Summary
Protracted inflammation leading to dysregulation of effector T-cell responses represents a common feature of a wide range of autoimmune diseases. The interleukin-12 (IL-12)/T-helper 1 (Th1) pathway was thought to be responsible for the pathogenesis of multiple chronic inflammatory diseases, including psoriasis, inflammatory bowel disease, arthritis, or multiple sclerosis, mainly through their production of interferon-γ and its effects on macrophage activation and chemokine production. However, this initial concept of T-cell-mediated chronic inflammation required an adjustment with the discovery of an IL-12-related cytokine, designated IL-23. IL-23 was rapidly recognized for its involvement in the establishment of chronic inflammation and in the development of a Th cell subset producing IL-17, designated Th17, which is distinct from the previously reported Th1 and Th2 populations. This review aims to describe the characterization of IL-23 and its receptor, its biological activities, as well as its involvement in the development of human Th17 cells and autoimmunity.
Keywords: IL-23, Th17 cells, inflammation, autoimmunity, human, cytokines
Interleukin-23, a novel heterodimeric cytokine involved in inflammation
Over 20 years ago, Mosmann and Coffman (1) described in mice the presence of two distinct populations of T-helper (Th) cells, designated Th1 and Th2, which were characterized by a specific cytokine signature. While interleukin-12 (IL-12) induces the development of Th1 cells that produce IL-2, interferon-γ (IFN-γ), and lymphotoxin-α, and elicit cell-mediated immunity against intracellular pathogens, Th2 cells differentiate in response to IL-4, produce IL-4, IL-5, and IL-13, and are involved in humoral immunity against parasites and allergy (2). Although it was relatively easy with the availability of T-cell receptor (TCR) transgenic mouse strains to polarize naive mouse T cells in vitro into pure Th1 and Th2 effector cells and to isolate effector T-cell clones from lymph nodes and organs of mice subjected to strongly polarizing disease models, studies on Th1 and Th2 cells in human were more challenging. A large percentage of T cells isolated from the blood of healthy individuals produce a mixed cytokine phenotype upon activation. Eventually human Th1 and Th2 cells were defined as differentiation protocols and culture conditions were optimized for human T cells with multiple rounds of priming (3–5). In addition, it turned out to be important that for the isolation of T-cells clones with polarized phenotypes, T cells were isolated from the actual site of inflammation from patients suffering from infectious or allergic diseases. The discovery of additional cytokines and mediators, such as thymic stromal lymphopoietin, IL-25, IL-33, IL-18, IFN-α, and Notch ligands that have the ability to modulate Th1/Th2 differentiation, further illustrates the complexity of the T-cell development process within and between different species (6, 7).
Protracted inflammation leading to dysregulation of effector T-cell responses represents a hallmark of a wide range of autoimmune diseases. Th1 cells have been associated with the development and maintenance of chronic inflammatory diseases, such as psoriasis, inflammatory bowel disease (IBD), multiple sclerosis (MS), and rheumatoid arthritis (RA), through their production of IFN-γ and its effects on macrophage activation and chemokine production. Enhanced expression levels of IFN-γ, IL-12, and other critical components of the Th1 pathway have indeed been demonstrated in these human inflammatory diseases and their appropriate corresponding mouse models (2, 8). However, this initial concept of T-cell-mediated chronic inflammation required an adjustment with the unexpected discovery that mice deficient in IFN-γ or IFN-γ receptor were not resistant to experimental autoimmune encephalomyelitis (EAE) but were actually more susceptible to central nervous system autoimmunity (9–11). Observations in mice with targeted disruptions in the genomic regions encoding the IL-12 subunits further questioned the association between IL-12 and inflammatory disorders. IL-12 was the first identified cytokine with a heterodimeric protein structure and is composed of a soluble cytokine receptor-like 40 kDa subunit p40 that is covalently linked to a cytokine-like 35 kDa subunit p35 (12). Surprisingly, mice with a targeted disruption of the gene encoding p35 were more susceptible to disease in models of chronic inflammation, whereas mice lacking the p40 subunit of IL-12 were resistant (8, 13). Conversely, IL-12p40-deficient mice were more susceptible than IL-12p35-deficient mice with respect to Cryptoccocus neoformans and Listeria monocytogenes bacterial infections (14, 15). Several years later, the identification of another cytokine-like binding partner for IL-12p40 would provide the first plausible explanation for these unexpected findings (16).
Based on a computational screen of cDNA and expressed sequence tag databases with structure-based algorithms modeled on the IL-6 helical cytokine family, we identified a novel cytokine called p19. Characterization of p19 protein proved difficult at first, as the protein was inefficiently secreted from transfected cells and did not show biological activity in various in vitro bioassays. However, when we realized that p19 could be part of another heterodimeric cytokine complex and evaluated potential binding partners in this family, we demonstrated that p19 could form a p19–p40 heterodimer. Furthermore, we showed that the p19–p40 heterodimer was expressed and secreted by primary dendritic cells (DCs) upon activation, and that this heterodimer had biological activity on T cells, which, all together, justified its designation as IL-23 (16).
The discovery of IL-23 has had a tremendous impact on our understanding of the cytokines and T-cell pathways that govern chronic inflammation. Many previous studies that explored the influence of IL-12 on chronic inflammation were based on the use of antibodies neutralizing the IL-12p40 chain or of mice deficient in the IL-12p40 gene and needed to be revisited, because this approach neutralized the biological activities of IL-12 and IL-23. That IL-23, rather than IL-12, is crucial during the pathogenesis of autoimmune diseases became clear when p19 and p35 subunits were targeted. IL-23p19-deficient but not IL-12p35-deficient mice were resistant to EAE and collagen-induced arthritis (CIA) (17, 18). Cua and colleagues (19) further showed that IL-23p19-deficient mice were still able to mount a Th1 response but failed to produce the proinflammatory cytokine IL-17 (reviewed in 20).
Characterization of IL-23-induced signal transduction
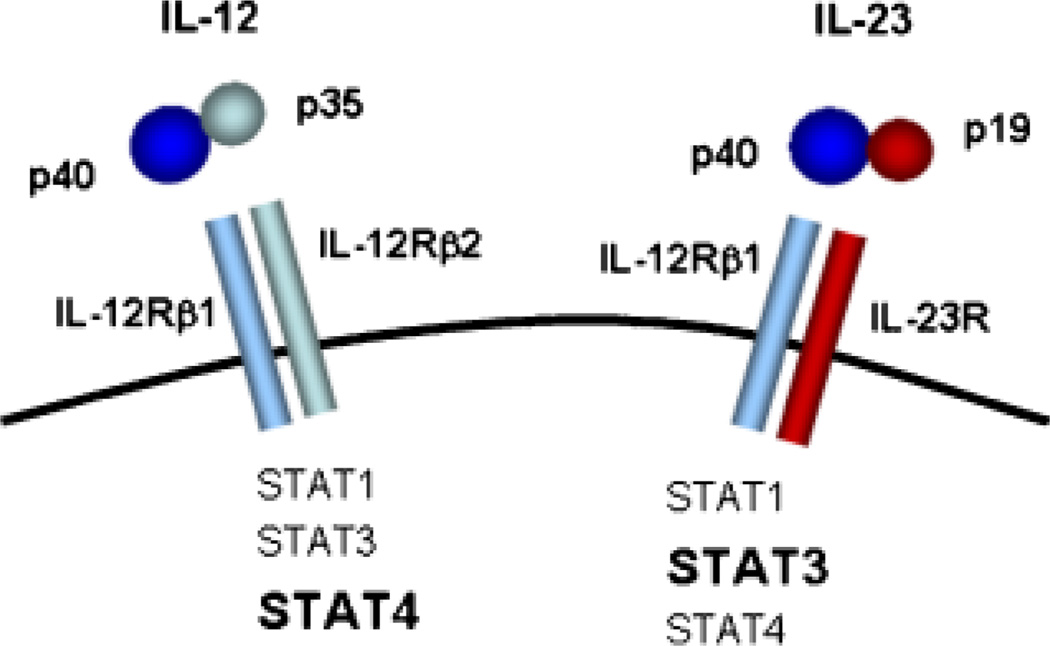
IL-23 exerts its biological activities through the interaction with a heterodimeric receptor complex composed of IL-12Rβ1 and IL-23R (21, 22) (Fig. 1). IL-23R is mainly expressed by T cells, natural killer cells, and to a lower extent by monocytes and DC populations (21). Like IL-12, IL-23 can directly bind the IL-12Rβ1 chain through its interaction with the IL-12p40 subunit. Whereas IL-12 uses IL-12Rβ2, IL-23 requires IL-23R as heterodimeric partner to allow signal transduction to occur. IL-23 and IL-12 activate the same Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling molecules. Non-receptor protein tyrosine kinase-2 is associated with IL-12Rβ1, and JAK2 is constitutively associated with the IL-23R chain. Binding of IL-12 and IL-23 to their receptor leads to phosphorylation of STAT1, STAT3, STAT4, and STAT5. However, STAT4 phosphorylation induced by IL-23 is much weaker than that induced in response to IL-12, and the formation of DNA-binding complexes are different, with mostly STAT4 homodimers formed in response to IL-12 and STAT3 homo- and heterodimers formed in response to IL-23. The responsiveness of cells to either IL-12 or IL-23 is thus determined by the respective expression of IL-12Rβ2 and IL-23R (21). Based on this pattern of receptor expression and signal transduction pathways, it can be expected that IL-12 and IL-23 would have overlapping but also unique biological activities.
Fig. 1. Overview of IL-12 and IL-23 ligand and receptor complexes.
Characterization of IL-23 bioactivity
In the initial characterization of IL-23 bioactivity, we compared the effects of IL-12 and IL-23 on sorted naive and memory mouse T cells from IL-10-deficient mice in the presence of anti-IL-2 monoclonal antibodies (16). Whereas CD4 CD45RBhigh naive T cells proliferated in response to anti-CD3 stimulation and IL-12 but not IL-23, CD4 CD45RBlow memory T cells proliferated in response to IL-23 but not to IL-12. These results are in agreement with the observation that CD45RBhigh cells expressed high levels of IL-12Rβ2 and lacked IL-23R, whereas CD45RBlow cells expressed IL-23R but low levels of IL-12Rβ2, and thus indicate that IL-23 preferentially acts as a growth factor for memory T cells (21). IL-10-deficient mice spontaneously develop enterocolitis resembling Crohn’s disease (CD), which can be blocked by treatment with anti-IL-12p40 monoclonal antibodies (23). In a follow-up study, we showed that IL-12p35 × IL-10-deficient mice but not IL-23p19 × IL-10-deficient mice spontaneously developed IBD, supporting a role of IL-23 in promoting intestinal inflammation (24). Furthermore, administration of IL-23 in a T-cell transfer model of colitis accelerated disease development irrespective of whether naive or memory cells from diseased IL-10-deficient mice were transferred into recombination-activating gene knockout recipients. Presumably, the naive T cells were converted to memory T cells in this inflammatory environment, making them responsive to IL-23. Furthermore, as shown in the EAE model (19), IL-23 promoted the production of IL-17 and IL-6 from the memory-activated T cells (24). These IL-17-producing T cells were present in the gut of IL-10-deficient mice but not in IL-10 × IL-23p19-deficient mice. Similar results on the role of IL-23 and production of IL-17 have also been described in other models of IBD and are discussed in (25) by Powrie et al. These results thus clearly identified a role for IL-23 in the proliferation of murine memory T cells, production of IL-17, and development of inflammation.
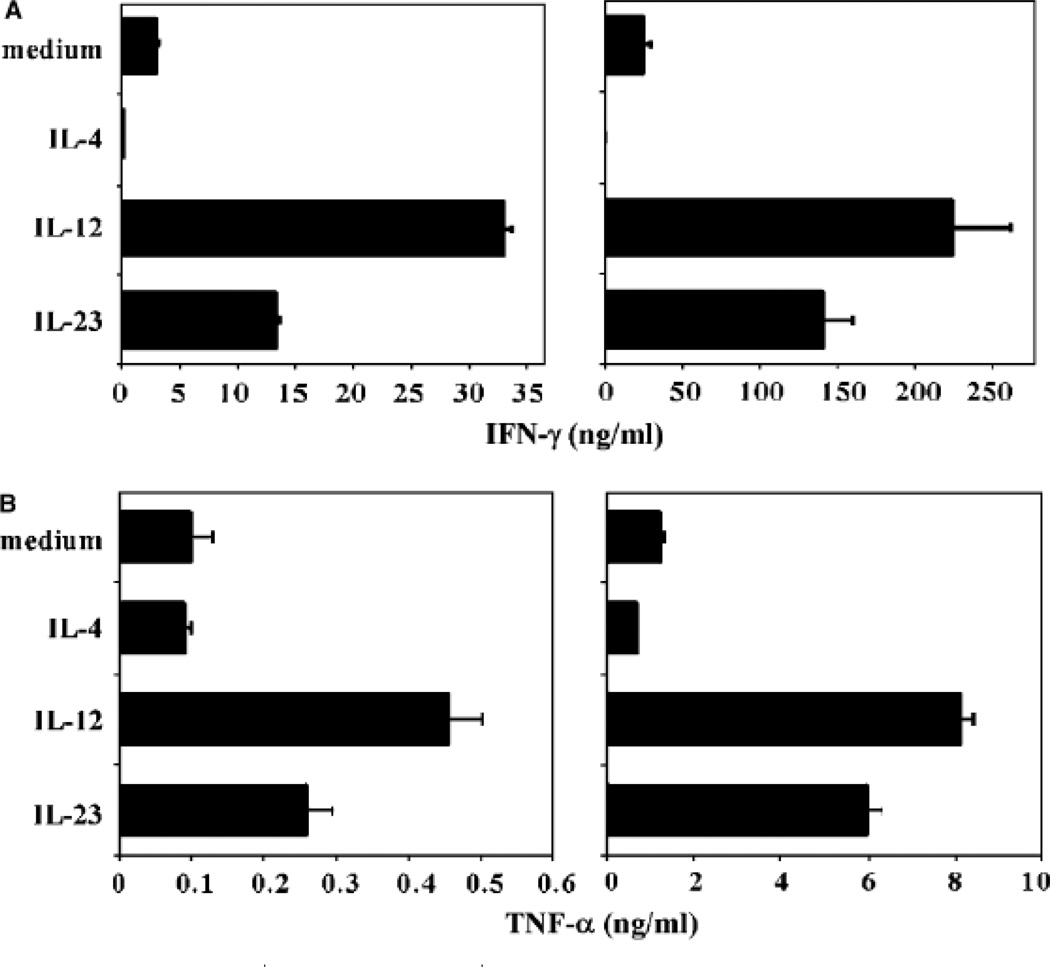
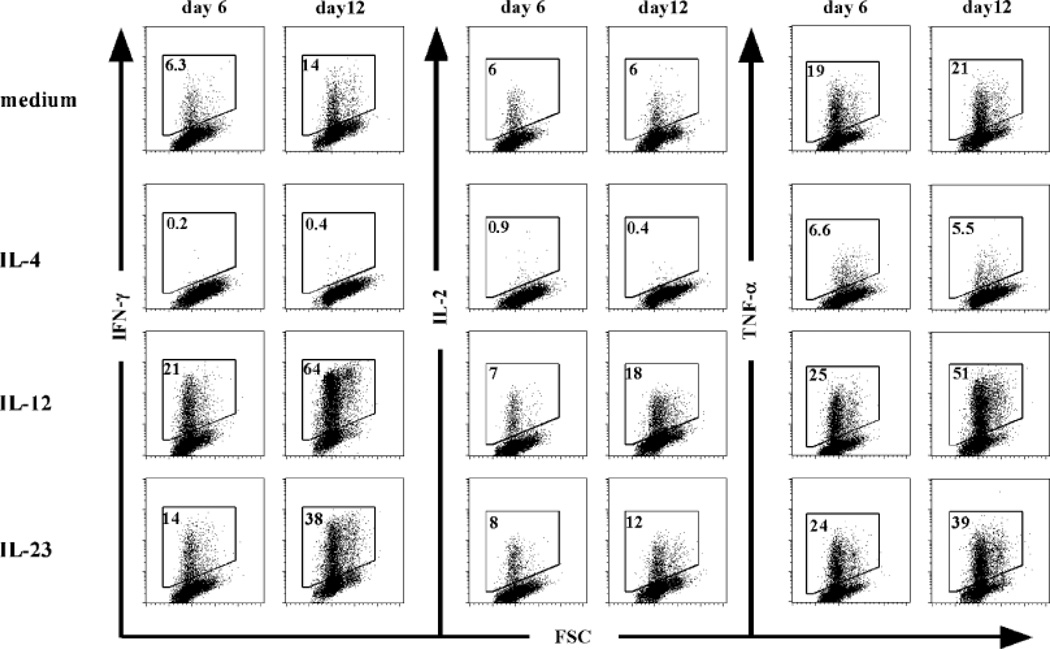
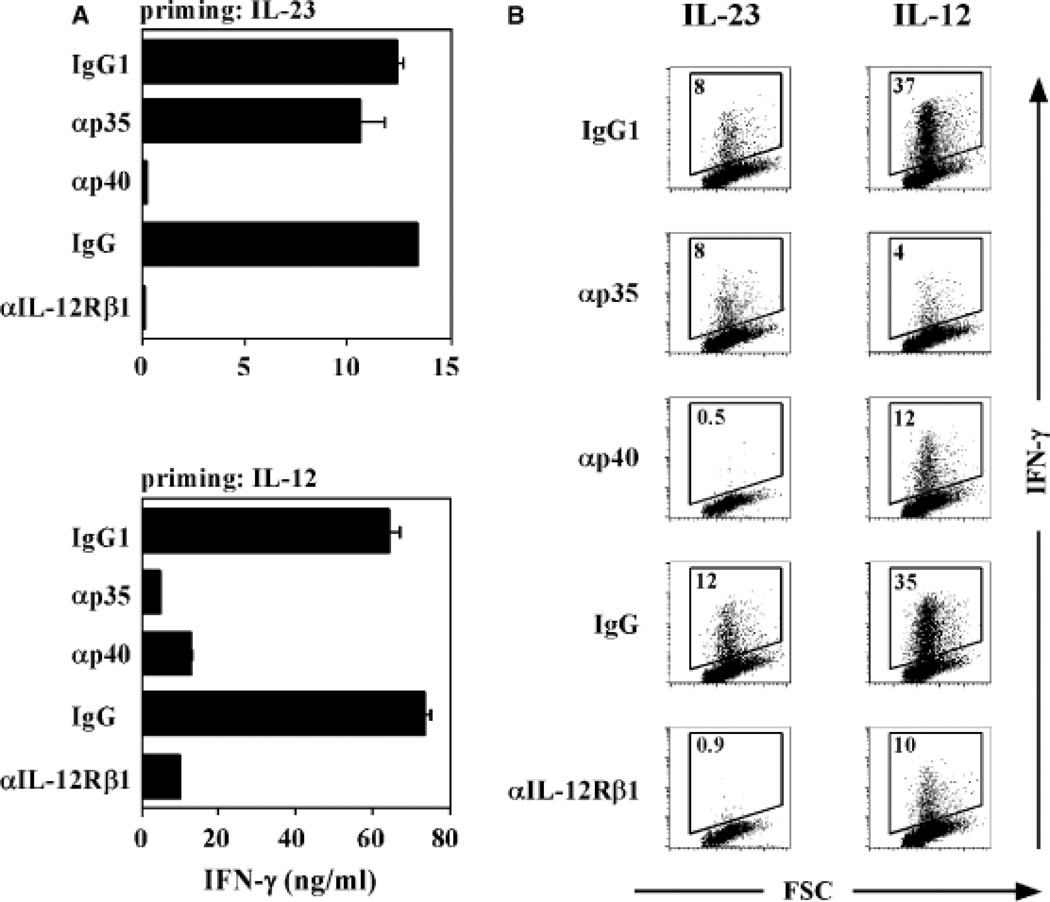
In our initial characterization of the effects of IL-23 on human T cells, we compared the activities of IL-23 and IL-12 on the proliferation and IFN-γ production by 7-day-old PHA blasts, a classical bioassay for IL-12 activity (12). Both IL-12 and IL-23 enhanced proliferation and IFN-γ production of anti-CD3- and anti-CD28-activated PHA blasts, which was blocked by anti-IL-12Rβ1 and anti-IL-12p40 monoclonal antibodies (16). However, the IL-23-induced activity could not be neutralized with anti-IL-12p35 monoclonal antibodies. We also compared the activities of IL-12 and IL-23 on fluorescence-assisted cell sorter (FACS)-sorted CD4+CD45RA+ naive and CD4+CD45RO+ memory T cells isolated from peripheral blood. In these experiments, T cells were activated by plate-bound anti-CD3 and soluble anti-CD28 in the presence of IL-2 and assessed for IFN-γ production at days 3 and 6. CD45RO+ memory T cells responded to IL-23 and IL-12 at both time points with enhanced IFN-γ production, although the levels were lower in response to IL-23. CD45RA+ naive T cells produced IFN-γ in response to IL-23 at day 6 but not at day 3, whereas IL-12 enhanced IFN-γ production at both time points (16). IL-23R is expressed on human memory T cells but is barely detected on naive cells (26, 27), suggesting that TCR activation of naive human T cells leads to an upregulation of low levels of IL-23R expression, rendering the cells sensitive to IL-23. In addition, IL-23 increases its own receptor expression on activated naive T cells (27). This is analogous to the TCR-mediated induction of IL-12Rβ2 expression in Th1 commitment (28) and further upregulation by IL-27 (29). In the next series of experiments, we extended these observations using purified cord blood CD4+CD45RA+ T cells in a culture system that was set up to study human Th1 and Th2 differentiation (4). T cells were cultured with anti-CD3 monoclonal antibody crosslinked onto irradiated FcγRII+ (CD32), CD58+, and CD80+ transfected L-cell fibroblasts in the presence of IL-2 and IL-23, IL-12, or IL-4, and expansion and differentiation was determined following restimulation with plate-bound anti-CD3, soluble anti-CD28, and IL-2 after 6 and 12 days. The addition of IL-2 to these cultures only affected cell survival and proliferation of T cells and did not affect the differentiation of the CD4+ T cells. Clonal expansion of naive CD4+ T cells stimulated in the presence of IL-23 was slightly higher compared with control cultures containing IL-2 only after 6 and 12 days of culture. The increase in cell number induced by IL-12 was higher compared with that induced by IL-23 or IL-2 but lower compared with that induced by IL-4. Culture of naive CD4+ T cells in IL-2 alone induced little IFN-γ after 6 or 12 days of stimulation, and addition of IL-4 to the cultures abolished the induction of IFN-γ production (Fig. 2). Strikingly, IL-23 induced the production of IFN-γ from naive CD4+ T cells after 6 days, and this was even more pronounced after 12 days of stimulation. Addition of IL-12 to the CD4+ T cells induced production of the highest levels of IFN-γ, both after 6 and 12 days. Interestingly, IL-23 also induced the production of tumor necrosis factor-α (TNF-α) from CD4+ T cells, which again was slightly lower compared with the IL-12-cultured cells. No significant effect of IL-23 on the production of the Th2 cytokines IL-4, IL-5, and IL-10 could be observed. Flow cytometric analysis was performed following intracellular cytokine staining to determine the effect of IL-23 on cytokine production at the single cell level. Clearly, after 6 days of priming, IL-23 induced a population of CD4+ T cells to produce IFN-γ (Fig. 3). Consistent with the results obtained by enzyme-linked immunosorbent assay, IFN-γ induction by IL-23 was more pronounced after 12 days of culture. IL-12 was a more potent inducer of IFN-γ-producing cells at both culture time points. IL-23 induced higher numbers of both IL-2 and TNF-α-producing T cells compared with the control-stimulated T cells, although prolonged stimulation with IL-23 was required to observe this effect. Similar observations were made in the presence of IL-12, with a more pronounced effect than IL-23. To determine the specificity of the IL-23-induced cytokine induction, experiments were carried out with neutralizing antibodies directed against IL-12p40, IL-12Rβ1, or IL-12p35 present during the priming conditions. Addition of anti-IL-12p40 monoclonal antibody and anti-IL-12Rβ1 polyclonal antibodies significantly blocked the IL-23- and IL-12-induced IFN-γ production (Fig. 4). The anti-IL-12p35 monoclonal antibody was not capable of blocking the IFN-γ production induced by IL-23 but did block IL-12-induced IFN-γ secretion. Taken together, the results of these early experiments on the biological activities of IL-23 in human indicated that it could act on both memory and naive T cells. The effects of IL-23 on naive T cells from peripheral blood and cord blood required prolonged exposure, consistent with the need to further upregulate the IL-23R on these cells. The read-out in these earlier experiments was the production of IFN-γ, mainly as we were comparing IL-23’s activity with that of IL-12. It is not uncommon for cytokines to have redundant activities such as IFN-γ production, especially for those that share receptor components and thus intracellular signaling pathways. There are many examples available, e.g. IL-4/IL-13 for immunoglobulin E (IgE) production, IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 as T-cell growth factors, IL-3/granulocyte-macrophage colony-stimulating factor for myeloid cell growth. However, the most important activity of IL-23 that distinguishes it from IL-12 is its ability to induce the production of IL-17, and differentiation of Th17 cells.
Fig. 2. IL-23 induces polarization of naive CD4+ T cells.
Naive CD4+ T cells were isolated from cord blood, primed for 6 days, and recultured for an additional 6 days in the presence of the indicated cytokines, anti-CD3, IL-2, and irradiated CD32/CD58/CD80-transfected L cells. Cells were harvested at day 6 (left) and day 12 (right) and stimulated for 24 h.
Fig. 3. IL-23 induces polarization of naive CD4+ T cells.
Naive CD4+ T cells were isolated from cord blood, primed for 6 days, and recultured for an additional 6 days in the presence of the indicated cytokines, anti-CD3, IL-2 and irradiated CD32/CD58/CD80-transfected L cells. Cells were harvested at days 6 and 12 and stimulated for 6 h.
Fig. 4. IL-23-induced polarization is inhibited by neutralizing antibodies against p40 and IL-12Rβ1 but not p35.
Discovery of a third Th cell subset: Th17 cells
IL-17 (30) was originally identified by Rouvier et al. (31) in rodent T-cell hybridoma clones as cytotoxic T-lymphocyte-associated antigen 8 and the human version was cloned from a CD4+ T-cell library (32). IL-17 is the founding member of a newly identified cytokine family comprising IL-17 (IL-17A), IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F. IL-17 is mostly known for its pro-inflammatory activities, both in vitro and in vivo, and its expression is increased in inflammatory tissues (33).
Initial findings showed that Borrelia burgdorferi induced IL-17 production by human T cells independently of Th1 or Th2 cytokine production (34). However, the concept that T cells producing IL-17 should be classified as a distinct Th cell subset emerged from mouse models of autoimmunity, such as EAE, IBD, or CIA, following the discovery of IL-23 (16, 17, 19, 35). Th17 cells are now recognized as a third T-effector cell subset (36–39), and the IL-23/IL-17 pathway is linked to mucosal host defense against extracellular pathogens (40–42) and to the induction and progression of a number of inflammatory diseases, including psoriasis, IBD, arthritis, and MS (17–19, 24, 26, 43–46). Following their identification in the mouse, we set out to define the development, cytokine profile, and function of human IL-17-producing T cells (26). The characterization of specific surface markers and cytokines defining human Th17 cells is critical to identify and ‘track’ those cells in the tissue during inflammation. Furthermore, the understanding of the cytokines involved in Th17 cell development and/or regulation is central in the perspective of new target discovery to cure inflammatory disorders.
Cytokine profile of human Th17 cells
Culture of naive T cells in the presence of IL-23 and or IL-1β induced the differentiation of human IL-17-producing T cells. In addition, based on the expression of the IL-23R, we identified human Th17 cells as a subpopulation of CD45RO+ T cells in the blood from normal healthy donors. Both the in vitro-derived Th17 cells and the in vivo-occurring Th17 cells were found to express a signature cytokine profile consisting of IL-17, IL-17F, IL-22, IL-26, and CCL20. In addition, a significant proportion of these cells coexpressed IL-17 and IFN-γ (26). This cytokine profile of human Th17 cells producing inflammatory cytokines, such as IL-17, IL-17F, and IL-22, was observed by other groups (26, 27, 40, 47–49) and was previously also shown in the mouse system (19, 35, 50, 51). Two groups independently isolated IL-17-producing CD4+ memory T cells from peripheral blood or intestine of healthy individuals as well as patients with active CD (40, 46), and these cells were found in both central (CCR7+) and effector (CCR7−) memory CD4+ pools (40, 52). Both IL-17 and IL-17F have major pro-inflammatory effects on epithelial cells and are important for the recruitment of neutrophils (26, 33, 51, 53). IL-22 is a member of the IL-10 family, largely described for its pro-inflammatory activities on keratinocytes and its upregulated expression during inflammatory disorders (51, 54–59). Moreover, IL-22 was recently shown to be a crucial cytokine during IL-23-induced dermal inflammation and acanthosis (59). We showed that human Th17 cells also express IL-26 (26), an IL-10 family member recently reported to induce inflammatory genes expression in intestinal epithelial cells and to be upregulated in colonic retinoid-related orphan receptor γt (RORγt)-expressing Th17 cells in CD patients (60). The work of Liu and Rohowsky-Kochan (52) suggests that the majority of IL-17 producers also express TNF-α and IL-6.
Importantly, as we discussed above from our early IL-23 experiments, human Th17 cells can also express IFN-γ (26, 27, 40, 46, 61). IFN-γ has been largely described for its inflammatory activities, and its expression is elevated in a number of inflammatory diseases, such as psoriasis and IBD (62–64). Moreover, IL-17 IFN-γ− (Th17) and IL-17+ IFN-γ+ (Th17/Th1) cells were identified in the intestine of patients with active CD (46). A preliminary study addressing this question showed that Th17 and Th17/Th1 populations expressed a similar chemokine receptor expression profile, at least for CCR4, CCR6, CXCR3, and CXCR6 (65). A more extensive characterization of the surface markers and cytokine profile expressed by these populations may improve the understanding of the specific function of these two subsets of IL-17 producers.
Phenotype of human Th17 cells
Chemokines are differentially expressed in inflamed tissues by epithelial and immune cells and induce the recruitment of specialized effector cells through the expression of specific chemokine receptors. Whereas Th1 cells specifically express CXCR3, CCR5, and CXCR6, Th2 cells express CCR4, CCR8, the prostaglandin D2 receptor CRTh2, and to a lesser extent CCR3 (66). It is now well documented that IL-17-producing cells express CCR6 (40, 46, 47, 52, 65, 67). CCR6 expressed by Th17 cells is functional, as its ligand CCL20/macrophage inflammatory protein-3a induced calcium influx in Th17 but not Th1 clones (46). Acosta-Rodriguez et al. (40) further showed that expression of CCR6 and CCR4 defines a population of Th17 cells expressing IL-17 but not IFN-γ, whereas cells expressing CCR6 and CXCR3 produce IL-17 and IFN-γ (Th1/ Th17) or IFN-γ only (Th1). CCR6 and CCL20 are highly expressed in inflammatory tissues and are involved in the recruitment of pathogenic T cells in MS, RA, CD, and psoriasis (46, 68–72).
Whether CCR2 could be used as a ‘marker’ of Th17 cells remains unclear. Sato et al. (73) described IL-17 producers as memory CD4+ T cells expressing CCR2. These cells secreted IL-17 and IFN-γ. Further analyses using CCR5 indicated that CCR2+ CCR5− T cells expressed high levels of IL-23R and IL-17 but not of IFN-γ. In contrast, CCR2+CCR5+ T cells produced IFN-γ but not IL-17 (73). Singh et al. (67) also observed an increased frequency of IL-17 producers in the CCR2+ CCR5− T cells compared with CCR2+ CCR5+ T cells; however, only a minority of CCR2+ CCR5− T cells could be induced to make IL-17. In addition, few Th17 cells from peripheral blood were found within the CCR2+CCR5− subset. Whether the CCR6 Th17 population overlaps with CCR2+CCR5− T cells was not examined.
It was recently shown that in vitro-derived Th17 cells have a higher expression of CCR9 and CXCR6 as compared with Th1 and Th2 cells (67). Thus, CCR9 and CXCR6 may also be expressed preferentially on Th17 cells. In line with this observation, CCR9+ lymphocytes from the lamina propria have been described to produce IL-17 (74), and CCR9 together with its ligand CCL25 have been reported to play an important role in small bowel immunity and inflammation (75, 76).
Besides expression of a specific set of chemokine receptors, we and others identified IL-23R as a specific marker for the Th17 population (26, 46, 47). In contrast, the specific IL-12 receptor subunit IL-12Rβ2 was expressed by all Th cell subsets (46). We showed that circulating IL-23R+ memory CD4+ T cells isolated from healthy donors produced higher levels of IL-17 than their IL-23R− counterparts, whereas IFN-γ levels were similar. IL-23R–expressing cells also express the ‘signature’ cytokine profile of Th17 cells, including IL-17F, IL-22, IL-26, and CCL20 (26). Thus, as for Th1 and Th2 subsets, Th17 cells are characterized by expression of specific chemokine receptors inducing their recruitment to inflammatory sites expressing their specific ligands. In addition, the expression of lineage specific surface markers, such as IL-23R, allows us to identify and track those cells in inflamed tissues as well as in the periphery.
Human Th17 cell differentiation
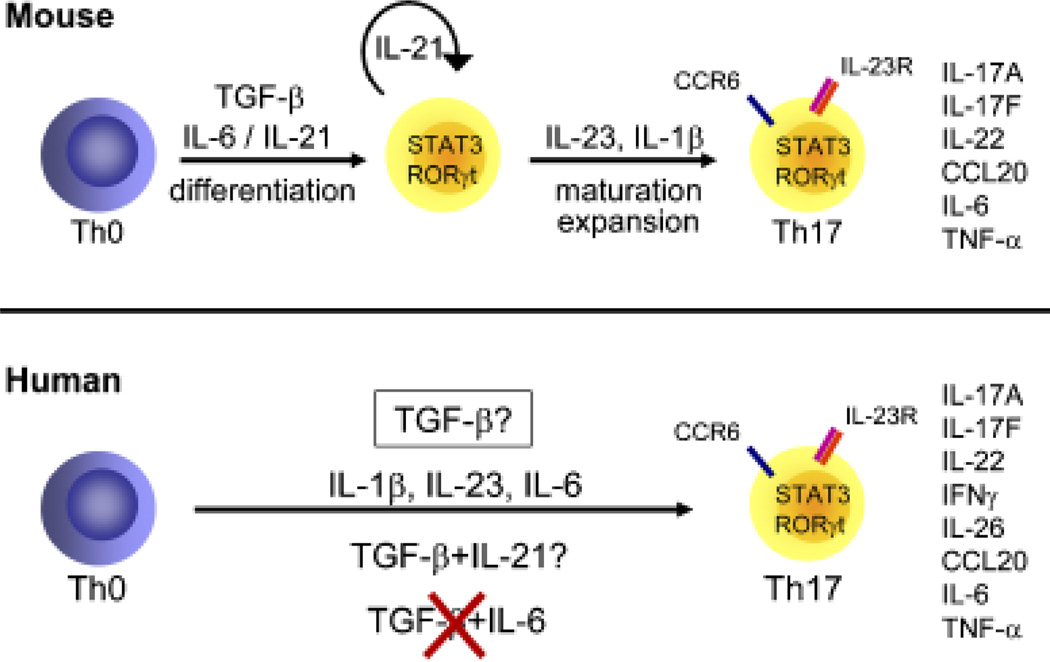
Although initial studies showed a crucial role of IL-23 in production of IL-17 and Th17-mediated autoimmunity, it became evident that IL-23 did not drive differentiation of naive T cells into Th17 cells in the mouse system (77–79). These observations were not unexpected, as naive T cells do not express IL-23R (21). Instead, transforming growth factor-β (TGF-β) was identified as a critical factor for mouse Th17 cell differentiation. Indeed, whereas TGF-β alone induces the development of regulatory T cells (Tregs) expressing Forkhead box protein 3 (FoxP3) (79, 80), the presence of IL-6 or IL-21 will prevent the generation of FoxP3+ cells and lead to the development of Th17 cells and upregulation of the IL-23R (77–79, 81–83). Consequently, IL-23 is not required for early Th17 development; it is however strictly necessary for the maintenance and pathogenicity of Th17 cells (50).
Several studies assessed the different cytokines possibly involved in the differentiation of human Th17 cells (Fig. 5); however, it remains difficult to obtain a consensus between groups, reminiscent of the early controversy with Th1 and Th2 cells in humans. The section below highlights the main findings observed by different groups.
Fig. 5. Th17 cell differentiation in mouse and human.
IL-1β and IL-23
We and others (26, 27, 49, 61, 84) have shown that the combination of TGF-β and IL-6 does not drive human Th17 development. Instead, the culture of peripheral blood naive T cells in the presence of IL-23 or IL-1β was sufficient to drive Th17 development (26, 27, 61). Moreover, Acosta-Rodriguez et al. (61) further showed that IL-6 could enhance IL-1β-driven IL-17 production. Furthermore, we (26) and O’Shea’s group (27) also described IL-23 as a potent promoter of Th17 development. In vitro-derived Th17 cells also express RORγt, IL-22, IL-17F, IL-26, CCL20, CCR6, and IL-23R. In addition, these cells are characterized by the presence of both IL-17+ and IL-17+ /IFN-γ+ producers.
TGF-β or no TGF-β?
All the studies published so far agree that in contrast to mice, the combination of TGF-β and IL-6 alone was not sufficient to drive human Th17 cell differentiation. Whether TGF-β is required for human Th17 cell differentiation is still a matter of controversy. Indeed, three independent groups (48, 49, 84) recently reported an absolute necessity of TGF-β to drive Th17 cell development in human. Volpe et al. (48) detected IL-17 production when naive T cells were cultured in the presence of IL-23, IL-1β, IL-6, and TNF-α, but observed that IL-17 levels were strongly increased when TGF-β was added to this inflammatory cytokine cocktail. This was true for naive T cells selected by magnetic cell sorting or FACS-sorted from peripheral blood and cord blood cells. Manel et al. (49) showed that the combination of TGF-β, IL-1β, and IL-23 was optimal to induce IL-17 producers from naive cord blood CD4+ T cells and that endogenous levels of IL-6 or IL-21 were not required for Th17 cell development. Th17 cells expressed increased levels of IL-17, IL-17F, IL-26, and IL-23R. In contrast, IL-22 production was downregulated in the presence of TGF-β (49). In their study, Volpe et al. (48) showed that IL-17 and IL-6 were associated with Th17 cells, whereas IL-22, TNF-α, IL-21, and IFN-γ were expressed by both Th17 and Th1 cells. However, Th17 cells polarized in the presence of TGF-β were IL-17 single producers, and no IL-17+ IFN-γ+ cells were detected. As both IL-17+ IFN-γ− and IL-17+ IFN-γ+ have been identified in inflamed tissues, for example the brain of mice after EAE induction (50, 85) or the intestine of patients with active CD (46), one could ask whether in vitro Th17 cells polarized in the presence of TGF-β reflect the Th17 populations present in vivo at sites of inflammation. The work of Yang et al. (84) suggests that in a serum-free medium, the combination of TGF-β and IL-21 drives the development of Th17 cells expressing increased levels IL-17 but not IFN-γ or IL-22 from peripheral blood and cord blood naive T cells. In contrast, Manel et al. (49) failed to see any induction of IL-17 production in the presence of TGF-β and IL-21 from naive cord blood cells cultured in medium either containing serum or not. They further showed that the neutralization of IL-21 during Th17 cell development did not regulate the proportion of IL-17 producers. We also failed to detect any upregulation of IL-17 production when peripheral blood naive T cells were stimulated in the presence of TGF-β and IL-21 (unpublished data). The recent observation that IL-21 plays an important role in the generation of T-follicular helper cells and is a potent inducer of Ig production would challenge its physiological importance for Th17 biology (86, 87). Interestingly, we and others (26, 27, 61, 77) showed that TGF-β inhibited IL-17 production induced by IL-23 or by the combination of IL-1β and IL-6.
How can the discrepancies observed in human Th17 development between groups be explained? One possibility could be the presence of endogenous TGF-β in cultures containing serum. Indeed, the use of serum-free medium leads to an increased proportion of IL-17 producers compared with medium containing serum (49), suggesting that TGF-β present in the serum can downregulate to some extent IL-17 production. Volpe et al. (48) showed that the use of medium with or without serum required the same cytokines for Th17 development, although IL-17 levels were lower in a medium containing serum. Another explanation could be the time of culture. The effects of IL-1β and/or IL-23 without addition of exogenous TGF-β were analyzed in a 2-week culture assay. In contrast, cultures performed in the presence of TGF-β were usually harvested after a 5-day culture period. Despite the differences observed among the different studies, IL-23 and IL-1β emerge as critical factors in the induction of IL-17-producing cells.
Transcription factors associated with human Th17 cell development
As T-bet/STAT4 and GATA-3/STAT6 are respectively related to Th1 and Th2 cells, Ivanov et al. identified RORγt as a transcription factor specifically expressed in mouse and human Th17 cells (26, 88, reviewed in 20). Transduction of human cord blood CD4+ T cells with RORγt is sufficient to induce IL-17 production, and conversely knockdown of RORγt results in much lower IL-17 production (49). As previously shown in mice (89), the involvement of other ROR family members is conceivable, as RORα also induces IL-17 expression when overexpressed in primary human T cells (49). Interestingly, forced expression of RORγt is not sufficient to induce IL-22 production (49), suggesting the requirement of additional transcription factors. In this regard, Veldhoen et al. (77) identified the aryl hydrocarbon receptor (AHR), a ligand-dependent transcription factor, as a critical factor in the induction of IL-22 production in mice. They further showed that human Th17 cells express AHR. In addition to RORγt, human Th17 cells express also T-bet, which is consistent with the observed production of IFN-γ (46).
An increasing body of evidence shows the requirement of STAT3 for Th17 cell development. Indeed, in mice STAT3 is absolutely required for the induction of IL-17, IL-17F, and RORγt (90–92). Moreover, patients suffering from autosomal dominant hyper-IgE syndrome, associated with negative mutations in STAT3, had impaired Th17 cell differentiation (93–96). These subjects secreted much lower IL-17 and IL-22 than control individuals, showing the requirement of STAT3 to induce the production of these cytokines in vivo in human. Thus, although there is still debate on the cytokines involved in Th17 differentiation in mice and human, there is a consensus that STAT3 activation is essential. In this light, it is relevant that IL-6, IL-21, and IL-23 are all able to induce STAT3 phosphorylation.
Negative regulators of human Th17 cell development
When looking at the strong potential of Th17 cells to cause damage to the host, it was expected that this subset would be subjected to many regulatory processes. Initial studies assessing lineage commitment of naive CD4+ T cells into Th1 and Th2 cells revealed that these subsets cross-regulate each other through their specific signature cytokines IFN-γ and IL-4, respectively (97–99). The same principle applies to Th17 cell regulation both in the mouse and in the human systems. We showed that IL-4 and IL-12 prevented Th17 differentiation induced by IL-23 from human naive CD4+ T cells (26).
Regulation of Th17 responses can also happen in a cytokine-independent context. For example, retinoic acid, an active metabolite of vitamin A, is a potent inhibitor of Th17 commitment in mice, while enhancing FoxP3+CD4+ Tregs (100–103). In contrast, sphingosine 1-phosphate can enhance development of Th17 cells in mice (104). Thus, non-cytokine immunomodulatory agents produced during inflammation also play an important role in regulating Th17 responses, and further studies will undoubtedly help to better understand how such agents positively or negatively modulate Th17 function.
Function of human Th17 cells
The link of Th17 cells to pathologic inflammation is much more established than our understanding of their role in normal immune defense mechanisms. Several lines of evidence support their involvement in mucosal immunity in mice, particularly against extracellular bacterial infections. For example, IL-17, IL-22, and IL-23 are all necessary to elicit full immune response to Klebsiella pneumoniae (105–107); mice deficient in IL-17RA have enhanced susceptibility to Toxoplasma gondii and Candida albicans infection but not to Mycobacterium tuberculosis or L. monocytogenes (106, 108, 109), and IL-22 is critical for host defense against Citrobacter rodentium infection (110). However, while little is known regarding the role of IL-23, IL-17, and IL-22 in resistance to infection in humans, what has emerged in the last few years is indirect evidence that patients suffering from diseases associated with various infections, including hyper-IgE syndrome, chronic mucocutaneous candidiasis, or Mendelian susceptibility to mycobacterial diseases, have a defect in Th17 cells (93–95, 111). In addition, both IL-17 and IL-22 induce anti-microbial peptides production from various epithelial cell types in human (26, 51, 57, 107, 112), suggesting their participation in host defense. Napolitani and colleagues (40) showed that human memory T cells specific for C. albicans were mainly CCR6+CCR4+ Th17 subset, whereas T cells specific for M. tuberculosis were present in the CCR6+CXCR3+ Th1 population, suggesting that Th17 and Th1 cells exhibit different immune functions in response to pathogens. C. albicans in the hyphal form primed Th17 responses in vitro and induced production of IL-23 but not IL-12 by human DCs (40).
Annunziato et al. (46) showed that Th17 cells exhibit poor proliferative capacity, low cytotoxicity, and reduced susceptibility to suppressive activity of CD4+FoxP3+ Tregs compared with Th1 and Th2 cells. They are also able to help B cells to induce the production of IgG, IgM, and IgA, but not IgE (46). Accordingly, a recent study showed that human Th17 cells, but not Th1 or Th2 cells, expressed B-cell chemoattractant CXCL13 (113). Thus, the Th17/B-cell interaction could lead to the production of Igs by B cells, which would help in resolving the infection.
IL-23/Th17 and/or IL-12/Th1-mediated autoimmune diseases?
While nature’s plan certainly envisioned a contribution of Th17 cells to host defense mechanisms, this inflammatory Th cell subset is better known for its role in promoting destructive tissue inflammation. Th17 cells are key mediators of chronic inflammation in various animal models (17–19, 24, 45). However, even after the establishment of Th17 cells as pathologic mediators, evidence remained for a contribution of the Th1 pathway during inflammatory processes, reflecting the complexity of inflammatory diseases. For example, IFN-γ producers are found in the brain of mice during EAE, inhibition of T-bet expression by RNA interference ameliorates EAE, and IFN-γ has been described for its pathogenic role in different mouse models of inflammation (50, 114–116).
The situation in human inflammatory diseases comes with an even higher degree of complexity: Pène et al. (47) isolated CD4+ T cells from lesions of patients suffering from chronic diseases, including psoriasis, CD, RA, and severe asthma, and identified Th1, Th2, as well as Th17 cells in inflamed tissues. Th17 clones, selected for their elevated IL-17 and IL-22 production, expressed higher levels of RORγt, IL-17F, IL-26, CCL20, TNF-α, CCR6, IL-1R1, and IL-23R than Th1 and Th2 clones. Interestingly, Th17 clones contained low levels of IFN-γ. To further understand the mechanisms of human inflammatory diseases, it becomes then critical to determine the importance of the distinct Th cell subsets in each particular disease.
A new line of evidence favors a role of IL-23/Th17 rather than IL-12/Th1 pathway during the pathogenesis of autoimmune disorders, for example in MS. Increased levels IL-17 were found in the cerebrospinal fluid and blood of MS patients (117, 118), and IL-23 but not IL-12 production was increased in monocyte-derived DCs isolated from MS patients (118). Prat and colleagues (44) directly investigated the involvement of human Th17 cells in central nervous inflammation. IL-23-driven Th17 cells generated from peripheral blood CD4+ T cells from healthy donors migrated more efficiently across the blood–brain barrier than did IL-12-driven Th1 cells. These Th17 cells were IL-17, IL-22, or IL-17/ IL-22 producers. IL-17+IL-22+ cells preferentially expressed granzyme B and had enhanced cytolytic activity toward neuronal cells isolated from fetal brain compared with nonactivated lymphocytes (44).
If psoriasis was traditionally associated with IL-12/Th1-exacerbated responses, this conclusion was mainly based on the upregulation of IL-12p40 expression in lesional skin. However, we and others (26, 119–121) reported that IL-23 but not IL-12 expression is increased in psoriatic lesions, as shown by upregulated expression of IL-23p19, IL-12p40, but not IL-12p35. The expression of IL-1β, RORγt, and Th17 cytokines IL-17, IL-22, IL-17F, IL-26, and of IFN-γ was also upregulated in psoriatic skin (26, 56, 58, 122, 123). Overexpression of Th17 cytokines also led to the production of anti-microbial peptides and chemokines. All together, this inflammatory milieu sets the stage for pathology (Fig. 6). The involvement of IL-23 during cutaneous inflammation is also corroborated in vivo, as intradermal injection of IL-23 in mice induced a psoriasis-like phenotype (59, 119, 124). The most compelling evidence indicating a major role of the IL-23/Th17 pathway during psoriasis came from the improvement in psoriasis area-and-severity index in patients when anti-IL-12p40-neutralizing antibodies were administered (125–129). We now know that neutralization of IL-12 by blocking IL-12p40 also neutralizes the function of IL-23, raising the question for the actual mechanism of action. Cooper and colleagues (125) further showed that the clinical improvement was associated with reduced expression of IL-12p40 and IL-23p19 but not of IL-12p35. Furthermore, genetic studies revealed an association between IL23R and IL12B genes and susceptibility to psoriasis (130–132), making it likely that the IL-23/Th17 pathway plays a dominant role in this disease.
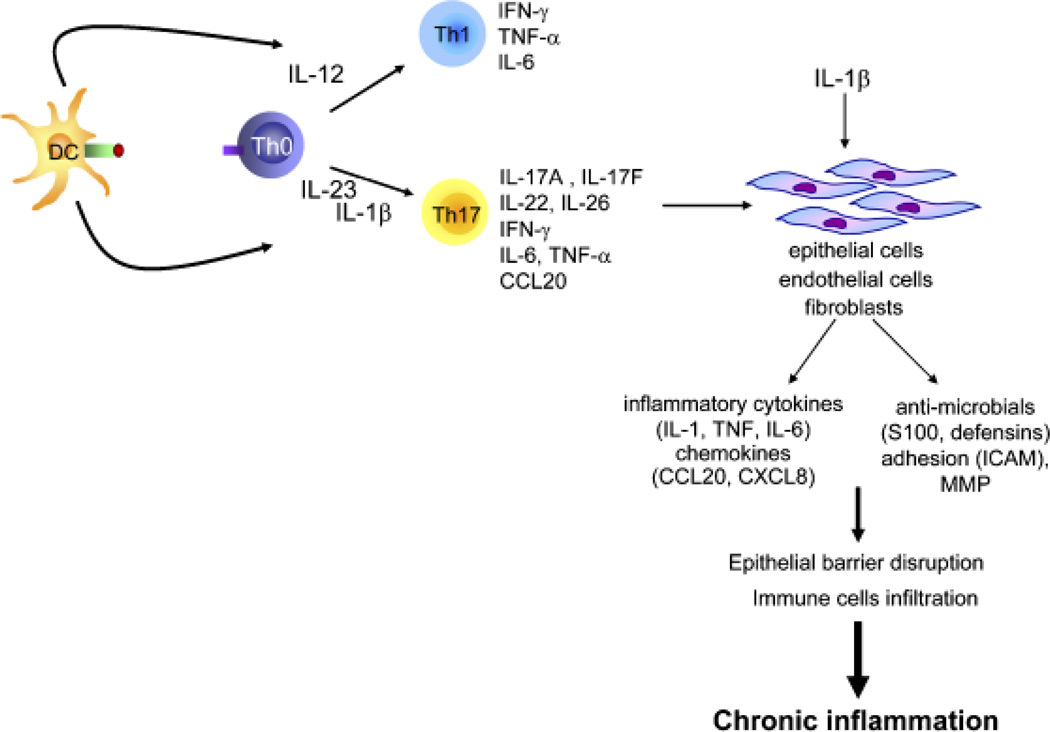
Fig. 6. IL-23/Th17 pathway and inflammation.
In response to inflammatory stimuli, dendritic cells produce IL-23 and IL-1β, which induce development of Th17 cells that produce IL-17, IL-22, IL-17F, and other proinflammatory cytokines. This inflammatory milieu, through its action on epithelial cells, will contribute to epithelial barrier disruption and recruitment of immune cells.
Mounting evidence supports a major role of IL-23 and Th17 cells in the pathogenesis of IBD. IL-12, IFN-γ, IL-23, and IL-17 expression is increased in the colonic lamina propria of CD patients (62, 63, 133–136). Clinical trials using anti-IL-12p40-neutralizing antibodies are showing promising results in the treatment of CD (137, 138). Investigating how IL-12p40 neutralization modulates the inflammatory response in CD patients, Mannon and colleagues (133) identified reduced secretion of IL-23, IL-12, IL-17, and IL-6 by mononuclear cells in the colonic lamina propria of patients that received anti-IL-12p40-neutralizing antibodies.
Strong evidence for the importance of IL-23 in CD pathogenesis emerged from genetic studies. Performing genome-wide analysis of single nucleotide polymorphisms in healthy subjects and CD patients, Cho and colleagues (139) established the association between IL23R gene polymorphisms and susceptibility to CD. Several studies performed in different patient cohorts confirmed those results (139–145). In addition, it was recently reported that IL23R gene polymorphisms correlate with IL-22 serum levels in CD patients (146). The established link between IL-23 and CD raises the question for a biological involvement of Th17 cells. Two groups recently cloned Th17 cells from the inflamed lamina propria of CD patients and compared them to Th1 or Th2 clones isolated from the same tissue (46, 47). These elegant studies were instrumental in characterizing the phenotype of human Th17 cells. The study of T-cell clones, however, provided only limited insight into the involvement of Th17 cells in the inflammatory process itself. The increasing knowledge about the phenotypical features of Th17 cells should soon enable studies of this important immune pathway in health and disease.
Conclusion
The discovery of IL-23 has led to the identification of a new Th cell subset that complements the Th1/Th2 paradigm. It is now clear that this Th17 pathway, with all its complexity, is essential for protection against infectious agents but also for the pathogenesis of inflammatory disorders. However, many questions still need to be answered. The precise role played by TGF-β during mouse and human Th17 cell development remains to be analyzed at a molecular level to get full insight in the importance of this factor. In addition, we have not discussed in this review the interaction and relationship between human Th17 and Treg cells. It is known that the regulation of FoxP3 expression is different between mice and humans (147) and that the interplay between FoxP3 and RORγt is important for the decision of mouse Th17 versus Treg cell lineage commitment (148). In this respect it is interesting that human Treg cells seem to be able to differentiate into IL-17-producing cells when stimulated with IL-2 and IL-15 in the presence of monocytes (149).
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 3.Del Prete GF, et al. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1T helper or type 2T helper) profile of cytokine production. J Clin Invest. 1991;88:346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD41 T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanen JB, et al. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991;174:583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Invest. 2002;109:431–435. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 9.Krakowski M, Owens T. interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 10.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 11.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- 12.Kobayashi M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brombacher F, et al. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 15.Decken K, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 17.Murphy CA, et al. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 19.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tato CM, Cua DJ. Reconciling id, ego, and superego within ILinterleukin-23. Immunol Rev. 2008;226:103–111. doi: 10.1111/j.1600-065X.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- 21.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 22.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 23.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(1) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahern PP, Izcue A, Maloy KJ, Powrie F. The IL-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogge L, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-αlpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 30.Yao Z, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 31.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 32.Yao Z, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 33.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 36.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 37.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 38.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 40.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 41.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelante T, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 43.Maloy KJ. The interleukin-23/interleukin-17 axis in intestinal inflammation. J Intern Med. 2008;263:584–590. doi: 10.1111/j.1365-2796.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 44.Kebir H, et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pène J, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 48.Volpe E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 49.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgam-mat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 51.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR61 effector memory T cells. J Immunol. 2008;180:7948–7957. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 53.Yang XO, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolk K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 56.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proin-flammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 58.Boniface K, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 60.Dambacher J, et al. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2008 doi: 10.1136/gut.2007.130112. [DOI] [PubMed] [Google Scholar]
- 61.Acosta-Rodriguez EV, Napolitani G, Lanza-vecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 62.Camoglio L, Te Velde AA, Tigges AJ, Das PK, Van Deventer SJ. Altered expression of interferon-gamma and interleukin-4 in inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:285–290. doi: 10.1002/ibd.3780040406. [DOI] [PubMed] [Google Scholar]
- 63.Fuss IJ, et al. Disparate CD4+lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 64.Gearing AJ, et al. Cytokines in skin lesions of psoriasis. Cytokine. 1990;2:68–75. doi: 10.1016/1043-4666(90)90045-u. [DOI] [PubMed] [Google Scholar]
- 65.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3 + regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 66.Annunziato F, et al. Chemokine receptors and other surface molecules preferentially associated with human Th1 or Th2 cells. Microbes Infect. 1999;1:103–106. doi: 10.1016/s1286-4579(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 67.Singh SP, Zhang HH, Foley JF, Hedrick MN. Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 68.Kaser A, et al. Increased expression of CCL20 in human inflammatory bowel disease. J Clin Immunol. 2004;24:74–85. doi: 10.1023/B:JOCI.0000018066.46279.6b. [DOI] [PubMed] [Google Scholar]
- 69.Homey B, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 70.Kohler RE, Caon AC, Willenborg DO, Clark-Lewis I, McColl SR. A role for macrophage inflammatory protein-3 alpha/CC chemokine ligand 20 in immune priming during T cell-mediated inflammation of the central nervous system. J Immunol. 2003;170:6298–6306. doi: 10.4049/jimmunol.170.12.6298. [DOI] [PubMed] [Google Scholar]
- 71.Ruth JH, et al. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 72.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato W, Aranami T, Yamamura T. Cutting edge: human Th17 cells are identified as bearing CCR2+CCR5– phenotype. J Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- 74.Saruta M, Yu QT, Avanesyan A, Fleshner PR, Targan SR. Papadakis KA. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. 2007;178:3293–3300. doi: 10.4049/jimmunol.178.5.3293. [DOI] [PubMed] [Google Scholar]
- 75.Papadakis KA, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 76.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 79.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 80.Chen W, et al. Conversion of peripheral CD4+CD25– naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 82.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 83.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17 + T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 89.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harris TJ, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoim-munity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 91.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 92.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 93.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renner ED, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 98.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-gamma-producing CD41 T cells following activation of naive CD41 T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 100.Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao S, et al. Retinoic acid increases Foxp31 regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 103.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 104.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 105.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelly MN, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorpho-nuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 110.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 111.Eyerich K, et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 112.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takagi R, et al. B cell chemoattractant CXCL13 is preferentially expressed by human Th17 cell clones. J Immunol. 2008;181:186–189. doi: 10.4049/jimmunol.181.1.186. [DOI] [PubMed] [Google Scholar]
- 114.Gocke AR, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 115.Egwuagu CE, et al. IFN-gamma increases the severity and accelerates the onset of experimental autoimmune uveitis in trans-genic rats. J Immunol. 1999;162:510–517. [PubMed] [Google Scholar]
- 116.Haas C, Ryffel B, Le Hir M. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. J Immunol. 1997;158:5484–5491. [PubMed] [Google Scholar]
- 117.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 118.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 119.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee E, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 122.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 123.Li J, Li D, Tan Z. The expression of interleukin-17, interferon-gamma, and macrophage inflammatory protein-3 alpha mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technolog Med Sci. 2004;24:294–296. doi: 10.1007/BF02832018. [DOI] [PubMed] [Google Scholar]
- 124.Kopp T, Lenz P, Bello-Fernandez C, Kastelein RA, Kupper TS, Stingl G. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J Immunol. 2003;170:5438–5444. doi: 10.4049/jimmunol.170.11.5438. [DOI] [PubMed] [Google Scholar]
- 125.Toichi E, et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 126.Gottlieb AB, et al. A phase 1, double-blind, placebo-controlled study evaluating single subcutaneous administrations of a human interleukin-12/23 monoclonal antibody in subjects with plaque psoriasis. Curr Med Res Opin. 2007;23:1081–1092. doi: 10.1185/030079907x182112. [DOI] [PubMed] [Google Scholar]
- 127.Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 128.Leonardi CL, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 129.Krueger GG, et al. A human interleukin-12/ 23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 130.Capon F, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 131.Cargill M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nair RP, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fuss IJ, et al. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006;12:9–15. doi: 10.1097/01.mib.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt C, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 135.Fujino S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nielsen OH, Kirman I, Rudiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and −17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 137.Sandborn WJ, et al. A randomized trial of ustekinumab a human interleukin-12/23 monoclonal antibody, in patients with moderate to severe Crohn’s disease. Gastro-enterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 138.Mannon PJ, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 139.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dubinsky MC, et al. IL-23 receptor (IL-23R) gene protects against pediatric Crohn’s disease. Inflamm Bowel Dis. 2007;13:511–515. doi: 10.1002/ibd.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Glas J, et al. 1004819 is the main disease-associated IL23R variant in German Crohn’s disease patients: combined analysis of IL23R, CARD15, and OCTN1/2 variants. PLoS ONE. 2007;2:e819. doi: 10.1371/journal.pone.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Limbergen J, et al. IL23R Arg381Gln is associated with childhood onset inflammatory bowel disease in Scotland. Gut. 2007;56:1173–1174. doi: 10.1136/gut.2007.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tremelling M, et al. IL23R variation determines susceptibility but not disease pheno-type in inflammatory bowel disease. Gastroenterology. 2007;132:1657–1664. doi: 10.1053/j.gastro.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cummings JR, et al. Contribution of the novel inflammatory bowel disease gene IL23R to disease susceptibility and pheno-type. Inflamm Bowel Dis. 2007;13:1063–1068. doi: 10.1002/ibd.20180. [DOI] [PubMed] [Google Scholar]
- 146.Schmechel S, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 147.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 148.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koenen HJ, Smeets RL, Vink PM, Rijssen EV, Boots AM, Joosten I. Human CD25high-Foxp3pos regulatory T-cells differentiate into IL-17 producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]