of protein-protein interactions of apoptosis pathways
Another hallmark of cancer cell survival is the evasion of apoptosis, which is commonly due to the overexpression of multiple anti-apoptotic Bcl-2 family proteins [8]. These anti-apoptotic proteins such as Bcl-2, Bcl-XL, and Mcl-1 can bind to the Bcl-2 homology (BH) α-helical domains of pro-apoptotic proteins such as Bax, Bak, Bad and Bim and inhibit their function. This observation prompted the development of molecular scaffolds to mimic the pro-apoptotic BH3 domain and target them to inhibit the anti-apoptotic Bcl-2 proteins in order to facilitate apoptosis [9]. As obtaining potent small molecule inhibitors to block such protein-protein interaction was difficult, Korsmeyer and coworkers devised an elegant chemical hydrocarbon “stapling” strategy to generate stabilized alpha-helix of Bcl-2 domains (SAHBs) helical mimetics that bound with increased affinity to multidomain Bcl-2 member pockets [10]. “Peptide stapling” is a strategy to stabilize α-helices by incorporating a ring-closing metathesis reaction to make a carbon-carbon bond “staple” between the turns of the α-helix to optimize helicity while preserving selectivity with increased protease resistance and cell-permeability (Figure 1a) [9,11]. Notably, a SAHB helical mimetic derived from BH3 domain of the Bid protein was shown to specifically activate the apoptotic pathway and effectively inhibit the growth of human leukemia xenografts in vivo[10]. More recently, building on the prototype of “peptide stapling” strategy, Walensky and coworkers have designed a Bim SAHB that associates with Bax at a novel interaction site that is distinct from its canonical binding site, which leads to the direct activation of apoptosis [12].
Another example is the discovery of ABT-737 by using NMR-based screening and structure-based design on the linkage of proximal fragments to achieve high binding affinity (Ki ≤ 1 nM) to anti-apoptotic proteins Bcl-2, Bcl-XL and Bcl-ω [13,14]. To prevent cell survival triggered by the Bcl-2 family, ABT-737 does not directly activate the pro-apoptotic proteins Bax and Bak. Moreover, ABT-737 and related compounds display synergism with chemotherapeutics and radiation, such as enhanced cytotoxicity with paclitaxel against A549 NSCLC (non small-cell lung carcinoma) cells by a factor of four. Similarly, ABT-737 triggered dose-dependent (10-100 nM) apoptosis in primary patient-derived chronic lymphocytic leukemia. Preclinical studies of ABT-737 demonstrated efficacy against leukemia and lymphoma, as well as small-cell lung cancer (SCLC). Other types of tumors, however, proved to be more responsive to the combination of ABT-737 with other suppressors of Mcl-1 expression than to ABT-737 monotherapy. An orally available derivative of ABT-737, ABT-263 [15], is currently under studies for the treatment of chronic lymphocytic leukemia, non-Hodgkin lymphoma (NHL) and SCLC [14,16].
ABT-737 lacked potent affinity in binding to Mcl-1 [13], which has emerged as a major chemoresistance factor in many human cancers. From a screen of a library of SAHBs, Stewart et al. has recently identified a Mcl-1 BH3 helix mimetic as a potent and exclusive Mcl-1 inhibitor [17]. Mcl-1 SAHB specifically targets Mcl-1 and antagonizes the inhibitory interaction of pro-apoptotic Bak for the promotion of downstream caspase-dependent apoptosis. On the other hand, Sebti and coworkers had also reported the evaluation of BH3-M6, a spatial and functional BH3 α-helical mimetic based on a synthetic terphenyl scaffold (Figure 1a), to be a “pan-Bcl-2” inhibitor that blocks Bcl-2, Bcl-XL and Mcl-1 binding to Bax, Bak, Bad and Bim [18]. Disruption of these protein-protein interactions results in the release of cytochrome c, which in turn leads to the activation of downstream caspases and Bax- and Bim-dependent apoptosis in human cancer cells.
Death receptor agonists
Tumor necrosis factor - related apoptosis-inducing ligand (TRAIL, also known as Apo2L or TNFSF10) is a type II transmembrane protein which is a member of the TNF family of death ligands [19]. TRAIL interacts with specific death receptors - TRAIL-R1 and TRAIL-R2 – to form the death-inducing signaling complex (DISC), which then recruits and activates the caspase cascades with the help of an intracellular adaptor, Fas-associated death domain (FADD) [20,21]. TRAIL-induced apoptosis occurs preferentially in various types of tumor cells while leaving normal cells unharmed [21]. In recent years, TRAIL receptor agonists have emerged as promising therapeutic strategies to selectively promote the apoptosis of tumor cells by activating the extrinsic pathway. TRAIL agonists have demonstrated in vitro and in vivo activity against multiple cell lines derived from colon and lung tumors, NHL, and multiple myeloma. Selected agonists in the form of recombinant, humanized, or fully human antibodies have been advanced into clinical development either as monotherapy or in combination [22-24]. Considerable crosstalk between the extrinsic and intrinsic death signaling pathways may explain the synergistic effect observed between TRAIL agonists and conventional chemotherapeutics in preclinical models. Amongst the death receptor agonists that are furthest advanced into clinical trials, mapatumumab and lexatumumab are fully human monoclonal antibodies that target TRAIL-R1 and TRAIL-R2 respectively to promote apoptosis. Interestingly, Lapatinib – a known EGFR- and HER2-targeting agent that was approved for treating metastatic breast cancer – has recently been shown to sensitize colon cancer cells through off-target upregulation of TRAIL-R2 [25].
Novel macrocyclics and synthetic mimetics as antiviral inhibitors
HCV infection is a devastating epidemic that afflicts approximately 200 million individuals worldwide, with 3-4 million new cases per year only to be exacerbated by the absence of vaccination and effective therapies. Chronically infected patients are often at risk of developing liver cirrhosis and/or hepatocellular carcinoma (HCC), and HCV-associated HCC mortality rate is on the rise globally [26]. In order to address the pressing medical need for more effective and better-tolerated therapies, a number of innovative agents that target specific HCV macromolecules are being developed. Most recently, the FDA approved the first-in-class inhibitors of the HCV NS3·4A serine protease – telaprevir and boceprevir [27]. Future combinations of different classes of anti-HCV drugs will be necessary to gradually shift away from the current interferon-centered regimens. Previously, Cheng et al. reported the discovery of a novel class of tetrabiphenylporphyrins as potent HCV inhibitors (Figure 1c). Designed as a potential protein surface binder (Figure 1b), the most potent inhibitor – meso-tetrakis-(3,5-dicarboxy-4,4′-biphenyl) porphyrin has an EC50 of 24 nM against the HCV genotype 1b replicons. Notably, this compound demonstrated the potential of preventing viral rebound and achieved an additive to synergistic effect when combined with IFN-α or HCV protease inhibitor BILN-2061 [28]. Moreover, this porphyrin derivative could directly inhibit the HCV NS3·4A protease in a noncompetitive manner, and may possibly disrupt the assembly of the HCV replication complex (manuscript under review). These proof-of-concept studies revealed the therapeutic potentials of tetrabiphenylporphyrins as potent disruptors of the crucial protein-protein interactions involved in viral pathogenesis.
Breakthroughs in the development of anti-HIV mimetics are best exemplified in the recent design of HIV fusion inhibitors. Gellman and coworkers have devised a strategy to combine alpha- and unnatural cyclic beta-amino acid residues to create “foldamers” that mimic the structural and functional properties of gp41 C-heptad repeat (CHR) domain [29]. Through protein surface association to the helix bundle, the gp41-mimetic alpha/beta-peptides were shown to effectively block HIV-cell fusion and prevent HIV infection similar to the action of natural gp41-derived alpha-peptides. Furthermore, this mimetic turned out to be less susceptible to proteolytic degradation with enhanced site-specific backbone rigidity owing to the beta-amino acid replacements. On the other hand, Arora and coworkers, using the hydrogen bond surrogate design, have also synthesized an artificial α helix mimetic of the N-terminal domain of gp41 to inhibit gp41-mediated cell fusion [30]. Lastly, “peptide stapling” mimetics were also exploited for the design of potent HIV-fusion inhibitors by Walensky and coworkers [31].
Meanwhile, Tsou et. al. have identified a novel anti-HIV and anti-HCV compound based on a tetrabutoxy-calix[4]arene scaffold (Figure 1c) [32]. Structure-activity relationship studies demonstrated that the aromatic isophthalate spacers at the upper rim are essential to the anti-HIV activities of the compounds, whereas the projected diacid groups are necessary for exerting anti-HCV effects. Moreover, maintaining the cone conformation of the tetrabutoxy-calix[4]arene scaffold was shown to be crucial to the activity against both viruses. The inhibitor also retains potency against different HIV strains in different cell lines while maintaining low cytotoxicity and was shown to have no effect on the replication of herpes simplex virus-1 (HSV-1), HSV-2, and hepatitis B virus (HBV). Further structural studies revealed that the inhibitor could bind to HIV-1 gp120 and block viral entry [33].
Conclusion
Molecular recognition with the aim of targeting protein surfaces has become an increasingly important field over the past decade owing to the potential of modulating protein-protein interactions that are key to cellular functions. At present, about 70% of the approved small-molecule drugs are targeting enzyme active sites or G-protein coupled receptors [34]. Despite the advantages in drug delivery options over some synthetic mimetics, small-molecule compounds often lack the therapeutic advantage due to rapid emergence of resistance. In contrast, synthetic topological mimetics not only offer multi-residue interactions with target proteins to improve selectivity but also may raise the genetic barrier for the emergence of high-level resistance. Although the selective disruption of protein-protein complexes through topological recognition by synthetic mimetics is still an underexplored area in medicinal chemistry, advances in structural biology, nanoparticle-aided drug delivery, and functional proteomics will undoubtedly bring protein surface recognizing agents to the frontline of drug discovery.
Highlights.
Synthetic “hydrogen bond surrogate” as alpha helix mimetics targeting Ras-Sos interaction.
The specific Stabilized Alpha-Helix of Bcl-2 domains (SAHBs) helical mimetic targets chemoresistance factor Mcl-1.
Design of “foldamer” with alpha- and unnatural cyclic beta-amino acid backbone modification as a HIV fusion inhibitor.
Synthetic calix-[4]-arene derivatives are blockers of PDGF and VEGF binding to their extracellular receptors.
A novel class of tetrabiphenylporphyrins as potent HCV inhibitors.
Acknowledgments
Y-C.C. thanks NCI PO1CA154295-01A1 for funding. Y-C.C. is a fellow of the National Foundation for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394(6691):337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 2.Patgiri A, Yadav KK, Arora PS, Bar-Sagi D. An orthosteric inhibitor of the Ras-Sos interaction. Nat Chem Biol. 2011;7(9):585–587. doi: 10.1038/nchembio.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patgiri A, Jochim AL, Arora PS. A hydrogen bond surrogate approach for stabilization of short peptide sequences in alpha-helical conformation. Acc Chem Res. 2008;41(10):1289–1300. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henchey LK, Jochim AL, Arora PS. Contemporary strategies for the stabilization of peptides in the alpha-helical conformation. Curr Opin Chem Biol. 2008;12(6):692–697. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 6.Blaskovich MA, Lin Q, Delarue FL, Sun J, Park HS, Coppola D, Hamilton AD, Sebti SM. Design of GFB-111, a platelet-derived growth factor binding molecule with antiangiogenic and anticancer activity against human tumors in mice. Nat Biotechnol. 2000;18(10):1065–1070. doi: 10.1038/80257. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Wang DA, Jain RK, Carie A, Paquette S, Ennis E, Blaskovich MA, Baldini L, Coppola D, Hamilton AD, Sebti SM. Inhibiting angiogenesis and tumorigenesis by a synthetic molecule that blocks binding of both VEGF and PDGF to their receptors. Oncogene. 2005;24(29):4701–4709. doi: 10.1038/sj.onc.1208391. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3(6):614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 9.Verdine GL, Walensky LD. The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL-2 family members. Clin Cancer Res. 2007;13(24):7264–7270. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 10.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305(5689):1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird GH, Bernal F, Pitter K, Walensky LD. Synthesis and biophysical characterization of stabilized alpha-helices of BCL-2 domains. Methods Enzymol. 2008;446:369–386. doi: 10.1016/S0076-6879(08)01622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 16.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115(16):3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6(8):595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, Rodriguez JM, Becerril J, Berndt N, Hamilton AD, Wang HG, et al. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J Biol Chem. 2011;286(11):9382–9392. doi: 10.1074/jbc.M110.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277(5327):818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature reviews Cancer. 2002;2(6):420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 22.Fox NL, Humphreys R, Luster TA, Klein J, Gallant G. Tumor Necrosis Factor-related apoptosis-inducing ligand (TRAIL) Receptor-1 and Receptor-2 agonists for cancer therapy. Expert opinion on biological therapy. 2010;10(1):1–18. doi: 10.1517/14712590903319656. [DOI] [PubMed] [Google Scholar]
- 23.Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis: an international journal on programmed cell death. 2009;14(4):607–623. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- 24.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(6):1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 25.Dolloff NG, Mayes PA, Hart LS, Dicker DT, Humphreys R, El-Deiry WS. Off-target lapatinib activity sensitizes colon cancer cells through TRAIL death receptor up-regulation. Science translational medicine. 2011;3(86):86ra50. doi: 10.1126/scitranslmed.3001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira AA, Jacobson IM. New and experimental therapies for HCV. Nature reviews Gastroenterology & hepatology. 2009;6(7):403–411. doi: 10.1038/nrgastro.2009.92. [DOI] [PubMed] [Google Scholar]
- 27.Pawlotsky JM. The results of Phase III clinical trials with telaprevir and boceprevir presented at the Liver Meeting 2010: a new standard of care for hepatitis C virus genotype 1 infection, but with issues still pending. Gastroenterology. 2011;140(3):746–754. doi: 10.1053/j.gastro.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Tsou LK, Cai J, Aya T, Dutschman GE, Gullen EA, Grill SP, Chen AP, Lindenbach BD, Hamilton AD, Cheng YC. A novel class of meso-tetrakis-porphyrin derivatives exhibits potent activities against hepatitis C virus genotype 1b replicons in vitro. Antimicrobial agents and chemotherapy. 2010;54(1):197–206. doi: 10.1128/AAC.01206-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, Gellman SH. Structural and biological mimicry of protein surface recognition by alpha/beta-peptide foldamers. Proc Natl Acad Sci U S A. 2009;106(35):14751–14756. doi: 10.1073/pnas.0902663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Lu M, Arora PS. Inhibition of HIV-1 fusion by hydrogen-bond-surrogate-based alpha helices. Angew Chem Int Ed Engl. 2008;47(10):1879–1882. doi: 10.1002/anie.200704227. [DOI] [PubMed] [Google Scholar]
- 31.Bird GH, Madani N, Perry AF, Princiotto AM, Supko JG, He X, Gavathiotis E, Sodroski JG, Walensky LD. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc Natl Acad Sci U S A. 2010;107(32):14093–14098. doi: 10.1073/pnas.1002713107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsou LK, Dutschman GE, Gullen EA, Telpoukhovskaia M, Cheng YC, Hamilton AD. Discovery of a synthetic dual inhibitor of HIV and HCV infection based on a tetrabutoxy-calix[4]arene scaffold. Bioorg Med Chem Lett. 2010;20(7):2137–2139. doi: 10.1016/j.bmcl.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsou LK, Chen CH, Dutschman GE, Cheng YC, Hamilton AD. Blocking HIV-1 entry by a gp120 surface binding inhibitor. Bioorg Med Chem Lett. 2012 doi: 10.1016/j.bmcl.2012.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]