Abstract
Purpose
These studies explored questions related to the potential use of Laromustine in the treatment of solid tumors and in combination with radiotherapy.
Materials and Methods
These studies used mouse EMT6 cells [both parental and transfected with genes for O6-alkylguanine transferase (AGT)], repair-deficient human Fanconi Anemia C and Chinese hamster VC8 (BRCA2−/ −) cells and corresponding control cells, and EMT6 tumors in mice assayed using cell survival and tumor growth assays.
Results
Hypoxia during Laromustine treatment did not protect EMT6 cells or human fibroblasts from this agent. Rapidly proliferating EMT6 cells were more sensitive than quiescent cultures. EMT6 cells expressing mouse or human AGT, which removes O6 -alkyl groups from DNA guanine, thereby protecting against G-C crosslink formation, increased resistance to Laromustine. Crosslink-repair-deficient Fanconi Anemia C and VC8 cells were hypersensitive to Laromustine, confirming the importance of crosslinks as lethal lesions. In vitro, Laromustine and radiation produced additive toxicities to EMT6 cells. Studies using tumor cell survival and tumor growth assays showed effects of regimens combining Laromustine and radiation that were compatible with additive or subadditive interactions.
Conclusions
The effects of Laromustine on solid tumors and with radiation are complex and are influenced by microenvironmental and proliferative heterogeneity within these malignancies.
Keywords: Laromustine, Onrigin, radiation, O6-Alkylguanine transferase, combined modalities, experimental radiotherapy
Introduction
Laromustine (also called onrigin, cloretazine, VNP40101M, and 101M), a sulfonylhydrazine prodrug first synthesized in the Sartorelli laboratory, undergoes base-catalyzed activation, to generate two classes of reactive electrophiles: one is an array of alkylating species with chloroethylating activity and the other is methyl isocyanate with carbamoylating activity (Baumann et al. 2004, Finch et al. 2001, Penketh et al. 2000, Shyam et al. 1996). Assays in biochemical systems and intact cells showed that Laromustine chloroethylates DNA at the O6-position of guanine, ultimately resulting in the formation of an interstrand crosslink with cytosine on the opposite strand (Baumann et al. 2004, 2005, Ishiguro et al. 2005, 2006, Penketh et al. 2000, 2004, 2008, Rice et al. 2005). These G-C ethane crosslinks are considered to be the lethal lesions produced by Laromustine. Measurements of crosslinking and of IC50 values (concentrations needed to inhibit cell growth by 50%) showed that <10 such crosslinks per cellular genome resulted in 50% mortality in three different leukemia cell lines (Penketh et al. 2008).
The formation of the lethal interstrand crosslinks is inhibited by the activity of the repair protein O6 -alkylguanine-DNA alkyltransferase (AGT), which transfers guanine O6 -alkyl groups to the cysteine in the active site of AGT and thereby restores the O6 -position of the guanine to its native state (Baumann et al. 2005, Ishiguro et al. 2006, 2010, Penketh et al. 2004, 2008, Rice et al. 2005). Because this transfer irreversibly inactivates AGT in a stoichiometric reaction, one AGT molecule can repair only a single O6-guanine lesion. The ability of a cell to repair alkylation through this route is therefore limited by the intracellular levels of AGT. Previous studies (Ishiguro et al. 2005, 2006, Penketh et al. 2008) showed a quantitative relationship between the number of AGT molecules present in cells and the resistance of the cell populations to Laromustine. The resistance of a panel of tumor cell lines with naturally varying levels of AGT expression increased progressively with AGT levels. Moreover, the IC50 for wild-type EMT6 cells, which lack demonstrable levels of AGT, was shown in cell growth studies to increase progressively with AGT expression level when EMT6 cells were transfected with the genes for either human or mouse AGT (Ishiguro et al. 2005). Tumors modified to express AGT likewise showed increased resistance to the effects of Laromustine (Ishiguro et al. 2005). Differences in the levels of AGT in tumor cells and in the cells of critical normal tissues may provide a basis for achieving selective antitumor effects with Laromustine in vivo (Ishiguro et al. 2005, 2010, Penketh et al. 2008).
Phase I/II trials showed that Laromustine had significant activity in the treatment of acute myeloid leukemia (AML) and high risk myelodysplastic disorders, with a therapeutic ratio and toxicity profile suggesting that the drug might have value for the treatment of patients with refractory disease and in the treatment of these diseases in the elderly (Giles 2007, Giles et al. 2007, Steensma 2010, Vey and Giles 2010). Furthermore, there are data to suggest that variability in the response of individual AML patients to Laromustine may be related to variations in the levels of AGT in the tumors (Giles 2007). Clinical studies examining the use of Laromustine in the treatment of solid tumors are limited to Phase I trials (Murren et al. 2005) that provide little insight into the potential efficacy of this agent for solid malignancies. Although Laromustine has been shown to be very effective in vivo in several rodent and human tumor models (Finch et al. 2001), the effects of the unique microenvironments within solid tumors and of the proliferative perturbations induced by microenvironmental heterogeneity in solid tumors have not yet been explored.
The work reported here extends preclinical studies of this novel investigational anticancer agent to explore additional questions related to its potential use in the treatment of solid tumors. We examined the effects of hypoxia on the cytotoxicity of Laromustine. Hypoxic cells are a common feature of solid malignancies, sometimes comprising the majority of the cells in the tumors (Moulder and Rockwell 1987, Rockwell et al. 2009). These cells are resistant to radiation and to many anticancer drugs; they are also viable and clonogenic, and cause tumors to recur after intensive therapeutic regimens. The response to Laromustine in hypoxia will therefore be important for determining its efficacy in solid tumors. In addition, we compared the effects of Laromustine on proliferating and quiescent cells, because solid tumors generally contain large numbers of non-proliferating clonogenic cells, which are resistant to the many anticancer drugs that target pathways critical to cell proliferation. These therapeutically resistant quiescent cells resume proliferation as the tumor microenvironment changes after treatment and cause recurrences (Hahn et al. 1974, Ray et al. 1973, Rockwell et al. 2009). As Laromustine moves toward broader clinical use, it will be important to better define the effects of regimens combining Laromustine with other therapeutic agents, so as to optimize these combined modality regimens. We therefore examined the interactions of Laromustine with radiation, since 65% of patients with solid cancers receive radiotherapy during the course of their treatment, often in combination with chemotherapy and often with curative intent.
Materials and Methods
Cell lines and Cell culture techniques
The cell culture experiments described here used EMT6 mouse mammary tumor cells; human Fanconi Anemia C fibroblasts and control fibroblasts; and Chinese hamster VC8 cells (deficient in BRCA2) and control VC8 cells complemented with the wild-type BRCA2 gene. All cells were maintained as monolayer cultures at 37°C in an atmosphere of 95% air / 5% CO2. EMT6 cells have been used extensively in the authors’ laboratories and their characteristics are well defined (Ishiguro et al. 2010, Rockwell 1977, Rockwell et al. 1972). Two features of EMT6 cells are important in these studies. First, they lack detectable AGT (Ishiguro et al. 2010) and second, they can be grown as solid tumors in mice as well as in culture (Rockwell 1977). EMT6 cells were maintained in Waymouth’s Medium with 15% Fetal Plex™ animal serum complex and antibiotics. EMT6 cells transfected with murine or human AGT cDNAs were developed and characterized by Ishiguro et al. (2010) and Baumann et al. (2010), respectively, and were cultured in McCoy’s 5A medium with 10% Fetal Bovine Serum (FBS) and antibiotics. VC8 cells (BRCA2 −/ −) and control VC8 cells transfected with and expressing the wild type BRCA2 gene were a gift of Dr. Graeme C. M. Smith (KuDOS Pharmaceuticals Ltd., Cambridge, United Kingdom) and were maintained in Dulbecco’s Minimal Essential Medium (high glucose) supplemented with 10% FBS and antibiotics (Rockwell et al. 2009). Established cell lines derived from human Fanconi Anemia C fibroblasts (PD331) and the corresponding control fibroblasts (PD751) were generously provided by Barbara Cox at the Fanconi Anemia Repository at Oregon Health Sciences University, Portland OR, USA, and were maintained in Alpha Minimal Essential Medium supplemented with 10% FBS, and antibiotics as described previously (Paz et al. 2008). All cell culture media, trypsin, and antibiotics were obtained from GIBCO (Invitrogen), Carlsbad CA, USA; all sera were obtained from Gemini Bioproducts, West Sacramento CA USA.
Unless otherwise noted, cell cultures were plated in standard 60 mm cell culture dishes using the media described above for each cell line, and treated in mid exponential growth under aerobic conditions (95% air / 5% CO2) in a standard cell culture incubator. Cultures were treated with drug and radiation as described below and trypsinized immediately after treatment, and the suspended cells were assayed for survival using colony formation assays as described previously (Rockwell 1977, Rockwell and Liu 2009). Surviving fractions were calculated relative to the plating efficiencies of untreated control cultures plated in the same experiment on the same day. Cell numbers in treated and control cultures were compared to detect any rapid cell death occurring during treatment, which could compromise the clonogenic assays; no decrease in cell numbers in treated cultures was detected in these studies.
In experiments examining the effects of hypoxia, cells were planted in glass milk dilution bottles and used in exponential growth. Cultures were made hypoxic by sealing the bottles with rubber gaskets, inserting needles for the influx and efflux of gases, and gassing with a humidified mixture of 95% nitrogen / 5% CO2 containing < 1 ppm O2 for two hours before treatment. Laromustine was injected through the septum of the gasket, without breaking the hypoxia, and cultures were incubated with this agent under hypoxia for 2 hours as described previously (Rockwell and Liu 2009). Controls exposed to hypoxia plus vehicle and cultures treated with Laromustine under aerobic conditions were treated simultaneously, in the same experiments.
In experiments comparing exponentially growing and quiescent cultures, EMT6 cells were plated in cell culture dishes as described above. One set of cultures, plated 3 days before treatment, were in mid exponential growth at the time of exposure to Laromustine, with a density of 1.2 ± 0.5 × 106 cells/dish. A second set of cultures was plated 7 days before exposure to Laromustine and medium was changed daily beginning on day 4. This procedure produced cultures in mid plateau phase (density 9.6 ± 0.1 × 106 cells per dish) at the time of treatment; cell number had ceased increasing two days before Laromustine, but the plating efficiency of these cultures remained the same as those of the exponential cultures that were processed simultaneously.
Tumor studies
All experiments with tumors in vivo were performed using BALB/c Rw mice 2.5–3.5 months of age that had been bred and raised in our specific pathogen free production colony. All protocols used with experimental animals were reviewed and approved by the Yale Institutional Animal Care and Use Committee. All experiments were performed in full compliance with the regulations and policies of the government, Yale University, and the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and with the principles outlined in the Guide for the Care and Use of Laboratory Animals. Tumors were produced by inoculating 2 × 105 EMT6 cells, harvested from exponentially growing cell cultures, intradermally into the flank, and were treated with Laromustine or radiation as described below approximately 2 weeks later, when the volumes were about 100 mm3 (Donnelly et al. 2007).
Tumor cell survival was assayed using a colony formation assay. Mice were euthanized using CO2, a euthanasia technique approved by the American Veterinary Medical Association. Tumors were then explanted sterilely and a single cell suspension was created by mincing and trypsinization as described in detail previously (Donnelly et al. 2007, Rockwell et al. 1977). Cells were collected by centrifugation, resuspended in Waymouth’s medium, counted under a phase contrast microscope using trypan blue to determine the number of cells damaged during the suspension process, and plated for colony formation as described above. Surviving fractions were calculated using the plating efficiencies of cells from untreated control tumors plated on the same day, which averaged 24 ± 2 % in the experiments reported here.
In tumor growth delay studies, the three diameters of each tumor were measured three times per week with vernier calipers and tumor volumes were calculated using the formula for the volume of a hemiellipsoid. When the tumors reached an average volume of 100 mm3, they were stratified by volume into control and treatment groups, which were treated with radiation and/or Laromustine as described below. Mice were then monitored and tumor volumes were measured three times per week until each tumor reached a volume of 1000 mm3. Tumor growth was compared by assessing the times needed for control and treated tumors to grow from the treatment volume to four times the treatment volume (Donnelly et al. 2007).
Drug and radiation treatments
Laromustine was provided without cost by Vion Pharmaceuticals. For cell culture studies, Laromustine was dissolved in dimethyl sulfoxide (DMSO; JT Baker, Phillipsburg, NJ, USA) and used within 1 hour of preparation. For studies in mice, Laromustine was dissolved in DMSO, then diluted in sterile pyrogen-free physiologic saline immediately before intraperitoneal injection. Because DMSO has biological effects and is a radioprotector, groups treated with the vehicle used to dissolve the highest dose of Laromustine were included in the cell culture and tumor cell survival experiments to detect any effects of the vehicle; none were observed, probably because the final levels of DMSO in the cell culture medium (< 0.8%) or injected into mice after dilution in saline (< 0.007 ml/gm) were low.
Cell cultures were irradiated with 320 kV X-rays produced by an XRAD (Precision x-ray, Branford CT, USA) at 12.5 mA, 2 mm Al filtration, and a dose rate of 2.4 Gy/min. In tumor growth studies, mice were anesthetized with ketamine/xylazine and positioned with the body shielded, and the tumors were irradiated locally with 250 kV X-rays produced by a Siemens Stabilipan (Malvern PA, USA) at 12.5 mA, 2 mm Al filtration and a dose rate of 6.4 Gy/min. Because the X-ray doses received by the intestines, bone marrow and other critical normal tissues were less than 5% of the tumor dose, these mice had no significant systemic injuries from the radiation. In tumor cell survival studies, mice were loosely confined in individual chambers of a well ventilated Lucite irradiation box and whole body irradiated with 250 kV X-rays produced by a Siemens Stabilipan at 12.5 mA, 2 mm Al filtration and a dose rate of 1.1 Gy/minute.
For regimens combining Laromustine and radiation in cell culture, drug was added to the culture medium 2 hours before suspension of cells to assay cell survival. Cultures were irradiated 5 minutes before the end of drug exposure and cell survival was assayed immediately after irradiation. In tumor studies, mice were treated with Laromustine 5 minutes before the start of irradiation. Tumor cell survival was measured 2 hours after injection of Laromustine; groups receiving radiation alone were assayed using similar times between treatment and measurement of cell survival. The effects of regimens combining the two agents were analyzed by considering the dose response curves for each agent individually, as well as the data for the combined treatments (Steel, 1979).
Results
Effects of the cellular environment on the survival of cells treated with Laromustine
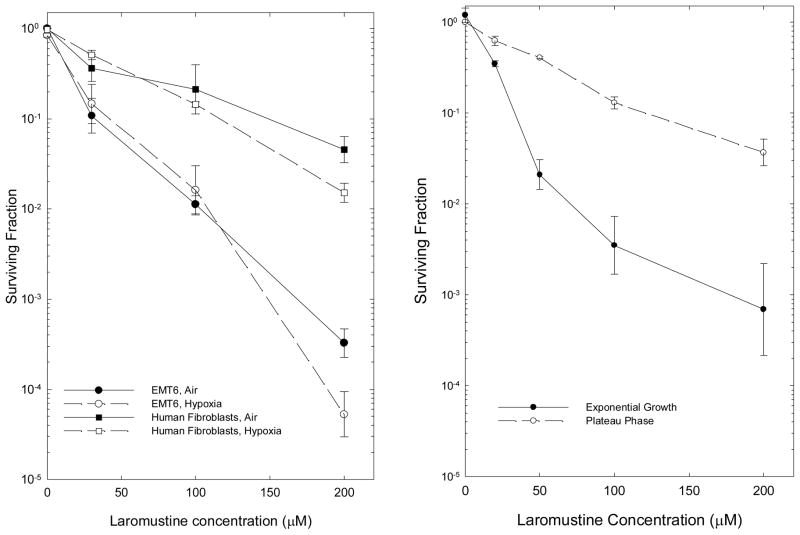
The effects of Laromustine on cell survival were examined using EMT6 mouse mammary tumor cells and using an established human fibroblast cell line; both cell lines were examined in exponential growth in monolayer cultures, under both aerobic and severely hypoxic conditions (Figure 1, left panel). Laromustine produced exponential dose-response curves for both cell lines; EMT6 cells were more sensitive than the human fibroblasts. Acute, severe hypoxia initiated 1 hour before the beginning of Laromustine treatment and continuing throughout the 2 hour exposure did not produce statistically significant changes in the survival curve for either cell line from that observed under aerobic conditions.
Figure 1.
Effect of hypoxia and proliferative state on the response of cells in vitro to Laromustine. Left panel: Hypoxia was induced 2 hr before treatment and continued throughout the 2 hr drug treatment. Points are means ± SEM of 4–8 independent measurements for EMT6 cells (circles) and of 3–4 measurements for human fibroblasts (squares). Surviving fractions are calculated relative to untreated control cultures; surviving fractions shown at zero dose are those for vehicle-treated control cultures subjected to all experimental manipulations. Right Panel: Survival of EMT6 cells treated with Laromustine in exponential growth or plateau phase for 2 hrs, then plated for colony formation immediately after treatment. Points are means ± SEM from 3 independent experiments.
Effects of cell proliferation on the survival of cells treated with Laromustine
The proliferative state of the cells was important in determining their sensitivity to Laromustine (Figure 1, right panel). Density-inhibited, quiescent, plateau phase EMT6 cultures were much less sensitive to Laromustine than cells in exponential growth.
Effects of AGT levels on the survival of cells treated with Laromustine
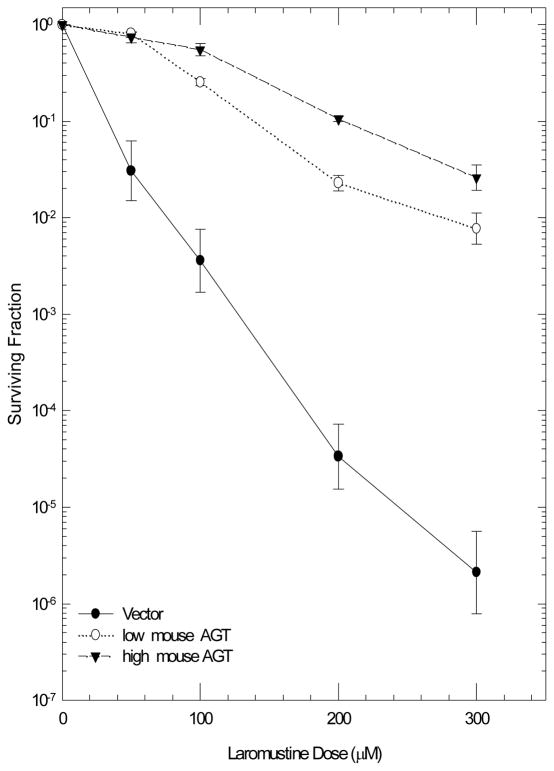
As shown previously by Ishiguro et al. (2010) using a cell growth assay, the sensitivity of EMT6 cells to Laromustine (Figure 1) reflects in part the absence of detectable AGT in these cells. EMT6 cells transfected to express low or high levels of mouse AGT (4,000 and 10,000 molecules/cell, respectively) or high levels of human AGT (18,000 molecules/cell) showed greatly increased resistance to killing by Laromustine over that seen in the respective vector-transfected controls. Resistance was seen over the full range of Laromustine concentrations examined (Figure 2), which include and extend beyond those achieved in clinical Phase I studies with the drug (60 μM) (Vey and Giles 2010), confirming the importance of the cellular AGT levels in determining the response of cells to Laromustine concentrations in the clinical range. EMT6 cells were more resistant to Laromustine than other AGT negative cell lines (Ishiguro et al. 2005, 2010, Penketh et al. 2008). This probably reflects the robust DNA repair capacity of EMT6 cells, which are resistant to radiation and many anticancer drugs.
Figure 2.
Effect of the AGT level on the response of EMT6 cells to Laromustine. Left panel: EMT6 transfected with an empty vector (●) or with a vector containing mouse AGT and expressing the AGT at a low (○) or high (▼) level. Right Panel: EMT6 cells transfected with an empty vector (●) or with a vector containing human AGT (○). Cells were treated with Laromustine for 2 hours. Points are means ± SEM from 3–4 experiments.
Effects of DNA repair on the survival of cells treated with Laromustine
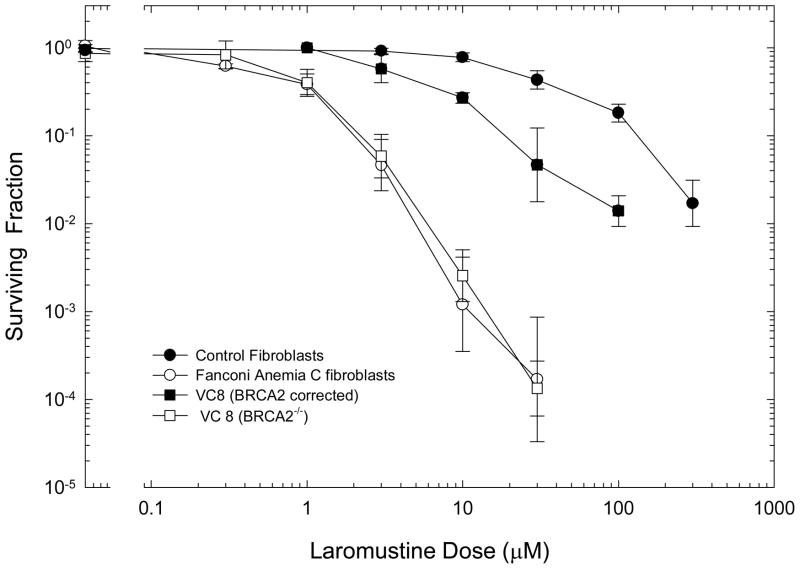
The ability of cells to repair DNA crosslinks influenced the cytotoxicity of Laromustine dramatically (Figure 3). A human cell line with a Fanconi anemia C mutation was hypersensitive relative to the corresponding control cell line. Similarly, the Chinese hamster line VC8, which is deficient in BRCA2, showed greatly enhanced sensitivity to the cytotoxic effects of Laromustine relative to the control cell line corrected by transfection with the normal BRCA2 gene and expressing this repair protein.
Figure 3.
Effect of repair capacity on the cytotoxicity of Laromustine. Normal repair-proficient human fibroblasts (●) or fibroblasts with a Fanconi Anemia C mutation (○) were treated with Laromustine for 2 hours and assayed for survival using a clonogenic assay. BRCA2 deficient VC8 cells (□) and the control cells transfected to express BRCA2 (■) were treated for 2 hours with graded doses of Laromustine and assayed for survival using clonogenic assays. Points are mean ± SEM for 3 independent determinations for human cells and 4–6 independent determinations for VC8 cell lines.
Effects of Laromustine on cellular radiosensitivity in vitro
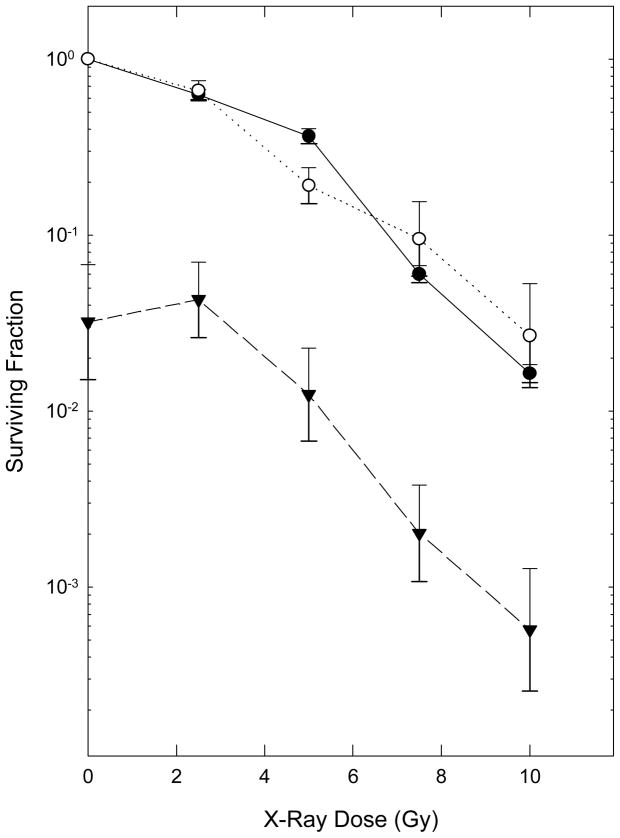
To test whether treatment with Laromustine altered cellular radiosensitivity, EMT6 cells were treated with a relatively toxic concentration of this agent (60 μM for 2 hours, which reduced the surviving fraction to 0.032) and irradiated with graded doses of X-rays during the final few minutes of exposure to Laromustine. Figure 4 compares the survival curves for X-rays alone with those for radiation delivered along with Laromustine. To allow easy visual comparison of the two survival curves, the data for radiation plus Laromustine are shown both as absolute surviving fractions and also normalized to the Laromustine-only group. Laromustine did not change either the shape or slope of the radiation dose-response curve significantly. In addition, isobologram analyses of the interactions (not shown) using the full survival curves for Laromustine alone (Figure 1) and radiation alone (Figure 4) and the data for radiation plus Laromustine shown on Figure 4 indicated that the two agents had additive cytotoxicities. These studies using exponentially-growing EMT6 cell populations under aerobic conditions in vitro provide no evidence for mechanistic interactions between the cytotoxic effects of Laromustine and those of radiation.
Figure 4.
Effect of Laromustine on the radiation dose-response curve for EMT6 cells. Cells were treated with graded doses of radiation alone (●) or in combination with a 2 hour treatment with 60 μM Laromustine (▼). The survival curve for Radiation plus Laromustine is also shown normalized to the survival of cells treated with Laromustine alone (○) to facilitate comparison of the two survival curves. Points are means ± SEM of 3–5 independent determinations.
Effects of Laromustine on EMT6 tumors
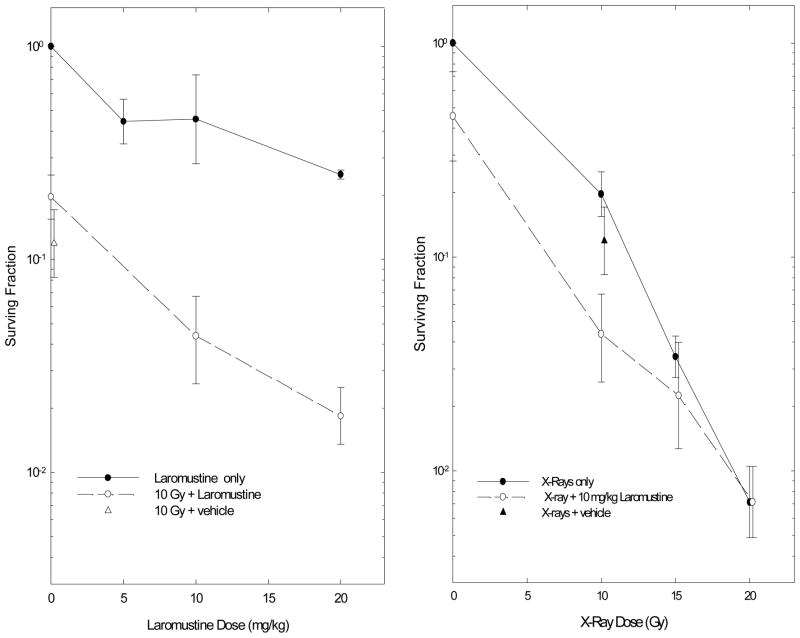
The effects of Laromustine were also examined using EMT6 tumors in mice. In one set of experiments, the surviving fractions of the tumor cells were measured after large single doses of Laromustine, large single doses of radiation, or regimens combining Laromustine and radiation. Laromustine alone had moderate toxicity to cells in EMT6 tumors (Figure 5); the doses given in these studies were limited by the solubility of Laromustine, which precluded giving higher doses of drug in a single injection. When given in combination with 10 Gy of X-rays, 10 or 20 mg/kg of Laromustine reduced the survival of the tumor cells by similar proportions in unirradiated and irradiated tumors and indicated additive effects of these regimens combining the two agents. When a constant dose of Laromustine (10 mg/kg) was given in combination with graded doses of radiation, the incremental effects of Laromustine decreased as the radiation dose increased (Figure 5), and the cell survivals observed at 15 and 20 Gy were compatible with subadditive cytotoxicities.
Figure 5.
Survival of cells from solid EMT6 tumors treated with radiation and Laromustine. Left panel: graded doses of Laromustine alone or in combination with a constant dose (10 Gy) of radiation. Right panel: graded doses of radiation alone or in combination with a constant dose (10 mg/kg) of Laromustine. Surviving fractions are means ± SEM of 3 to 7 independent determinations.
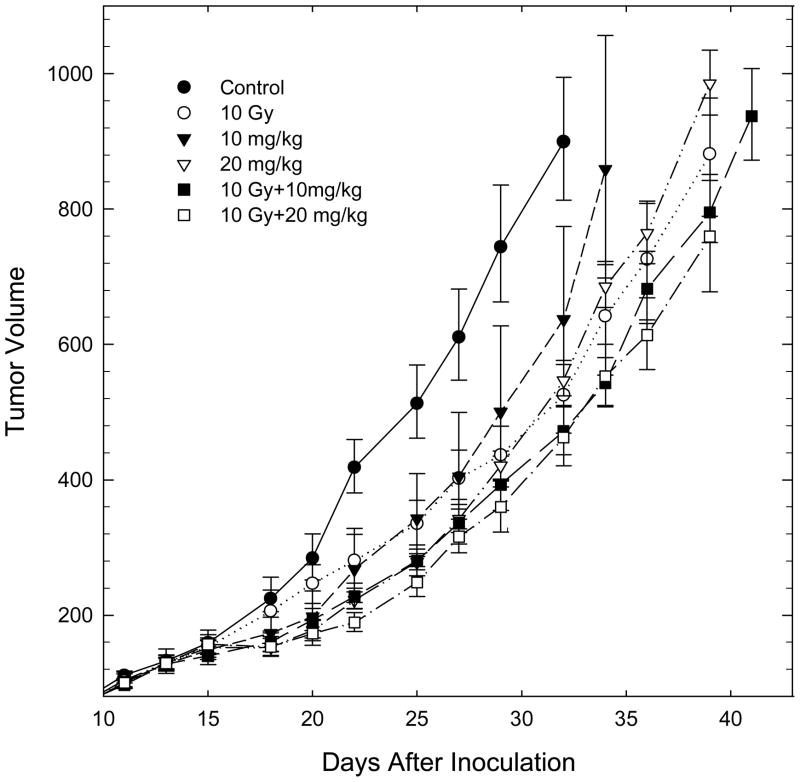
Experiments examining the growth of treated and control tumors showed modest delays in tumor growth with Laromustine alone and with 10 Gy of radiation alone (Figure 6 and replicate experiments, not shown). Regimens combining radiation (10 Gy) with 10 and 20 mg/kg of Laromustine showed tumor growth delays that were consistent with either additive or subadditive toxicities of the two agents, in agreement with findings from the more extensive tumor cell survival data shown in Figure 5.
Figure 6.
Representative experiment showing the growth of EMT6 tumors treated on day 13 with large single doses of Laromustine and radiation, alone or in combination. Points are means ± SEM for 5–6 tumors within a single experiment; a replicate experiment gave similar findings. The growth delays, calculated as the excess time needed for treated tumors to reach 4 times the treatment volume, were 6.2 ± 0.8, 3.4 ± 1.8, 6.8 ± 0.8, 7.6 ± 0.4 and 7.9 ± 0.8 days for 10 Gy, 10 and 20 mg/kg Laromustine, and 10 Gy + 10 and 20 mg/kg Laromustine, respectively.
Discussion
Clinical trials with Laromustine have focused on its use in combination chemotherapy regimens for hematologic malignancies. The experiments reported here begin to explore questions important in the use of Laromustine for the treatment of solid tumors and in combination with radiation therapy. Severe hypoxia is present in most solid tumors and induces resistance to many widely-used anticancer drugs (Teicher et al. 1981, Rockwell et al. 2009). Severe hypoxia shortly before and during Laromustine exposure did not protect cells in culture from the cytotoxic effects of Laromustine. This finding confirmed the expectation, based on the mechanism of action of Laromustine (Baumann et al. 2005, Ishiguro et al., 2006, Penketh et al. 2008), that hypoxia would not alter the effects of this agent. Hypoxia should not be a barrier to the effective treatment of solid malignancies with Laromustine.
Laromustine was markedly more toxic to EMT6 cells in exponentially growing cultures than to quiescent plateau phase cells. This differs from our findings for the crosslinking agents mitomycin C and porfiromycin (Rockwell and Hughes 1994) and those of Twentyman and Bleehan (1975) for carmustine, lomustine, bleomycin, and cis-platin, which all showed similar cytotoxicities to exponentially growing and plateau phase EMT6 cells. The mechanism underlying the greater cytotoxicity of Laromustine to rapidly proliferating EMT6 cells is not yet known; it could imply a difference in initial alkylation, in crosslink formation after alkylation, or in recognition and repair of crosslinks caused by differences in the proliferative status, cell cycle distribution, or extracellular milieu of the exponential and plateau phase cultures. In our experiments, the medium on the plateau phase cultures was changed daily and medium on both exponential and plateau phase cultures was changed 1 hour before addition of Laromustine to ensure that differences in the pH, serum protein content, and nutrient content of the media during treatment were minimized. Because EMT6 cells are not contact inhibited, cells in plateau phase cultures pile up and drugs must penetrate through the upper cell layers to reach cells nearest to the growth surface; penetration effects must therefore be considered. Cultures were trypsinized and plated for colony formation immediately after treatment. Re-plating causes plateau phase EMT6 cells to begin rapid proliferation and prevents the repair of potentially lethal damage (PLD) that could occur if the plateau phase cells remained quiescent after treatment (Hahn et al. 1974, Ray et al. 1973). Thus, although plateau phase EMT6 cells are proficient in PLD repair when held in quiescence after treatment with radiation or drugs (Hahn et al. 1974, Rockwell 1977), PLD repair could not be responsible for the difference seen here. Moreover, PLD repair was not observed in EMT6 cells treated with mitomycin C or porfiromycin and may be less important for crosslinks than for some other lesions (Rockwell and Hughes 1994). Laromustine inhibits DNA polymerase β (Frederick et al. 2009), a principal enzyme in base excision repair; a direct effect of Laromustine on the enzymatic pathways that repair the DNA crosslinks therefore could be responsible for the unanticipated proliferation-dependence of Laromustine cytotoxicity. The biological basis for the differential cytotoxicity of Laromustine to proliferating and quiescent EMT6 cells, the generality of the effect in other cell lines, and the implication of this effect for the treatment of solid tumors, which contain large numbers of non-cycling clonogenic cells, all merit further investigation.
The importance of DNA crosslinks to the toxicity of Laromustine was shown in two sets of experiments. The studies shown in Figure 2 confirm the importance of the resistance protein AGT in determining the cytotoxicity of Laromustine. Expression of either mouse or human AGT in EMT6 cells increased the resistance of these cells over that of cells not expressing AGT throughout the range of Laromustine concentrations examined, confirming previous studies (Ishiguro et al. 2005, 2010, Penketh et al. 2008) suggesting that pretreatment measurements of tumor AGT levels could be used to guide therapy with Laromustine.
The importance of DNA crosslinks was also examined by using cell lines with different mutations in pathways implicated in the repair of DNA crosslinks: a Fanconi anemia C mutation in a cell line derived from a human patient and a BRCA2 mutation in Chinese hamster VC8 cells. We showed previously (Paz et al. 2008, Rockwell et al. 2009) that both mutations induce hypersensitivity to the DNA crosslinking agent mitomycin C. Both mutations produced hypersensitivity to Laromustine relative to the corresponding repair-proficient control cells. This suggests that screening for repair defects could be used to identify tumors that have increased sensitivity to Laromustine and other alkylating agents, such as BRCA2−/ − breast cancers. Normal tissues in patients with these tumors generally have a wild-type repair phenotype and therefore normal resistance to the toxic side effects of the drugs, providing the potential for therapeutic gain. (The rare patients with homozygous or dominant negative germline mutations would also be at increased risk of toxic side effects.) As our understanding of the genotypes of tumors and normal tissues in cancer patients improves, information on DNA repair pathways of individual tumors and patients may be increasingly useful in developing individualized therapeutic regimens (Li et al. 2010).
Regimens combining large single doses of Laromustine and radiation produced additive toxicities to exponentially growing EMT6 cells in vitro (Figure 4). The data provide no evidence for interactions between the lesions produced by radiation and those produced by Laromustine in rapidly proliferating cells in vitro.
The effects of Laromustine on EMT6 tumors in mice were more complex. Laromustine killed cells in solid tumors (Figure 5) and produced significant tumor growth delays (Figure 6), but both effects were relatively modest. In addition, increasing the dose of Laromustine from 10 to 20 mg/kg produced only modest gains in cell killing. Regimens combining Laromustine with 10 Gy of radiation (Figure 5, left panel) produced tumor cell killing that was consistent with additivity. However, regimens combining 10 mg/kg of Laromustine with higher doses of radiation (15 and 20 Gy; Figure 5, right panel) were not correspondingly more effective in killing tumor cells, and had cytotoxic effects that were compatible with either additive or subadditive cytotoxicities. Experiments using a tumor growth endpoint produced similar results. Large single doses of Laromustine produced modest growth delays. Regimens combining these treatments with 10 Gy (which alone produces only modest growth delays in this radiation-resistant tumor) produced additional delays in tumor growth, which were consistent with either additive or subadditive toxicities.
The interactions between Laromustine and radiation in tumors were different from those in cell cultures. In vitro studies of radiation/Laromustine interactions were performed using aerobic, exponentially-growing monolayer cultures. The malignant cell populations in solid tumors are much more complex, and many additional factors influence their response to treatment. In the studies reported here, the solubility and relatively short in vivo lifetime of Laromustine (Finch et al. 2001) may have contributed to the limited anti-tumor effects of Laromustine and the subadditive effects of some radiation-Laromustine combinations, by limiting the time the cells were in contact with cytotoxic concentrations of Laromustine and by limiting its distribution into poorly perfused areas of the tumors. EMT6 tumors are poorly vascularized; in tumors of the size used in these studies ~ 30% of the viable tumor cells are sufficiently hypoxic to be resistant to radiation (Rockwell 1977, 1998, Rockwell et al. 1972, 2009). The vast majority (90–99%) of the cells surviving the radiation doses used in these studies would be cells that had been severely hypoxic during irradiation. Failure of Laromustine to reach the unperfused areas containing hypoxic cells in effective concentrations would therefore result in subadditive cytotoxicities for the radiation-Laromustine combinations used here. The proliferative status of cells in EMT6 tumors would also affect the interactions between Laromustine and radiation. The cell cycle times of the proliferating cells in EMT6 tumors are longer and more variable than those of EMT6 cells in exponential growth in vitro (Rockwell 1977, Rockwell et al. 1972). Moreover, 50% of the cells in solid EMT6 tumors are quiescent, and the proportion of quiescent tumor cells is greater in areas that show histologic evidence of poor perfusion (Rockwell 1977; Rockwell et al. 1972). Importantly, the quiescent tumor cells are viable and resume proliferation when their environment improves, allowing them to form colonies or contribute to tumor growth and metastasis (Hahn et al. 1974, Rockwell 1977). Like the quiescent cells in plateau phase cell cultures, non-proliferating cells in solid tumors would be resistant to Laromustine. Tumors in vivo would therefore contain a mixture of Laromustine-sensitive and Laromustine-resistant cells; many Laromustine-resistant cells would be in poorly perfused areas that would also be hypoxic and therefore radioresistant as well.
Several aspects of the microenvironmental heterogeneity within solid EMT6 tumors could therefore limit the efficacy of the Laromustine treatments and combination regimens used in the experiments reported here. Many of these effects could be circumvented by the use of fractionated regimens combining multiple doses of Laromustine and multiple treatments with localized radiation at doses similar to those used in radiotherapy (1.8–2.0 Gy/fraction). Experiments testing such regimens represent a logical next step in evaluating the potential value of regimens combining Laromustine with radiation for the treatment of solid malignancies.
Footnotes
Declaration of Interest
This work was supported by grants P01 CA129186 and CA129186-03S2 from the National Cancer Institute (NCI). Core facilities supported by the Yale Cancer Center and NCI center grant 16359 were used in the performance of the studies.
None of the authors presently have conflicts of interest related to the work reported in this paper.
References
- Baumann RP, Penketh PG, Ishiguro K, Shyam K, Zhu YL, Sartorelli AC. Reductive activation of the prodrug 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine (KS119) selectively occurs in oxygen-deficient cells and overcomes O6-alkylguanine-DNA alkyltransferase mediated KS119 tumor cell resistance. Biochemical Pharmacology. 2010;79:1553–15561. doi: 10.1016/j.bcp.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann RP, Seow HA, Shyam K, Penketh PG, Sartorelli AC. The antineoplastic efficacy of the prodrug Cloretazine™ is produced by the synergistic interaction of carbamoylating and alkylating products of its activation. Oncology Research. 2005;15:313–325. doi: 10.3727/096504005776404553. [DOI] [PubMed] [Google Scholar]
- Baumann RP, Shyam K, Penketh PG, Remack JS, Brent TP, Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamino)carbonyl]hydrazine (VNP40101M): II. Role of O6-alkylguanine-DNA alkyltransferase in cytotoxicity. Cancer Chemotherapy Pharmacology. 2004;53:288–295. doi: 10.1007/s00280-003-0739-0. [DOI] [PubMed] [Google Scholar]
- Donnelly ET, Liu Y, Rockwell S. Efaproxiral (RSR13) plus oxygen breathing increases the therapeutic ratio of carboplatin in EMT6 mouse mammary tumors. Experimental Biology and Medicine. 2006;231:317–321. doi: 10.1177/153537020623100312. [DOI] [PubMed] [Google Scholar]
- Finch RA, Shyam K, Penketh PG, Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylamino)carbonylhydrazine (101M): A novel sulfonylhydrazine prodrug with broad-spectrum antineoplastic activity. Cancer Research. 2001;61:3033–3038. [PubMed] [Google Scholar]
- Frederick AM, Davis ML, Rice KP. Inhibition of human DNA polymerase β activity by the anticancer prodrug Cloretazine. Biochemical and Biophysical Research Communications. 2009;378:419–423. doi: 10.1016/j.bbrc.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles FJ. Bendamustine and cloretazine: Alkylators with sharply contrasting activity in AML. Leukemia & Lymphoma. 2007;48:1064–1066. doi: 10.1080/10428190701332464. [DOI] [PubMed] [Google Scholar]
- Giles F, Rizzieri D, Karp J, Vey N, Ravandi F, Faderl S, Khan KD, Verhoef G, Wijermans P, Advani A, Roboz G, Kantarjian H, Bilgrami SF, Ferrant A, Daenen SM, Karsten V, Cahill A, Albitar M, Mufti G, O’Brien S. Cloretazine (VNP40101M), a novel sulfonylhydrazine alkylating agent, in patients age 60 years or older with previously untreated acute myeloid leukemia. Journal of Clinical Oncology. 2007;25:25–31. doi: 10.1200/JCO.2006.07.0961. [DOI] [PubMed] [Google Scholar]
- Hahn GM, Rockwell S, Kallman RF, Gordon LF, Frindel E. Repair of potentially lethal damage in vivo in solid tumor cells after x-irradiation. Cancer Research. 1974;34:351–354. [PubMed] [Google Scholar]
- Ishiguro K, Seow HA, Penketh PG, Shyam K, Sartorelli AC. Mode of action of the chloroethylating and carbamoylating moieties of the produrg cloretazine. Molecular Cancer Therapy. 2006;5:969–976. doi: 10.1158/1535-7163.MCT-05-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Shyam K, Penketh PG, Sartorelli AC. Role of O6-alkylguanine-DNA alkyltransferase in the cytotoxic activity of cloretazine. Molecular Cancer Therapy. 2005;5:1755–1763. doi: 10.1158/1535-7163.MCT-05-0169. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Zhu Y-L, Shyam K, Penketh PG, Baumann RP, Sartorelli AC. Quantitative relationship between guanine O6-alkyl lesions produced by Onrigin™ and tumor resistance by O6-alkylguanine-DNA alkyltransferance. Biochemical Pharmacology. 2010;80:1317–1325. doi: 10.1016/j.bcp.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Sjolund A, Harris L, Sweasy JB. DNA repair and personalized breast cancer therapy. Environmental and Molecular Mutagenesis. 2010;51:897–908. doi: 10.1002/em.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Rockwell S. Tumor hypoxia: Its impact on cancer therapy. Cancer and Metastasis Reviews. 1987;5:313–341. doi: 10.1007/BF00055376. [DOI] [PubMed] [Google Scholar]
- Murren J, Modiano M, Kummar S, Clairmont C, Egorin M, Chu E, Sznol M. A phase I and pharmacokinetic study of VNP40101M, a new alkylating agent, in patients with advanced or metastatic cancer. Investigational New Drugs. 2005;23:123–135. doi: 10.1007/s10637-005-5857-6. [DOI] [PubMed] [Google Scholar]
- Paz MM, Ladwa S, Champeil E, Liu Y, Rockwell S, Boamah EK, Bargonetti J, Callahan J, Roach J, Tomasz M. Mapping DNA adducts of mitomycin C and decarbamoyl mitomycin C in cell lines using liquid chromatography electrospray tandem mass spectrometry. Chemical Research Toxicology. 2008;21:2370–2378. doi: 10.1021/tx8002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh PG, Shyam K, Sartorelli AC. Comparison of DNA lesions produced by tumor-inhibitory 1,2-Bis(sulfonyl)hydrazines and chloroethylnitrosoureas. Biochemical Pharmacology. 2000;59:283–291. doi: 10.1016/s0006-2952(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Penketh PG, Shyam K, Baumann RP, Remack JS, Brent TP, Sartorelli AC. 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamino)carbonyl]hydrazine (VNP40101M): II. Direct inhibition of O6-alkylguanine-DNA alkyltransferase (AGT) by electrophilic species generated by decomposition. Cancer Chemotherapy and Pharmacology. 2004;53:279–287. doi: 10.1007/s00280-003-0740-7. [DOI] [PubMed] [Google Scholar]
- Penketh PG, Baumann RP, Ishiguro K, Shyam K, Seow HA, Sartorelli AC. Lethality to leukemia cell lines of DNA interstrand cross-links generated by Cloretazine derived alkylating species. Leukemia Research. 2008;32:1546–1553. doi: 10.1016/j.leukres.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray GR, Hahn GM, Bagshaw MA, Kurkjian S. Cell survival and repair of plateau-phase cultures after chemotherapy; Relevance to tumor therapy and to the in vitro screening of new agents. Cancer Chemotherapy Reports. 1973;57:473–475. [PubMed] [Google Scholar]
- Rice KP, Penketh PG, Shyam K, Sartorelli AC. Differential inhibition of cellular glutathione reductase activity by isocyanates generated from the antitumor prodrugs Cloretazine™ and BCNU. Biochemical Pharmacology. 2005;69:1463–1472. doi: 10.1016/j.bcp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Rockwell S. In vivo-in vitro tumor systems: New model for studying the response of tumors to therapy. Laboratory Animal Science. 1977;27:831–851. [PubMed] [Google Scholar]
- Rockwell S, Dobrucki IT, Kim EY, Vu V. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Current Molecular Medicine. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell S, Hughes CS. Effects of mitoymcin C and porfiromycin on exponentially growing and plateau phase cultures. Cell Proliferation. 1994;27:153–163. doi: 10.1111/j.1365-2184.1994.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Rockwell S, Kallman RF, Fajardo LF. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. Journal of the National Cancer Institute. 1972;49:735–749. [PubMed] [Google Scholar]
- Rockwell S, Liu Y. Aplidin as a potential adjunct to radiation therapy: In vitro studies. International Journal of Radiation Biology. 2010;86:63–70. doi: 10.3109/09553000903264531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyam K, Penketh PG, Loomis RH, Rose WC, Sartorelli AC. Antitumor 2-(aminocarbonyl)-1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-hydrazines. Journal of Medicinal Chemistry. 1996;39:796–801. doi: 10.1021/jm9505021. [DOI] [PubMed] [Google Scholar]
- Steel GG. Termonology in the description of drug-radiation interactions. International Journal of Radiation Oncology Biology and Physics. 1979;5:1145–1150. doi: 10.1016/0360-3016(79)90634-5. [DOI] [PubMed] [Google Scholar]
- Steensma DP. Novel therapies for myelodysplastic syndromes. Hematology and Oncology Clinics of North America. 2010;24:423–441. doi: 10.1016/j.hoc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Lazo JS, Sartorelli AC. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Research. 1981;41:73–81. [PubMed] [Google Scholar]
- Twentyman PR, Bleehen NM. Changes in sensitivity to cytotoxic agents occurring during the life history of monolayer cultures of a mouse tumour cell line. British Journal of Cancer. 1975;31:417–423. doi: 10.1038/bjc.1975.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey N, Giles F. Laromustine (cloretazine) Expert Opinions in Pharmacotherapy. 2010;11:657–667. doi: 10.1517/14656561003621232. [DOI] [PubMed] [Google Scholar]