Abstract
Microbeads of alginate cross-linked with Ca2+ and/or Ba2+ are popular matrices in cell-based therapy. The aim of this study was to quantify the binding of barium in alginate microbeads and its leakage under in vitro and accumulation under in vivo conditions. Low concentrations of barium (1 mM) in combination with calcium (50 mM) and high concentrations of barium (20 mM) in gelling solutions were used for preparation of microbeads made of high-G and high-M alginates. High-G microbeads accumulated barium from gelling solution and contained higher concentrations of divalent ions for both low- and high-Ba exposure compared to high-G microbeads exposed to calcium solely and to high-M microbeads for all gelling conditions. Although most of the unbound divalent ions were removed during the wash and culture steps, leakage of barium was still detected during storage. Barium accumulation in blood and femur bone of mice implanted with high-G beads was found to be dose-dependent. Estimated barium leakage relevant to transplantation to diabetic patients with islets in alginate microbeads showed that the leakage was 2.5 times lower than the tolerable intake value given by WHO for high-G microbeads made using low barium concentration. The similar estimate gave 1.5 times higher than is the tolerable intake value for the high-G microbeads made using high barium concentration. In order to reduce the risk of barium accumulation that may be of safety concern, the microbeads made of high-G alginate gelled with a combination of calcium and low concentration of barium ions is recommended for islet transplantation.
Keywords: Alginate, microcapsules, transplantation, barium, barium toxicity
INTRODUCTION
Ionically cross-linked alginate hydrogels form the basis for commonly used capsules for cell immobilization. Alginate provides entrapping of cells under physiological conditions, ensuring cell viability and function. Hence, alginate based capsules are popular candidates for the immunoisolation of cells in transplantation. Although a variety of diseases can be treated with cell therapy1, the most studied system is the encapsulation of pancreatic islets for transplantation of diabetic patients and by this offering a treatment for type 1 diabetes2–4.
Alginate is a linear copolymer of α-L-mannuronic acid (M) and β-D-guluronic acid (G). The composition of the natural polymer varies depending on its origin in different seaweed and some bacteria, and has been shown to strongly influence its physical properties. Alginate has strong affinity for divalent ions. In concentrated (>1%) solutions of high molecular weight alginate, divalent ions cross-link alginate polymers into a hydrogel network, which forms the matrix for encapsulation of cells. Although ionic cross-linking has mainly been attributed to long G-blocks5, alternating M and G sequences (MG-blocks) have also shown to participate in Ca-alginate gel formation6,7.
The affinity of alginates towards divalent ions has been shown to decrease in the order Pb > Cu > Cd > Ba > Sr > Ca > Co, Ni, Zn > Mn8,9. Recently, the affinity of different block structure towards Ca2+, Sr2+ and Ba2+ was shown10. More specifically, Ca2+ was found to bind to G- and MG-blocks, Ba2+ to G- and M-blocks, and Sr2+ to G-blocks solely. Different affinity was shown to influence the physical properties of ionically cross-linked alginate gels. High-G alginates were greatly influenced by using ions of high affinity (Ba2+ or Sr2+), whereas for high-M alginates no effect was seen on stability or permeability when using Ba2+ or Sr2+ in the gelling solution10.
Owing to its high affinity to alginate, Ba2+ has in recent years frequently been used as cross-linking ion in alginate hydrogels for cell encapsulation purposes, especially for capsules without the use of a stabilizing polycation4,11–13. However, care must be taken as barium is known to be toxic in high doses, and chronic toxicity includes cardiovascular diseases, and possibly hypertension and renal toxicity14.
Normally, the primary route of exposure to barium appears to be ingestion from food and drinking water. The WHO15 reported several published estimates of dietary intake of barium by humans; daily dietary intake ranged from 0.3 to 1.7 mg barium/day, with wide variations. Mammals tend to accumulate significant concentrations of the metals in their bones resulting in bones holding 90% of the body burden of barium15,16. Primary route of secretion of barium is via feces15,16. The toxicity is dependent on the water solubility of the barium compound; hence poorly soluble barium salts are commonly used as contrast agents in human medicine and are not considered a significant health risk15.
In spite of the potential risks of barium exposure following implantation of Ba-alginate gels, research on binding and leakage of barium from alginate gels has so far been lacking. The aim of this study was to determine the amount of calcium and barium bound in various alginate gel beads proposed for use in transplantation. Leakage of barium during washing and culturing of beads was also measured. A long-term storage study was performed to evaluate further loss of ions that may take place during prolonged culture and in vivo. Alginate beads made by exposure to two different BaCl2 concentrations were also implanted to Balb/c mice and barium was measured in blood and bone. Finally, the leakage data were compared to literature recommendation towards barium exposure.
MATERIALS AND METHODS
Alginates
A high-G alginate from Laminaria hyperborea (UP-MVG, FG=0.67, NovaMatrix, Sandvika, Norway) and a high-M alginate from Macrocystis pyrifera (FG=0.40, Sigma Chemicals, St. Louis, US) were used. Details of composition, molecular weight and endotoxin content are given in Table 1.
Table 1.
Chemical composition and sequences of high-G alginate from stipe of Laminaria hyperborea and high-M alginate from Macrocystis pyrifera obtained from 1H-NMR spectra, molecular weight and molecular weight distribution, and endotoxin content.
| Alginate source | FG | FGG | FMG/GM | FMM | FGGM/MGG | FMGM | FGGG | NG>1 | Mw (×103)a |
Mw/Mna | Endotoxin (EU/g)b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L. hyperborea, stipe | 0.67 | 0.56 | 0.11 | 0.22 | 0.05 | 0.08 | 0.51 | 13 | 238 | 2.2 | <43 |
| M. pyrifera | 0.40 | 0.21 | 0.20 | 0.40 | 0.05 | 0.18 | 0.16 | 5 | 170 | n.d. | n.d. |
SEC-MALLS measurements
Given by the manufacturer.
n.d.: not determined
Formation of alginate beads, washing and culturing
Alginate microbeads were made by dripping 5 ml of a filtered (by 0.22 µm syringe filter) 1.8% (w/v) alginate solution, dissolved in double distilled water (ddH2O), into 300 ml of either 50 mM CaCl2 (Ca-beads), or 50 mM CaCl2 + 1 mM BaCl2 (Ca/Ba-beads), or 20 mM BaCl2 (Ba-beads) solution (all in ddH2O). The size of capsules was controlled to approximately 500 µm in diameter using a high-voltage electrostatic bead generator (7 kV, 10 ml/hr flow, steel needle with outer diameter 0.35 mm). The beads were cured for 15 minutes in the gelling solution and transferred onto a filter and washed 3 times in 10 ml of cell culture media CMRL (CMRL 1066 without glutamine, Invitrogen) with the total washing time < 1 min. The washing solution was collected and 3 times 5 ml taken out for determination of barium and calcium concentrations. A volume of 50 ml CMRL with added Pen/Strep was used to transfer the beads from the filter to a 50 ml tube. After 24 hrs at room temperature, 3 times 5 ml was taken out for elemental analysis. The beads were washed 3 times in 100 ml CMRL on a filter at total washing time < 1 min and 3 times 5 ml of the collected washing solution was taken out for elemental analysis.
Barium bound in alginate beads after washing and culturing
50 ml CMRL with Pen/Strep were used to transfer the washed beads to a 50 ml tube and 3 times 1 ml of beads in CMRL were taken out. To determine the amount of divalent ions bound in the gel, beads were dissolved by adding EDTA (80 mM) and kept over night on a stirrer at room temperature before elemental analysis.
Barium leakage during long-term storage of beads
After washing and culturing as described above, microbeads made from 5 ml alginate solution were placed in a 50 ml tube and the tube was filled with CMRL media with added 0.01% NaN3 to prevent bacterial growth. The tubes were placed on a rotator (8 rpm) at room temperature. Once a week, the CMRL media was removed and fresh CMRL was added up to 50 ml. Before removal of CMRL, 3 times 0.5 ml of the media was taken out for elemental analysis. The leakage of barium was measured once a week over a period of five weeks with a total of 5 shifts of media.
Determination of calcium and barium concentrations from solutions
The content of barium and calcium in the various solutions (wash solutions, CMRL media and dissolved alginate beads) was determined by inductively coupled plasma mass spectrometry (high-resolution ICP-MS, Element 2, Thermo Scientific). As most samples contained high amounts of sodium, samples were diluted with deionized water (10–100 times, depending on the sodium concentration), and nitric acid added to 0.1 M HNO3. A calibration solution of 350 mg Na/L was used to verify matrix effects.
In vivo mice studies
Two types of high-G alginate beads (Ca/Ba-beads and Ba-beads) were fabricated at the Norwegian University of Science and Technology (NTNU) and shipped in CMRL media to the University of Illinois at Chicago (UIC) for implantation. Two separate experiments were conducted using healthy male Balb/c mice (22–25 g of body weight, Harlan Industries, Indianapolis, Indiana). All procedures were performed under the guidelines of the National Institutes of Health and approval by the Animal Care Committee at UIC. All the beads were washed with 100 ml of Hank’s Buffered Salt Solution (HBSS) for three times prior to implantation. In the first implantation study, different doses of each bead type were implanted into peritoneal cavity of mice (0.02, 0.05, 0.1, and 0.3 ml beads, 3 animals in each group). In addition, 5 mice were used as control without implantation. In the second experiment, implantation of only 0.3 ml of both capsule types was conducted (4 animals in each group). Again, 5 animals were used as control. After 16 weeks (1st experiment) and 9 weeks (2nd experiment), all the mice were sacrificed and alginate beads were retrieved. Whole blood samples and two femur bones from mice were taken. Most of the soft tissue was removed from the bone sample. All samples were then frozen at −80°C and sent to St. Olavs Hospital for storage in freezer until measurements of barium concentrations as described below.
Handling and analysis of mice blood and femur samples
Aliquots of approximately 250 mg room tempered samples were accurately weighed into polypropylene vials containing 1 mL of concentrated HNO3 (doubly distilled, Scan Pure, Elverum, Norway) and solution of indium (In) (CPI, Amsterdam, The Netherlands). In was used as an internal standard for the quantification of Ba (serial dilution from 1000 mg/L). The sample tubes were then put on a ModBlock heating block (CPI, Santa Rosa, California, USA) at 100 °C for 2.5 h. After cooling the samples were diluted to 10 mL to reach a final acid concentration of 10 % (v/v) and 1 µg In/L. Seronorm Whole Blood Level 1 and 3 (LOT MR4206 and 0512627, respectively from Sero, Billingstad, Norway) were treated as a normal sample and used to control accuracy and precision.
To our knowledge there are no previous published studies on barium determination in bone. A new method was developed for this purpose. This method was based on closed-vessel microwave digestion partly adopted from17 concerning lead determination. Femur samples were freeze-dried and the remaining traces of soft tissue removed with a plastic knife before accurately weighed (average 44 mg) into vials (Fluoropolymer) for Multiwave 3000 microwave oven (Anton Paar, Graz, Austria) containing 1 mL of HNO3 and solution of indium. The samples were then exposed to a microwave program of 1200 W in 20 minutes. This resulted in a complete digestion of the bone. After cooling a dilution to 10 mL resulted in a final acid concentration of 10 % (v/v) and 10 µg In/L. No certified reference material for barium in bones was available by the time. To control accuracy of calibration and contamination risk, Seronorm Whole Blood Level 3 and blank sample were added to every digestion run.
Determination of barium in whole blood and femur were carried out with a high resolution ICP-SFMS (Element 2, Thermo Scientific, Bremen, Germany) consisting of both magnetic and electric sector fields and three modes of predefined resolutions. The monitoring of barium was performed in low resolution (300 m/Δm at 10% valley definition). Concentric nebulizer and a cooled (5 °C) cyclonic spray chamber made of PFA-material (ESI, Omaha, USA) and a CETAC ASX-520 autosampler (CETAC, Omaha, USA) constituted the sample introduction system. Aquatic solutions containing certified concentrations of barium (serial dilutions from 1000 µg/ml, Spectrapure Standards, Oslo, Norway) were used for external calibration. Limit of detection for determination of barium was 0.30 µg/kg and 0.018 mg/kg in whole blood and bone, respectively. Linearity was proven for the concentration range from 1 to 50 µg/kg for whole blood and from 0.06 to 300 mg/kg for bone.
Statistics
A two way student t-tests were used to compare concentrations of barium found in blood and femur of mice assuming the same variance for all samples (Microsoft Office Excel 2003).
RESULTS
Binding of ions in alginate microbeads
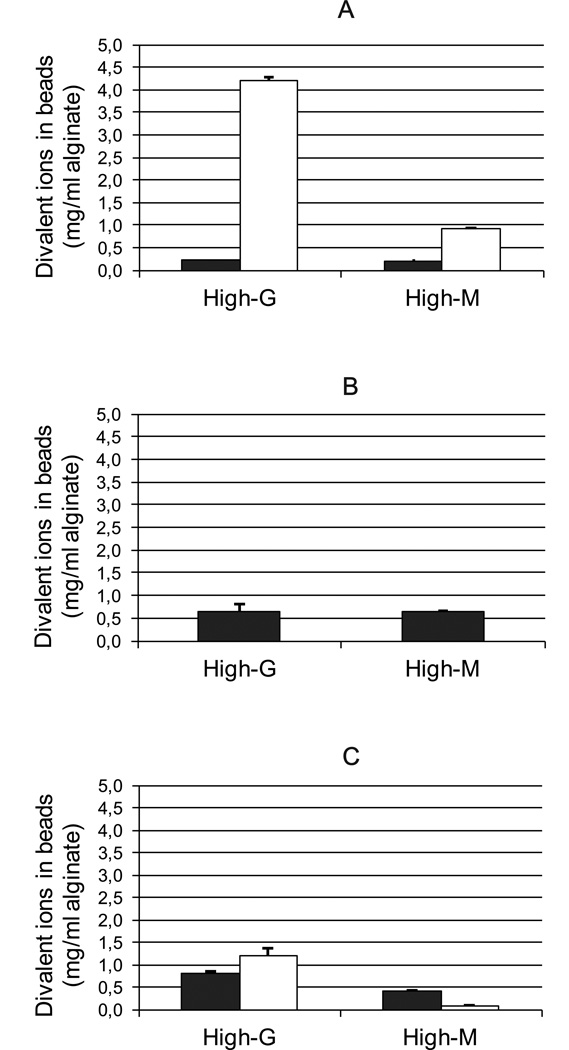
Microbeads of both high-G and high-M alginate cross-linked with Ca2+ and/or Ba2+, obtained after washing and culturing, were compared with regard to their divalent ion content by means of ICP-MS. As expected, Ba2+ bound to a higher extent to high-G alginate than to high-M alginate for both high and low concentrations of barium (Figure 1A and C). The binding of Ca2+ was similar for the two alginates when only calcium ions were used as gelling ions (Figure 1B) and an equal accumulation of Ca2+ could be seen for both alginates upon washing in culture media containing small amounts of calcium (Figure 1A). However, when a combination of Ca2+ and Ba2+ was used, more calcium was found in the high-G alginate compared to the high-M alginate sample in addition to increased levels of barium (Figure 1C). Indeed, although a high concentration of Ca2+ relative to Ba2+ was used in the gelling solution (50/1), more barium than calcium, given in mg/ml alginate, was bound in the gel of high-G alginate after washing illustrating the affinity of a high-G alginate towards barium.
Figure 1.
Content of divalent ions in alginate beads after wash and culture. A: alginate gelled in 20 mM BaCl2 (Ba-beads), B: alginate gelled in 50 mM CaCl2 (Ca-beads) and C: alginate gelled in 50 mM CaCl2 and 1 mM BaCl2 (Ca/Ba-beads). Closed bars: calcium, open bars: barium. For sample A, content of calcium in the beads results from accumulation of smaller contents of calcium in the washing solutions and culture media. Values are given as mean ± StDev for three measurements.
Calculations of the number of divalent ions per monomer of uronic acid in the polymer showed that for high-G alginates the number of divalent ions in the gel was doubled when Ba2+ was used in the gelling solution (Table 2). Surprisingly, this was also found for the gelling solution of 50 mM CaCl2 and 1 mM of BaCl2, showing the dramatic effect of adding 1 mM of Ba2+ to the gelling solution compared to using calcium ions solely. For the high-M alginate beads, the ratio of divalent ions per uronic acid monomer was similar to high-G alginates for the Ca-beads. However, for Ca/Ba- and Ba-beads the ratio of divalent ions per monomer was reduced for the high-M alginate, in contrast to the high-G alginate.
Table 2.
Molar ratio of divalent ion (Ca2+ and Ba2+) to uronic acid residue in alginate microbeads. The values are calculated from measured amount of calcium and barium in dissolved beads after wash and culture as mean ± StDev.
| divalent ion:uronic acid (mol/mol) | ||
|---|---|---|
| High-G alginate | High-M alginate | |
| Ca-beads | 0.181 ± 0.011 | 0.166 ± 0.004 |
| Ca/Ba-beads | 0.316 ± 0.014 | 0.120 ± 0.005 |
| Ba-beads | 0.404 ± 0.009 | 0.132 ± 0.009 |
By using the concentrations of ions found in the washing solutions (Table 3) and the concentrations of ions in the gels after wash and culture (Figure 1), the concentrations of ions in the gels just after formation could be calculated. From this the molar ratio of calcium vs. barium was calculated for the various beads after formation (Table 4). As is clearly seen from the results, an accumulation of barium occurred in the alginate beads exposed to the 50 mM CaCl2 and 1 mM BaCl2 gelling solution (Ca/Ba-beads). For the high-G alginate gel, the accumulation of barium in the gel was dramatic, changing the molar ratio of Ca:Ba from 50:1 in the gelling solution to 50:20 in the final gel. The high-G alginate gel exposed to 20 mM BaCl2 experienced a loss of barium after wash and culture steps apparently due to excess of barium in the gelling solution with respect to the available uronic acid units (Table 3). For the high-M alginate gel, the lower affinity towards Ba2+ was seen as a lower amount of barium accumulated in the gel from the Ca/Ba gelling solution. A molar Ca:Ba ratio of 15:20 was found for the high-M alginate beads gelled in 20 mM BaCl2 after loss of barium upon washing and accumulation of calcium from the culture media.
Table 3.
Divalent ions removed from alginate beads upon wash and culture post gel formation. Numbers are relative (in %) to what is bound in the alginate gel beads upon gelation (100 %, Figure 1) and are given as mean ± StDev.
| Bead treatment | High-G alginate | High-M alginate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca/Ba-beads | Ca-beads | Ba-beads | Ca/Ba-beads | Ca-beads | Ba-beads | |||||||
| Ba2+ | Ca2+ | Ba2+ | Ca2+ | Ba2+ | Ca2+ | Ba2+ | Ca2+ | Ba2+ | Ca2+ | Ba2+ | Ca2+ | |
| 1. wash in culture media (3 × 10 ml) | 5.8 ± 0.7 | 39 ± 2 | NA | 42 ± 2 | 20.1 ± 0.4 | NA | 29 ± 2 | 50 ± 2 | NA | 46 ± 2 | 36 ± 1 | NA |
| Culture | 4.7 ± 0.6 | 26 ± 1 | NA | 25 ± 1 | 18.4 ± 0.5 | NA | 15 ± 1 | 19 ± 2 | NA | 16 ± 2 | 33 ± 1 | NA |
| 2. wash in culture media (3 × 100 ml) | 2.0 ± 0.3 | 10 ± 3 | NA | 6 ± 2 | 7.7 ± 0.2 | NA | 12 ± 1 | 13 ± 1 | NA | 10 ± 4 | 17 ± 1 | NA |
NA – not applicable
Table 4.
Comparison of molar ratios of calcium versus barium ions in the alginate beads (1) from given experimental conditions with respect to the ratio in the gelling solution, (2) calculated after bead formation, and (3) measured after wash and culture steps.
| calcium:barium (mol/mol) | ||||||
|---|---|---|---|---|---|---|
| High-G alginate | High-M alginate | |||||
| Ca/Ba-beads | Ca-beads | Ba-beads | Ca/Ba-beads | Ca-beads | Ba-beads | |
| In gelling solution | 50:1 | 50:0 | 0:20 | 50:1 | 50:0 | 0:20 |
| After bead formation | 50:6.3 | - | 3.3:20 | 50:1.1 | - | 2.2:20 |
| After wash and culture | 50:20 | - | 4:20 | 50:2.8 | - | 15:20 |
Leakage of divalent ions from alginate beads
The content of barium in the washing solutions is given in Table 3 as percentage loss relative to the initial amount of divalent ions in gels after gelation (before wash and culture). As expected, the majority of ions were washed out during the first washing step, the leakage of ions gradually decreasing for each treatment of beads. The leakage of barium from gels of high-M alginate was significantly higher than from gels of high-G alginate during washing and culturing. For calcium, the leakage from gels was similar for high-G and high-M alginate as well as for the Ca- and Ca/Ba- beads.
Ba- and Ca/Ba-beads of high-G alginates were exposed to CMRL culture media resembling in vitro culture or in vivo situation. The solutions were analyzed for content of barium (Table 5), representing the barium leached from alginate beads within one week for five consecutive weeks, i.e. five media shifts. The leakage of barium from the alginate beads was significantly reduced compared to earlier treatments (Table 3). As expected, the leakage was reduced upon extensive wash/culture, and for both types of beads the leakage was reduced to less than 1 % of the overall amount bound in the beads after the first wash and culture steps (Figure 1). However, as the amount of barium in the beads was higher for the alginates gelled in 20 mM BaCl2 than for 1 mM BaCl2 (Figure 1), the concentrations of barium in the culture media were considerably higher in the former case (Table 5).
Table 5.
Barium released from beads of high-G alginate upon long time storage in CMRL media (numbers in % are relative to what is bound in the alginate microbeads upon gelation (100 %, Figure 1)). Alginate from L. hyperborea gelled in 50 mM CaCl2 and 1 mM BaCl2 (Ca/Ba-beads) and 20 mM BaCl2 (Ba-beads). Values are given as mean ± StDev.
| Capsule treatment | Ca/Ba-beads | Ba-beads | ||
|---|---|---|---|---|
| (µg/ml) | % | (µg/ml) | % | |
| 1. storage | 18.1 ± 0.3 | 1.5 ± 0.2 | 449 ± 5 | 10.7 ± 0.2 |
| 2. storage (1. media shift) | 13.9 ± 0.2 | 1.2 ± 0.2 | 197 ± 2 | 4.7 ± 0.1 |
| 3. storage (2. media shift) | 12.3 ± 0.1 | 1.0 ± 0.1 | 93.7 ± 0.8 | 2.23 ± 0.04 |
| 4. storage (3. media shift) | 10.6 ± 0.1 | 0.89 ± 0.11 | 55 ± 1 | 1.31 ± 0.04 |
| 5. storage (4. media shift) | 9.9 ± 0.1 | 0.82 ± 0.10 | 40.8 ± 0.9 | 0.97 ± 0.03 |
| 6. storage (5. media shift) | 9.1 ± 0.1 | 0.76 ± 0.10 | 35.5 ± 0.1 | 0.85 ± 0.02 |
Concentrations of barium in mouse blood and femur
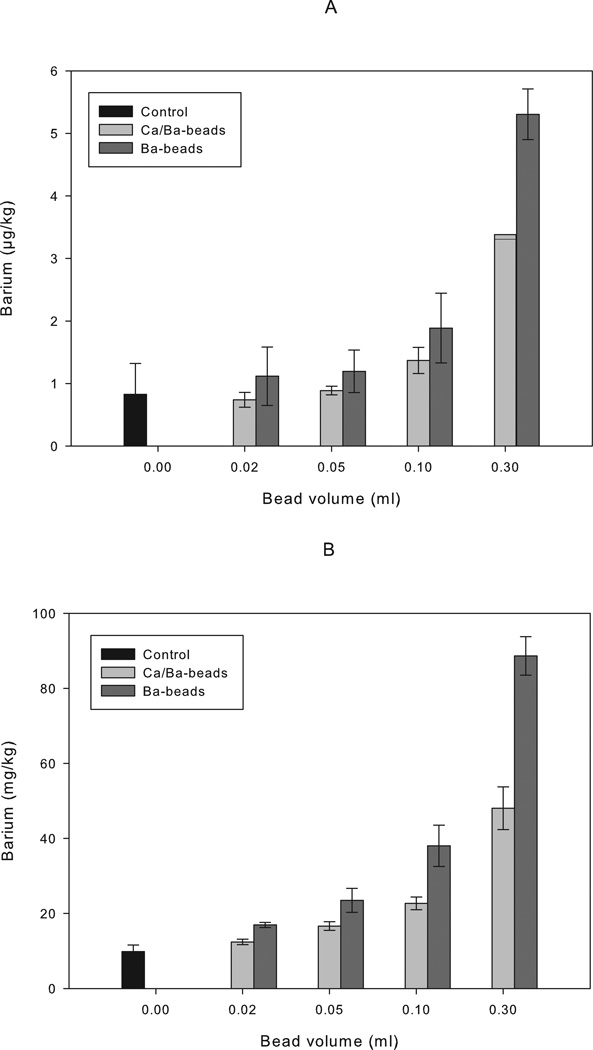
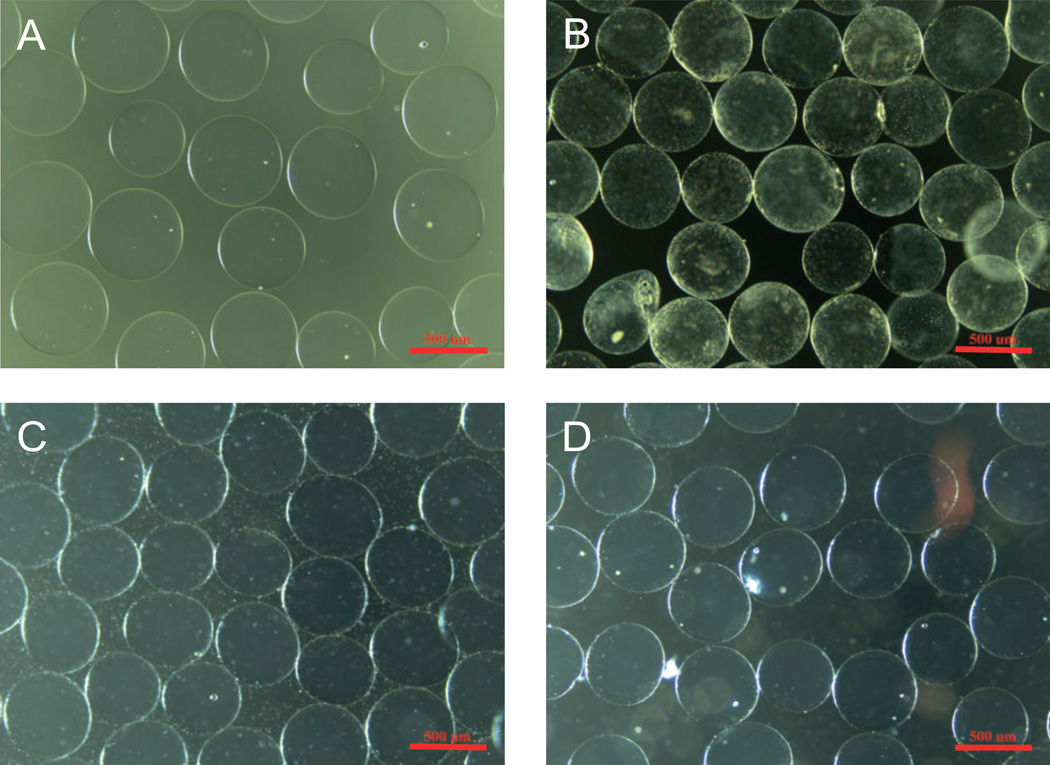
In vivo leakage of barium from Ca/Ba- and Ba-beads were evaluated upon implantation of microbeads into the peritoneal cavity of mice. Balb/c mice implanted with Ca/Ba-beads or Ba-beads showed a dose-dependent increase in the barium concentrations in blood and femur with the volume of implanted microbeads 16 weeks post implantation (Figure 2). In femur, the concentrations of barium from all beads were significantly different from the control for all doses of beads (p ≤ 0.005). For all doses of beads, the concentration of barium in femurs was significantly higher for the Ba-beads than the Ca/Ba-beads (p < 0.001). Also in blood, higher levels of barium were measured for both types of beads for doses of 0.30 ml beads (p ≤ 0.001). For Ba-beads also a dose of 0.10 ml beads showed higher levels of barium in the blood than the control (p < 0.05), but only at a dose of 0.3 ml beads there was a significant difference between Ca/Ba- and Ba-beads (p < 0.01). The experiment was repeated for the dose of 0.3 ml beads at 9 weeks post transplantation, showing significantly elevated concentrations of barium in the blood and femurs of both Ca/Ba-beads and Ba-beads compared to the control (Table 6, p < 0.05 and p < 1×10−6 for blood and femur, respectively). Again, a significant difference between the Ba- and Ca/Ba-beads was seen in bone (p < 1×10−8), however no significant difference was seen in blood. The integrity of the microbeads was evaluated by microscopy before implantation and after retrieval. Beads retrieved after 9 or 16 weeks were easily washed out from the peritoneum and showed no signs of overgrowth, swelling or destabilization. Figure 3 shows pictures of Ca/Ba- and Ba-beads before implantation and at retrieval 9 weeks post implantation.
Figure 2.
Barium measured in blood samples (A) and femur bone (B) from Balb/c mice 16 weeks post implantation with alginate beads. Ca/Ba-beads: high-G alginate gelled in 50 mM CaCl2 and 1 mM BaCl2, n = 3 for all samples (2 femurs per mouse) except for n = 2 for bead volume of 0.30 ml. Ba-beads: high-G alginate gelled in 20 mM BaCl2, n = 3 (2 femurs per mouse) for all samples. Control: not transplanted mice, n = 5 (9 femurs). Results are given as mean ± StDev, except for n = 2 where both values are shown.
Table 6.
Barium concentration measured in blood and femur of mice implanted with 0.3 ml beads of high-G alginate gelled in 50 mM CaCl2 and 1 mM BaCl2 (Ca/Ba-beads) and high-G alginate gelled in 20 mM BaCl2 (Ba-beads). Results are from 9 weeks post implantation and shown as mean ± StDev.
| Samples | Barium concentration | |
|---|---|---|
| Blood (µg/kg) | Femur (mg/kg) | |
| Control (n = 5) | 0.81 ± 0.09 | 10 ± 1 |
| Ca/Ba-beads (n = 4) | 2.4 ± 0.3 | 33 ± 8 |
| Ba-beads (n = 4) | 11 ± 8 | 93 ± 12 |
Figure 3.
Optical microscopy pictures of microbeads of high-G alginate before implantation (upper panel) and after retrieval (lower panel) from Balb/c mice. A, C: Ca/Ba-beads. B, D: Ba-beads. Scale bar is 500 µm.
DISCUSSION
Binding of ions in alginate microbeads
Beads of high-G alginate were found to bind significantly more barium compared to high-M alginate beads (Figure 1) both for high and low concentrations of barium. Long G-blocks of the high-G alginate are known to bind barium strongly and produce stable cross-links resulting in stiff gels10,18. The alginate from M. pyrifera (high-M), on the other hand, is dominated by shorter G-blocks and longer sequences of MG- and M-blocks (Table 1). Due to the cooperative binding of ions in junction zones, long sequences of G-blocks bind more strongly to divalent ions compared to short ones5,19. In addition, circular dichroism measurements have demonstrated that in contrast to Ca2+, Ba2+ ions do not seem to interact with MG-blocks10.
The strong affinity of long G-blocks towards Ba2+ is further seen when comparing the ratio of ion binding per monomer in the alginate (Table 2). The high divalent ion:uronic acid ratio found in the high-G beads with Ba2+ in the gelling solution could impose that more cross-links are formed by the divalent ions in these gels. This may be the case, as previous experiments have shown10 that Ba-gels of high-G alginates indeed exhibit a higher Youngs modulus compared to either Ca-alginate gels of the same alginate or Ba-gels of alginate from M. pyrifera, which indicates a higher degree of cross-linking. However, although the Ba- and Ca/Ba-beads of high-G alginate in this study end up with a similar ratio of divalent ions per uronic acid residues (0.32 and 0.40 mol/mol, respectively, Table 2) and are shown to have comparable stability10, the gels are shown to have different permeabilities towards IgG that may be due to a higher concentration of alginate at the surface of the Ba-beads 10.
It is well established that barium has a higher affinity to G-blocks compared to calcium8,9. Indeed, the dramatic decrease in the ratio of Ca:Ba in the Ca/Ba-beads of high-G alginate (Table 4) illustrates the accumulation of barium from the gelling solution by the long G-blocks in the polymer. When barium ions are used solely in the gelling solution, Ba2+ will be the only available counterion (except for the non-gelling Na+ occurring as a counterion in the initial sample of Na-alginate) also for MG- blocks and M-blocks having less affinity for Ba2+. Hence, loosely bound Ba2+ are exchanged by Na+ and Ca2+ acting as counterions upon wash and culture. As a result, the Ba-beads of high-G alginate loosing Ba2+, and Ca/Ba-beads of high-G alginate accumulating Ba2+, end up with four times difference in the content of barium (Figure 1a and c) after gelling and washing steps.
The viability of cells has been shown to be unaffected by using barium as a gelling ion for alginate beads20. Due to non-physiologial concentrations of divalent cations, the exposure time for the cells in the gelling solution should be reduced to a minimum. However, due to the strong affinity of alginate towards calcium and barium, the toxic effect of gelling ions is reduced within the alginate bead. Hence, no significant changes of viability and function of pancreatic islets encapsulated in Ba-alginate or Ca/Ba-alginate has been reported 4, 12–13
Leakage of divalent ions from alginate microbeads
The large number of ions lost in the first step of washing from all types of beads indicates that there is a substantial excess of divalent ions in the gel network, which are not bound in junction zones (Table 3). As the alginate hydrogels are composed of about 98% water, dissolved salt from the gelling solutions will be present in the gel network after microbead formation and, hence, will be removed during the first steps of washing. This stresses the importance of thorough washing of alginate microbeads after gel formation.
The leakage of divalent ions upon culture and following wash of the beads is influenced by the composition of the alginate and the initial binding of the ions (Table 3). The minimal loss of barium ions in Ca/Ba-beads of high-G alginate during washing indicates that most of the barium is tightly bound in the polymer network of these gels. The energetically favored formation of cross-links between long sequences of G residues and barium stabilizes the gel towards ion exchange with non-cross-linking Na+ ions in the cell culture media.
A long-term storage study of high-G alginate beads was performed (Table 5) to evaluate further loss of ions that may take place during prolonged culture and in vivo. Although the values after the 5th media shift are comparable of around 0.8 %, this represents the amount relative to bound barium in the beads (Figure 1). Hence, the absolute values of leaked barium differ by an order of magnitude. In the case of Ca/Ba-beads, only small amounts of barium leaked out, indicating that the three-step wash/culture/wash treatment of beads may be considered as sufficient to remove excess barium not tightly bound in the gel network. For Ba-beads, however, a substantial amount of barium was still leaking out from microbeads after the wash/culture/wash treatment indicating that additional steps of washing and culturing would be needed to reduce the leakage of barium from these beads.
Although declining, there were still detectable leakage of barium from both Ba- and Ca/Ba-beads after 5 shifts of media (Table 5). Hence, it is not clear from this study what would represent the final values of barium in the beads. Loss of cross-linking ions is connected to destabilization of the gel and swelling21, but no observation of swelling of the microbeads was observed during the extended culturing in this experiment (data not shown) or after 9 weeks in vivo (Figure 3) in agreement to previous findings10. Hence, the loss of barium seems to be connected to loss of not cross-linked ions. Indeed, the strong accumulation of barium by the high-G alginate from a solution of low Ba2+ content (1 mM BaCl2) indicates that the vast majority of barium is tightly bound to the alginate.
Concentrations of barium in mouse blood and femur and relevance for implantation to humans
Mice receiving Ba-beads and Ca/Ba-beads of high-G alginate showed significant increase in the barium concentrations in the blood and femurs at 16 weeks post implantation at different bead volumes (Figure 2), compared to the control. Increase in barium concentration in blood and femur was confirmed at 9 weeks post implantation for 0.3 ml of beads for both Ca/Ba- and Ba-beads (Table 6). Mice receiving Ba-beads had higher concentrations of barium, both in blood and bone, than mice transplanted with Ca/Ba-beads. This corresponds well with the findings of higher leakage of barium from the Ba-beads than from the Ca/Ba-beads. The value of 10 mg/kg found for barium concentration in bone for the control animals is in agreement with the value of 2 mg/kg previously reported for humans16. Also the mouse blood values of 0.9 µg/kg are in relative agreement to reported human values of 3–9 µg/kg for brain, lung and liver16. The dose of 0.3 ml beads corresponds with the dose of diabetic mice transplanted i. p. with human islets for reaching normoglycemia post transplantation given a concentration of islets of 10,000 IEQ/ml alginate13. No obvious effect of the increase in barium in blood or femur was observed in mice in this experiment or in previous experiments where also higher doses of Ca/Ba-beads were used (data not shown and Qi et al.13, respectively), however, this was not specifically examined in this work. Nevertheless, a lower dose of 0.02 ml of alginate beads in a mouse of 25 g corresponds closely to a clinically relevant situation where for 10 000 IEQ/ml22 of alginate solution only a dose of 0.86 ml of alginate beads per kg of body weight is estimated. At this dose (Figure 2), no significant increase in the barium concentration was seen in blood, however significant increase was observed in femurs of mice receiving either Ca/Ba- or Ba-beads. Still significantly higher barium concentrations were seen in blood and femur bone of mice receiving Ba-beads compared to Ca/Ba-beads.
Calcium was measured at the same time as barium in mice femur bone at 16 weeks post implantation, the concentrations of calcium being in the range of 2×104 times the concentrations of barium in the control animals. There was a small, but significant reduction in calcium in femur bone comparing mice implanted with Ba-beads to the control for all doses of microbeads: p = 0.007 – 0.05 (data not shown). No significant changes were found for mice implanted with Ca/Ba-beads for any dose (p < 0.05). Measurements of calcium were not performed on the blood sample of the animals. The concentration of barium in the blood of the control animals was approximately 1 µg/kg (Figure 2A). This is 125 times less than the normal concentration of free calcium in serum (1 mM). The highest concentration measured in the blood of the mice (5 µg/kg, Figure 2A), corresponds to 25 times less the normal concentration of free calcium in serum, and is 5 times the normal values. Hence, the concentration of barium is much lower than the concentration of calcium in blood, however, an influence of barium on the calcium concentration can not be ruled out.
Based on the results from this work, the concentration of barium ions leaking from the two types of high-G alginate beads can be estimated for a situation relevant for implantation of alginate microbeads to humans. It can then be correlated with the tolerable intake value as well as to other available barium exposure values related to humans (Table 7). The tolerable intake value is defined as an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure of the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime and is by WHO estimated as 0.02 mg barium/kg body weight per day15. A patient of 70 kg transplanted with e.g. 600,000 IEQ will receive 60 ml of alginate gel beads (10 000 IEQ/ml alginate solution). For Ca/Ba-beads, 0.76 % barium leakage (Table 5) from a content of 1.2 mg barium/ml alginate (Figure 1C) gives 0.009 mg barium/ml alginate resulting in 0.008 mg barium/kg taking into account the numbers given above (70 kg person and 60 ml alginate beads). This value is 2.5 times lower than the tolerable intake value. However, for the high-G Ba-beads, the similar estimate leads to the value of 0.03 mg barium/kg, which is about 1.5 times higher than the tolerable intake value. This demonstrates the importance of both selection of gelling conditions and increasing the number of washing steps to further reduce the leakage of barium before the in vivo use of alginate microbeads gelled in the presence of barium ions.
Table 7.
Human reference values for barium exposure.
| Reference values: | Barium (mg/day) |
Barium (mg/(kg - day)) |
Reference |
|---|---|---|---|
| Lethal dose | 800–5000 | - | [14, 22] |
| Observed acute toxic effect | 200 | - | [14, 22] |
| No observable adverse effect level (NOAEL) | - | 0.21 | [14, 22] |
| Subchronic oral reference dose (RfD) | - | 0.0700 | [14, 22, 23] |
| Chronic oral RfD | - | 0.200 | [14, 22, 24] |
| Tolerable intake | - | 0.02 | [15] |
| Daily intake, mainly from diet | 0.3–1.7 | 0.4–0.025 | [15, 16] |
The dose of microbeads may be reduced by e.g. increasing the concentration of islets in the alginate. Indeed, Cui et al. report the use of 20,000 IEQ/ml of alginate11. The increase in concentration gives a higher probability of increasing the number of islets per capsule, but also the benefit of reducing the number of capsules without islets. However, it is not unlikely that the dose of beads may need to be increased as re-transplantation of encapsulated islets is a relevant scenario. Ideally a combination of Ca2+ and Ba2+ should be used with a high-G alginate for alginate microbead formation to specifically target the cross-linking zones for Ba2+ and leave Ca2+ to bind in the regions of the alginate where barium ions are not participating in the cross-links.
Acknowledgments
This study was conducted as part of the Chicago Diabetes Project, an international effort for a functional cure for diabetes. The study was sponsored by the Christopher Foundation, the Efroymson Fund, the Wirtz Family, and the College of Medicine at UIC. J.O. is supported by NIH (ICR Grant 1 U42 RR023245-01) and JDRF (#5-2006-398 and #5-2006-424 and #5-2007-773), as well as by the Washington Square Health Foundation and the Grant Health Care Foundation. B.L.S. was supported by the Norwegian Diabetes Association/Norwegian Foundation for Health and Rehabilitation and Helse Midt-Norge. I.L. was also supported by the Slovak Research and Development Agency under the contract No. APVV-0486-10. We thank David Hunkeler (AQUA+TECH) for fruitful discussions.
References
- 1.Lanza RP, Jackson R, Sullivan A, Ringeling J, McGrath C, Kuhtreiber W, Chick WL. Xenotransplantation of cells using biodegradable microcapsules. Transplantation. 1999;67(8):1105–1111. doi: 10.1097/00007890-199904270-00004. [DOI] [PubMed] [Google Scholar]
- 2.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 3.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes - First two cases. Diabetes Care. 2006;29(1):137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 4.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. Safety and Viability of Microencapsulated Human Islets Transplanted Into Diabetic Humans. Diabetes Care. 2009;32(10):1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokke BT, Smidsrød O, Zanetti F, Strand W, Skjåk-Bræk G. Distribution of uronate residues in alginate chains in relation to alginate gelling properties. 2. Enrichment of beta-d-mannuronic acid and depletion of alpha-l-guluronic acid in sol fraction. Carbohydr. Polym. 1993;21(1):39–46. [Google Scholar]
- 6.Donati I, Holtan S, Mørch YA, Borgogna M, Dentini M, Skjåk-Bræk G. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules. 2005;6(2):1031–1040. doi: 10.1021/bm049306e. [DOI] [PubMed] [Google Scholar]
- 7.Draget KI, Strand BL, Hartmann M, Valla S, Smidsrød O, Skjåk-Bræk G. Ionic and acid gel formation of epimerised alginates; The effect of AlgE4. Int. J. Biol. Macromol. 2000;27(2):117–122. doi: 10.1016/s0141-8130(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 8.Haug A. The affinity of some divalent metals to different types of alginates. Acta Chem. Scand. 1961;15:1794–1795. [Google Scholar]
- 9.Haug A, Smidsrød O. Selectivity of some anionic polymers for divalent metal ions. Acta Chem. Scand. 1970;24:843–854. [Google Scholar]
- 10.Mørch YA, Donati I, Strand BL, Skjåk-Bræk G. Effect of Ca2+, Ba2+ and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7(5):1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 11.Cui H, Tucker-Burden C, Cauffiel SMD, Barry AK, Iwakoshi NN, Weber CJ, Safley SA. Long-Term Metabolic Control of Autoimmune Diabetes in Spontaneously Diabetic Nonobese Diabetic Mice by Nonvascularized Microencapsulated Adult Porcine Islets. Transplantation. 2009;88(2):160–169. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]
- 12.Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–58. doi: 10.1097/01.tp.0000149340.37865.46. [DOI] [PubMed] [Google Scholar]
- 13.Qi M, Strand BL, Morch Y, Lacik I, Wang Y, Salehi P, Barbaro B, Gangemi A, Kuechle J, Romagnoli T, et al. Encapsulation of Human Islets in Novel Inhomogeneous Alginate-Ca2+/Ba2+ Microbeads: In Vitro and In Vivo Function. Artificial Cells Blood Substitutes and Biotechnology. 2008;36(5):403–420. doi: 10.1080/10731190802369755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EPA. Toxological review of barium and compounds. Cincinnati, OH: United States Environmental Protection Agency; 2005. pp. 1–57. [Google Scholar]
- 15.WHO. Barium and Barium Compounds. Geneva, Switzerland: World Health Organization; 2001. pp. 1–31 p. On line: http://whqlibdoc.who.int/publications/2001/9241530332.pdf. [Google Scholar]
- 16.Baselt RC. Disposition of Toxic Drugs and Cehmicals in Man. Foster City, CA: Biomedical Publications; 2004. [Google Scholar]
- 17.Iavicoli I, Carelli G, Castellino N, Schlemmer G. The determination of low lead levels in the bone of lead-depleted mice by graphite furnace atomic absorption spectrometry. Fresenius Journal of Analytical Chemistry. 2001;370(8):1100–1104. doi: 10.1007/s002160100917. [DOI] [PubMed] [Google Scholar]
- 18.Smidsrød O. Molecular basis for some physical properties of alginates in the gel state. J. Chem. Soc. Faraday Trans. 1974;57:263–274. [Google Scholar]
- 19.Kohn R, Larsen B. Preparation of water-soluble polyuronic acids and their calcium salts, and the determination of calcium ion activity in relation to the degree of polymerization. Acta Chem. Scand. 1972;26:2455–2468. doi: 10.3891/acta.chem.scand.26-2455. [DOI] [PubMed] [Google Scholar]
- 20.Rokstad AM, Donati I, Borgogna M, Oberholzer J, Strand BL, Espevik T, Skjåk-Bræk G. Cell-compatible covalently reinforced beads obtained from a chemoenzymatically engineered alginate. Biomaterials. 2006 doi: 10.1016/j.biomaterials.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Donati I, Mørch YA, Strand BL, Skjåk-Bræk G, Paoletti S. Effect of Elongation of Alternating Sequences on Swelling Behavior and Large Deformation Properties of Natural Alginate Gels. Journal of Physical Chemistry B. 2009;113(39):12916–12922. doi: 10.1021/jp905488u. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]