Introduction
There is emerging consensus regarding the need to optimize the efficacy of HIV interventions by integrating evidence-based social, behavioral and biomedical interventions to produce a population-level impact on the HIV/AIDS epidemic. Unfortunately, there is limited discussion specifying how interventions can be integrated to yield maximal prevention impact. Of importance to program planners is the need to specify which combination of interventions to integrate and how interventions should be sequenced to achieve the prevention synergy needed to reduce the risk of HIV acquisition and transmission among diverse at-risk populations.
In this Supplement Vermund et al. explore biomedical HIV interventions that have shown efficacy in reducing HIV incidence, while Wingood, DiClemente and Blank, respectively, discuss combination interventions to reduce concurrency, technological interventions to reduce intervention decay, and intervention cascades to enhance medication adherence. Novel intervention settings within which to conduct HIV prevention research is highlighted by Rich’s collaborations with correctional facilities, Latkin’s research on social networks and DiClemente’s research in county health departments. Rhodes and colleagues discuss the use of community-based participatory research (CBPR) to access at-risk Latino men who have sex with men (MSMs) and Sullivan, Grey and Rosser highlight emerging technologies to access MSM to reduce HIV-related disparities. Additionally, this supplement addresses implementation science approaches to sustaining HIV interventions. Glasgow and colleagues advance the Evidence Integration Triangle, Brown and co-investigators discuss computational linguistics and system science and Wingood presents adaptation of evidence-based behavioral intervention as a strategy to enhance implementation.
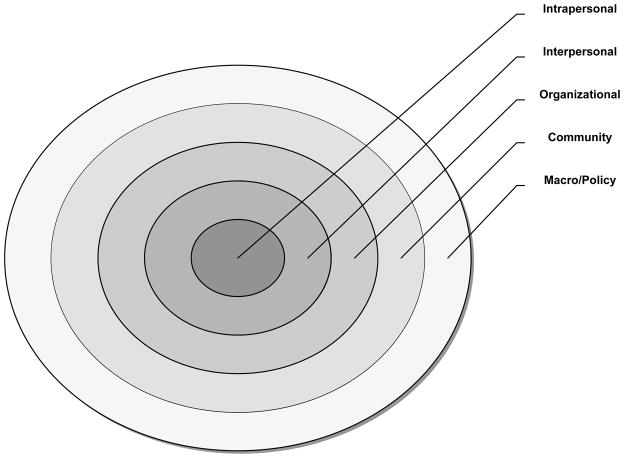
A social ecological perspective1 provides a framework for examining how the interaction of multiple interventions (e.g., pre-exposure prophylaxis (PrEP), male circumcision, antiretroviral therapy (ART), behavioral) and techniques (e.g., CBPR, technology) implemented in key venues (e.g., correctional facilities, health departments, social networks) when targeted at different levels of influence (Table 1) create HIV prevention synergy for specific at-risk populations (e.g., MSM, women, serodiscordant couples). The central principle of the social ecological perspective is that the risk of acquiring and/or transmitting HIV arises from a complex interaction of multiple determinants (or factors) corresponding to different “levels of influence,” often depicted as nested concentric circles (Figure 1). The circles represent contextual layers of increasing scope: intrapersonal, interpersonal, organizational, community, and macro/policy level determinants.2 This manuscript uses the social ecological framework to conceptualize how integrating HIV biomedical and behavioral interventions at these five levels can produce complementary or synergistic effects2. Without such a comprehensive framework, program planners may combine interventions that produce scattered, redundant or mutually opposing effects2. This perspective also provides a compelling approach for the scale-up of combination interventions integrated across different levels of influence to facilitate their reach and sustainability.
Table 1.
Patient and provider intervention strategies across different levels of influence
| Level of Influence | Possible Intervention Strategies |
|---|---|
| Intrapersonal |
|
| Interpersonal |
|
| Organizational |
|
| Community |
|
| Macro Policy |
|
Figure 1.
Levels of Influence nested within the socio-ecological model
The ecological perspective emphasizes interdependence among levels of influence. Thus, interventions at one level may influence interventions at another level. Hence, interdependence emerges as a central mechanism for integrating interventions at multiple levels. Below we examine three forms of interdependence – pooled interdependence, sequential interdependence and reciprocal interdependence3 – as applied to PrEP adherence interventions and other HIV prevention interventions.
Pooled Interdependence
The concept of pooled interdependence is derived from organization theory. This concept emphasizes that social organizations are complex systems composed of many interdependent parts, such as teams, departments, units, or subsidiaries3. Under pooled interdependence, organizational components are independent in terms of their everyday functioning, tasks, and routine processes; yet these components are interdependent in that they all contribute to organizational efficiency. If any component malfunctions, organizational efficiency is jeopardized3.
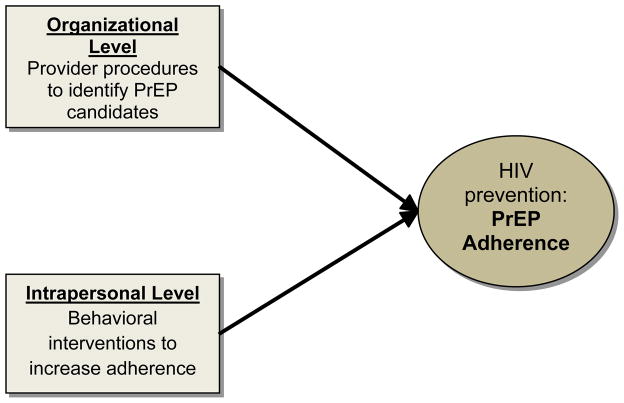
Similarly, pooled interdependence of integrated HIV interventions occurs when interventions at different levels of influence work independently of each other to provide HIV prevention services to a target population. For example, research shows that although many providers know about PrEP, few actually prescribe it in their clinical practice4. To increase prescribing practices providers need to successfully identify appropriate candidates, individuals at highest risk of HIV who may benefit most from PrEP, such as seronegative partners in HIV serodiscordant relationships5, MSM6,7, or heterosexual men and women engaging in risky sexual practices5. This, however, is not an easy task due to provider and patient discomfort with discussions involving sexual orientation, risky sexual practices, or drug use.4 An organizational-level (structural) intervention, designed to evaluate new procedures to facilitate providers’ identifying appropriate PrEP candidates, can work independently from an intrapersonal-level (behavioral) intervention to increase PrEP adherence among seronegative partners in HIV serodiscordant relationships. However, both contribute to the broader prevention goal of decreasing HIV transmission, demonstrating pooled interdependence (Figure 2).
Figure 2.
Pooled Interdependence Model (applied to enhancing PrEP adherence)
In general, biomedical interventions operate independently from behavioral interventions which, in turn, operate independently from structural interventions. Each intervention makes a discrete contribution to enhance PrEP adherence, without being dependent on each other. The efficacy of PrEP adherence is the sum, or the accumulation, of the intervention contributions at different levels. While deployed at different levels of influence, the interventions target the same outcome (PrEP adherence) and converge. Importantly, differential exposure to interventions across levels may produce scattered and noncumulative effects.
Sequential interdependence
Sequential interdependence is conceptualized by organizational theory as the type of interdependence that takes a serial form, whereby the outputs of one organizational component become the inputs of another3. This concept can also be translated for integrating HIV prevention interventions. Sequential interdependence exists when interventions at different levels of influence are delivered in a purposive, planned sequence. Interventions at one level produce an outcome and these outputs become inputs for interventions at another level of influence. Thus, a community level (structural) intervention designed to reduce HIV stigma may prompt a change in providers’ prescribing practices (behavior); they become more supportive of PrEP, which may enhance providers’ motivation to promote PrEP adherence among their patients. In essence, there is a cascading effect whereby the outputs of an HIV intervention at one level become the inputs of an HIV intervention at another level of influence.
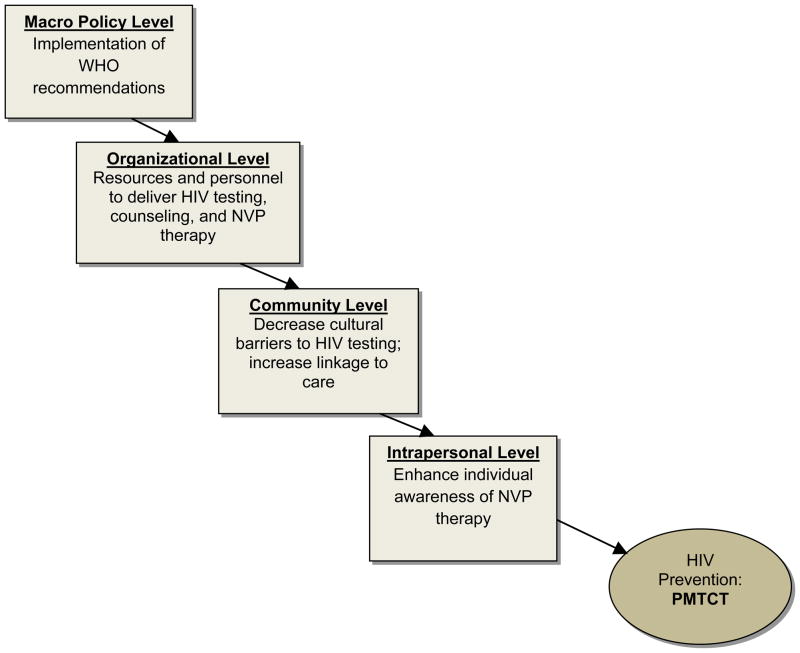
Application to prevention of mother-to-child transmission of HIV (PMTCT) in resource-challenged countries represents an example of sequential interdependence of HIV prevention interventions (Figure 3). Current evidence suggests that the use of ART in pregnant and breastfeeding women and in their breastfeeding infants markedly reduces mother-to-child HIV transmission8. In resource-challenged settings, single-dose intrapartum and neonatal nevirapine (NVP) therapy is a safe and cost-effective method of PMTCT9. Mothers’ adherence is an important component of program effectiveness9, and interventions are needed to increase women’s NVP awareness10 and PMTCT acceptability. Importantly, however, individual-level interventions may not be the optimal starting point to promote PMTCT in these settings. In resource-challenged countries, a large proportion of women, especially those living in less urban areas, are neither linked to antenatal care nor give birth at hospitals. Moreover, many maternity wards and antenatal care clinics do not have resources or established procedures and guidelines to implement HIV testing, counseling, and NVP administration9–11. Finally, even in cases when a medical infrastructure exists, community-level barriers may prevent pregnant women from accessing HIV testing or NVP therapy9.
Figure 3.
Sequential Interdependence Model (applied to enhancing PMTCT)
To increase PMTCT rates in this environment, an integrated intervention strategy is needed, where interventions at different levels are sequentially interdependent. Indeed, it is less productive to initiate behavioral interventions designed to increase mothers’ NVP adherence before NVP treatment and appropriate access to medical care are more widely available. An alternative strategy is to deliver interventions in a sequential order, starting from the higher structural levels (macro-level policy, organizational, and community), then moving down the intervention hierarchy to the interpersonal and intrapersonal levels. First, political will must exist at the level of state governments (e.g., Ministries of Health) to commit resources to PMTCT and implement relevant policies and procedures (e.g., those recommended by the World Health Organization) in national medical care systems11. As a second step, interventions need to be conducted at the organizational and community levels. Medical care facilities involved in antenatal and postnatal care should have trained medical personnel delivering HIV testing, counseling and NVP therapy; HIV testing should be routinely offered to pregnant women, and facilities must have the necessary equipment and resources. At the community level, further interventions are needed to ensure pregnant women have better access to medical care, for example, by providing reduced cost bus fare for antenatal visits10 and working to mitigate cultural barriers to access.
Prescribing PrEP for serodiscordant couples is another example of sequential interdependence of HIV prevention interventions. While individuals’ adherence is a crucial component of PrEP effectiveness5,13, previous interventions suggest that individual-level behavioral interventions may not be the optimal starting point when access to treatment is severely limited due to the lack of financial and organizational resources, or various cultural and physical barriers9–11. Therefore, when developing PrEP interventions for HIV serodiscordant couples in these settings, national policies, resources, organizational capabilities, and legal and cultural environments must be considered12. Specifically, before widespread implementation of PrEP, countries will need to develop policies and evaluation criteria, and determine how scarce HIV prevention resources should be prioritized to PrEP, in general, and to PrEP for HIV serodiscordant couples, in particular. Key issues to consider include whether PrEP is available free or at low (subsidized) cost, and whether and how that might contribute to discrimination against certain groups, such as residents with limited access to providers and resources12. Countries considering widespread PrEP implementation may also need to develop appropriate legal and regulatory frameworks12. For example, in the case of PrEP for HIV serodiscordant couples, legal questions exist, such as: 1) is an HIV seropositive partner in a serodiscordant relationship obligated to disclose their HIV status to the seronegative partner? And 2) how can we enhance the availability, accessibility and affordability of PrEP for the HIV seronegative partner? These macro-level policy interventions must be followed by the development of organizational-level capabilities and resources. PrEP should be accompanied by regular HIV testing and counseling to prevent behavioral disinhibition and to ensure PrEP use is avoided in the presence of a new HIV infection14. However, as discussed above, many health-care facilities may not be equipped to provide HIV testing and counseling9–11, and appropriate interventions are needed. Moreover, many communities may have limited access to medical care, so regular HIV testing as well as the requisite health monitoring necessary to detect and mitigate potential adverse side-effects of PrEP is not available. In particular, populations residing in less urban areas are often geographically distant from urban medical care facilities, which represents a physical barrier for individuals interested in accessing PrEP10. In addition, as in the case of PMTCT, cultural barriers may prevent individuals from accessing HIV testing or using PrEP. Community-level interventions may follow macro-level interventions designed to improve linkage to care and mitigate cultural barriers to HIV testing and PrEP uptake. Individual-level behavioral interventions designed to improve PrEP adherence may be more appropriate and effective following the implementation of macro-level and organizational- and community-level interventions.
Integrating interventions across levels of influence have a cascading effect and create a “circuit” through which the effects of the interventions combine and flow. However, in this model it is difficult to disaggregate intervention effects, since the distinct contribution of each intervention depends on the interventions at other levels to enhance PrEP adherence. Thus, “intervention packages” can be implemented, but not dissected, to examine individual-component effects.
Reciprocal interdependence
Reciprocal interdependence is the most complex type of interdependence, whereby different parts of the whole reciprocally influence each other, so that outputs of each component become the inputs of another3. Reciprocal interdependence of integrated HIV interventions exists when interventions at different levels of influence mutually reinforce each other by altering patterns of interaction amongst two or more target audiences. For example, physician-directed and patient-directed interventions may mutually reinforce each other, changing the quality of physician-patient interaction that, in turn, increases PrEP adherence. Reciprocal interdependence is indicated when coordinated behavior change is necessary to produce a desired outcome among different interdependent entities (e.g., patients, physicians, hospitals, and clinics) (Figure 4).
Figure 4.
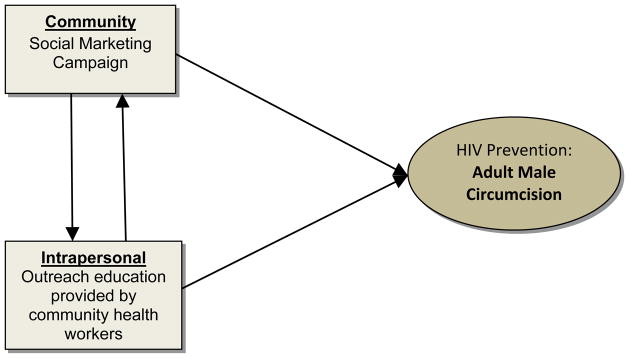
Reciprocal Interdependence Model (applied to enhancing adult male circumcision)
For example, male circumcision reduces the risk of HIV transmission. Where infant circumcision is not common, adult male circumcision is recommended in high prevalence HIV settings8. However, in some national contexts, social and cultural acceptability represents a barrier to male circumcision. In this case, an integrated intervention with reciprocal interdependence is a viable option. Social marketing campaigns designed to increase the cultural acceptability of male circumcision can be implemented simultaneously with interventions using outreach strategies by community health workers designed to inform individuals about adult male circumcision as an effective HIV prevention strategy. These interventions demonstrate reciprocal interdependence: as social marketing campaigns increase cultural acceptance of adult male circumcision, more adults will elect to undergo the procedure. Also, as outreach strategies enhance cultural acceptance of adult male circumcision, the practice of adult male circumcision becomes more socially acceptable and, as a consequence, may be more common.
Conclusions
In the absence of a highly effective vaccine, HIV prevention interventions will remain a mainstay of the public health armamentarium to combat the HIV epidemic. Numerous behavioral HIV prevention interventions have been developed and evaluated, demonstrating efficacy in reducing HIV-associated risk behaviors. More recently, the advent of biomedical prevention interventions such as pre-exposure prophylaxis, vaginal gel, male circumcision and effective ART treatment have shown efficacy in reducing HIV transmission.8 And, finally, HIV testing and linkage to effective care remains a critical component of any prevention strategy. While the degree of efficacy varies, it is clear that biomedical prevention interventions are an important component in the HIV prevention toolkit.
Recently, with the advent of biomedical prevention interventions, prevention program planners have suggested developing “combination or integrated intervention packages” rather than focusing solely on a single intervention modality.8 The concept of a “package” is a useful and appropriate analogy; however, as highlighted in this article, this concept needs to evolve further to optimize interventions and their impact. The integrated intervention approach needs to examine the role of interdependence between intervention modalities and their level of influence. This paradigm suggests that putting interventions into a package is not the same as the orderly arrangement and sequencing of interventions in a package; there is a qualitative and quantitative distinction. Thus, the goal is to create “packages” in which interventions are complementary, efficient, effective, and that maximize the potential for HIV prevention synergy. And although we have outlined three models of interdependence, we have purposely simplified the models by avoiding the complexities of considering techniques to enhance intervention efficacy (e.g. technology), moderator effects and mediators through which interventions exert that prevention effects.2 While significant strides in HIV prevention have been made, formulating HIV prevention intervention packages that address the role of interdependence may further facilitate a new generation of effective integrated interventions.
Acknowledgments
Financial Support: This research was supported by a grant from the National Institute of Mental Health (5R01 MH070537) to the third author. Additional support was provided by the Emory Center for AIDS Research (P30 AI050409), the Atlanta Clinical & Translational Science Institute (UL1TR000454), the Center for Contextual Genetics & Prevention (P03 DA027827) and the Women’s Interagency HIV Study (WIHS-V) (U01AI103408) and the University of Pennsylvania Center for AIDS Research.
Footnotes
Conflicts of Interest: None reported
References
- 1.McLeroy KR, Bibeau D, Steckler A, Glanz K. An Ecological Perspective on Health Promotion Programs. Health Education & Behavior. 1988 Dec 1;15(4):351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 2.Weiner BJ, Lewis MA, Clauser SB, Stitzenberg KB. In Search of Synergy: Strategies for Combining Interventions at Multiple Levels. Journal of National Cancer Institute Monographs. 2012;44:34–41. doi: 10.1093/jncimonographs/lgs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson JD. Organizations in Action. New York: Mc Graw-Hill Book Company; 1967. [Google Scholar]
- 4.Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Current Opinion in HIV and AIDS. 2012 Nov;7(6):593–599. doi: 10.1097/COH.0b013e3283590446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paxton LA. Considerations regarding antiretroviral chemoprophilaxis and heterosexuals in generalized epidemic settings. Current Opinion in HIV and AIDS. 2012 Nov;7(6):557–562. doi: 10.1097/COH.0b013e328359064a. [DOI] [PubMed] [Google Scholar]
- 6.Poynten MI, Zablotska I, Grulich AE. Considerations regarding antiretroviral chemoprophylaxis in MSM. Current Opinion in HIV and AIDS. 2012;7:549–556. doi: 10.1097/COH.0b013e3283582c71. [DOI] [PubMed] [Google Scholar]
- 7.Schackman BR, Eggman AA. Cost-effectiveness of pre-exposure prophylaxis for HIV: a review. Current Opinion in HIV and AIDS. 2012 Nov;7(6):587–592. doi: 10.1097/COH.0b013e3283582c8b. [DOI] [PubMed] [Google Scholar]
- 8.Vermund SH, Tique JA, Cassell HM, Johnson ME, Ciampa PJ, Audet CM. Translation of biomedical prevention strategies for HIV: Prospects and pitfalls. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stringer EM, Sinkala M, Stringer JSA, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003 Jun 13;17(9):1377–1382. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltzer K, Mosala T, Shisana O, Nqueko A, Mngqundaniso N. Barriers to prevention of HIV Transmission from Mother to Child (PMTCT) an a Resource poor Setting in the Eastern Cape, South Africa. African Journal of Reproductive Health. 2007 Apr;11(1) [PubMed] [Google Scholar]
- 11.Uwimana J, Jackson D, Hausler H, Zarowsky C. Health system barriers to implementation of collaborative TB and HIV activities including prevention of mother to child transmission in South Africa. Tropical medicine and International Health. 2012 May;17(5):658–665. doi: 10.1111/j.1365-3156.2012.02956.x. [DOI] [PubMed] [Google Scholar]
- 12.International AIDS Society. PrEP Implementation Policy Forum. [Accessed: December 2012];Developing Country-Level Preparation and Capacity for PrEP Implementation. 2007 ( http://www.iasociety.org/Web/WebContent/File/ILF_ias_sydney_report_02.pdf)
- 13.Muchomba FM, Gearing RE, Simoni JM, El-Bassel N. State of Science of Adherence in Pre-Exposure Prophylaxis and Microbicide Trials. JAIDS. 2012 doi: 10.1097/QAI.0b013e31826f9962. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers GM, Mayer KH. Oral Preexposure Anti-HIV Prophylaxis for High-Risk U.S. Populations: Current Considerations in Light of New Findings. AIDS Patient Care and STDs. 2011;25(2):63–71. doi: 10.1089/apc.2010.0222. [DOI] [PubMed] [Google Scholar]