Short abstract
Quantitative measurements of cartilage wear have been challenging, with no method having yet emerged as a standard. This study tested the hypothesis that latest-generation particle analyzers are capable of detecting cartilage wear debris generated during in vitro loading experiments that last 24 h or less, by producing measurable content significantly above background noise levels otherwise undetectable through standard biochemical assays. Immature bovine cartilage disks (4 mm diameter, 1.3 mm thick) were tested against glass using reciprocal sliding under unconfined compression creep for 24 h. Control groups were used to assess various sources of contamination. Results demonstrated that cartilage samples subjected to frictional loading produced particulate volume significantly higher than background noise and contamination levels at all tested time points (1, 2, 6, and 24 h, p < 0.042). The particle counter was able to detect very small levels of wear (less than 0.02% of the tissue sample by volume), whereas no significant differences were observed in biochemical assays for collagen or glycosaminoglycans among any of the groups or time points. These findings confirm that latest-generation particle analyzers are capable of detecting very low wear levels in cartilage experiments conducted over a period no greater than 24 h.
Introduction
The primary function of articular cartilage is to serve as the bearing material in diarthrodial joints, transmitting loads while minimizing friction and wear. The friction coefficient of cartilage has been characterized extensively in the literature, using standard measurements of normal and tangential forces acting across a sliding interface [1–7]. Qualitative observations of cartilage wear debris have been made [8–15]; however, quantitative measurements of cartilage wear have proven to be more challenging, with only a few studies having reported such measurements. The primary quantitative approaches proposed to date include biochemical assaying of cartilage and test solutions [16–20], characterization of changing articular layer thickness [17,21–23], and changes in surface roughness [7,20,24–28]. One study examining polyethylene wear debris in hip arthroplasty reported the use of an automated particle analyzer [29]. The aim of this study was to test whether latest-generation particle analyzers are capable of detecting cartilage wear debris generated during in vitro loading experiments that last 24 h or less, by producing measurable content significantly above background noise levels. The longer-term objective of our studies is to test the hypothesis that elevated interstitial fluid pressurization, which is known to reduce the friction coefficient of cartilage [30,31], also reduces cartilage wear.
Materials and Methods
Sample Harvest.
Articular cartilage cylindrical explants were harvested from the tibial plateau of 2–3 month old calf knee joints (n = 9) obtained from a local abattoir. Full thickness cartilage discs (ø 4 mm) were excised, placed with articular surface down on a freezing stage microtome (Leica Instruments #SM2400, Nusslock, Germany), embedded and frozen in a water soluble sectioning gel (Thermo Scientific #1310APD, Rockford, IL), and trimmed of bone and deep zone tissue to obtain samples inclusive of an intact superficial zone with a final thickness between 1.2 and 1.4 mm (n = 9 per treatment group). After preparation, samples were stored at −20 °C for a period no greater than 30 days until use. Cartilage sample pairs harvested from adjoining regions were randomized into test and control groups to produce a study design with repeated measure.
Solution Preparation.
Phosphate buffered saline solution (PBS) supplemented with a protease inhibitor cocktail (Thermo Scientific #78438, Rockford, IL) and bactericide (Sigma-Aldrich #46878-U, St. Louis, MO) was used as a base solution for all testing. Base solution was passed through a 0.1 μm pore filter (Millipore #SLVV033RS, Billerica, MA) to eliminate background particulate, and care was taken to prevent contamination. Four solution groups were prepared, one each for the loaded cartilage wear (TEST), unloaded cartilage control (CTRL), environmental contamination control (ENVR), and base solution contamination control (BASE) groups (Fig. 1).
Fig. 1.
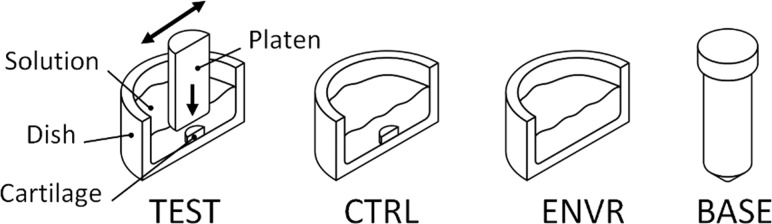
Schematic for TEST, CTRL, ENVR (cross-sectional views), and BASE (15 ml centrifuge tube) solution configurations
Mechanical Testing.
Test and control cartilage samples were thoroughly rinsed with base solution prior to testing and submerged in 10 ml of base solution, which was aspirated and replaced with fresh solution at 1, 2, 6, and 24 h time points. Test cartilage samples were subjected to friction using a smooth glass slide. The glass slide surface roughness was measured, Ra = 0.003 ± 0.002 μm, using an optical profilometer with a 10× objective and 1.81 × 1.36 mm scan area (Zygo #NV5000, Middlefield, CT). Measurements were performed in an unconfined compression creep configuration with a prescribed 4.48 N load (equivalent to 0.36 MPa) and intermittent sliding with translation range of ±10 mm and sliding velocity of 1 mm/s at room temperature, as used previously for the TEST group [7]. Control cartilage samples were placed in dishes without loading platens to determine the combined effects of natural particulate shedding from cartilage and environmental contamination for the CTRL group. Dishes without cartilage samples were used to determine environmental solution contamination for the ENVR group and were sampled in the same way as TEST and CTRL groups. The BASE solution group particulate content was also assessed to verify cleanliness over time (Fig. 1).
Particulate Analyzer.
Solutions were tested with a particle sizing and counting analyzer configured to measure particulates from 0.6 to 60 μm in diameter using two separate aperture tubes (30 and 100 μm) with a 300 μl total aspiration volume (Beckman Coulter #Multisizer 4, Brea, CA). After testing with the 100 μm aperture tube, solutions were then screened through a 10 μm filter (Spectrum Labs #148102, Rancho Dominguez, CA) prior to testing with the 30 μm aperture, to prevent clogging (the 10 μm filters are first cleaned by copious flushing with base solution). Counting analyzer calibration verification for both particulate size and number was performed per manufacturer specifications. Solution samples were placed in clean containers for testing, and agitated throughout test duration to keep particulates in suspension during characterization. For each group, cumulative particulate content was calculated at each time point by adding results from preceding time points.
Particulate Assay.
To concentrate wear particulate in preparation for biochemical assay, remaining sample solutions were centrifuged at 14,000 relative centrifugal force (RCF) for 45 min. The supernatant was removed and replaced with filtered de-ionized water to decrease the overall salt content prior to lyophilization. This centrifugation rinse cycle was repeated twice for each solution sample prior to lyophilization. Wear particulate lyophilisate was digested with Proteinase K for 12 h at 60 °C in 0.1 M sodium acetate containing 5 mM EDTA, 1 mM iodacetamide, 10 μg/ml pepstatin A, and 50 mM Tris. Minimal digestion solution volumes were used (250 μl) to maximize particulate concentration for increased assay sensitivity. Solution digests were then assayed for sulfated glycosaminoglycan (GAG) content using the 1,9-dimethylmethylene blue dye-binding assay [32]. Hydroxyproline content was determined from aliquots of the Proteinase K digest product. Samples were hydrolyzed by heating with equal volume of 12M HCl at 107 °C for 18 h and dried. The hydrolysates were assayed for hydroxyproline content using a colorimetric assay [33]. Collagen content was calculated from the aliquot fraction and hydroxyproline to total collagen ratio (7.64) [34].
Particulate Imaging.
Representative samples were obtained for TEST, CTRL, and ENVR solutions at the 24 h time point and concentrated according to the method above. Solutions were then incubated in 20 μM dichlorotriazinyl aminofluorescein (Invitrogen #D16, Eugene, OR) for 30 min then mixed one to one with a water soluble mounting gel to prevent particulate motion during imaging (Sigma-Aldrich #C 0612, St. Louis, MO). Gel solutions were mounted on coverslips for confocal imaging and sealed to prevent evaporation [35]. Z image stacks were obtained on a confocal microscope (Leica Microsystems #TCS SP5, Buffalo Grove, IL) and combined (NIH #ImageJ V1.44 p, Bethesda, MD) for qualitative visualization. Before and after testing, images were taken of the TEST cartilage tissue samples to assess potential macroscopic damage.
Statistical Analysis.
A two-way analysis of variance was performed for the factors of treatment (TEST, CTRL, ENVR, BASE) and time (1, 2, 6, 24 h) using repeated measures, with significance set at p ≤ 0.05. Post hoc testing of the means used Tukey correction. Analyses were performed for particulate number, particulate volume, GAG content, and collagen content (SAS Institute #SAS V9.2, Cary, NC).
Results
Particulate Number.
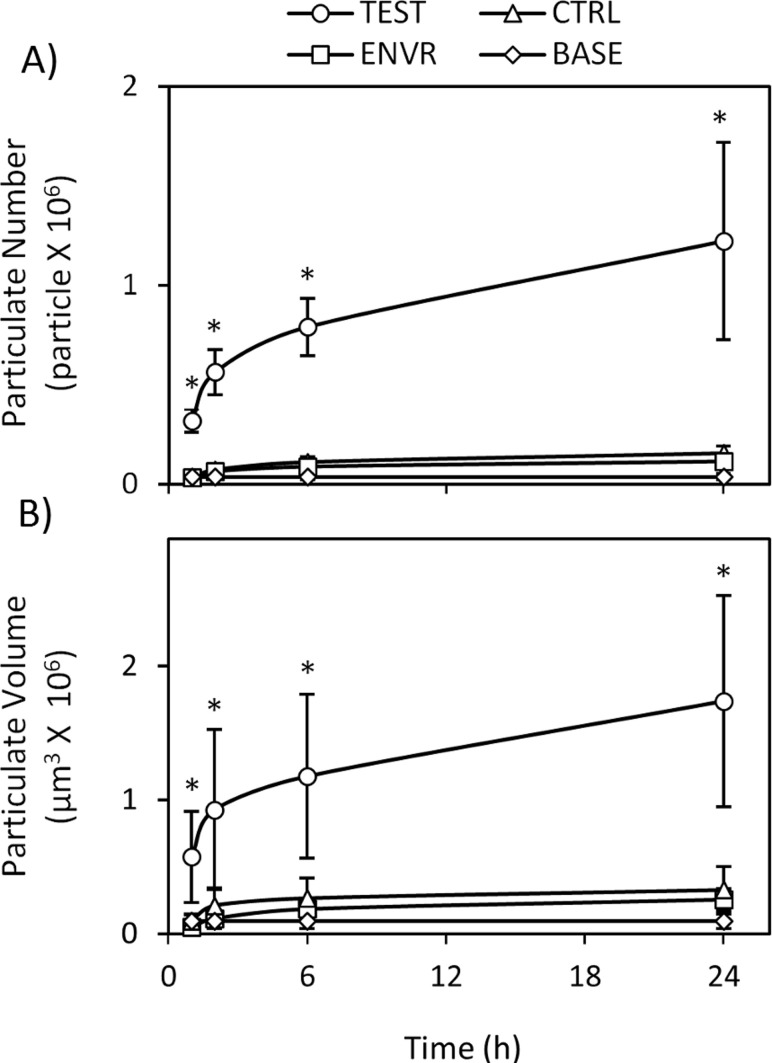
TEST solution cumulative wear particulate number was found to be significantly greater than CTRL, ENVR, and BASE solutions at all time points tested (p < 0.005, Fig. 2(a)). No differences were detected between CTRL, ENVR, and BASE solutions at any time points (p > 0.97).
Fig. 2.
Wear particulate measurements for bovine articular cartilage explants showing significant (*p < 0.05) increases in (a) particulate number and (b) volume when compared to unloaded CTRL, ENVR, and BASE groups at respective time points (n = 9 per group, 1, 2, 6, 24 h). No differences between CTRL, ENVR, or BASE groups were detected [(a) p > 0.97, (b) p > 0.90)].
Particulate Volume.
TEST solution cumulative wear particulate volume, similar to particulate number, was found to be significantly greater than CTRL, ENVR, and BASE solutions at the 2, 6, and 24 h time points (p < 0.042) (Fig. 2(b)). No differences were detected between CTRL, ENVR, and BASE solutions at any time points (p > 0.90).
Particulate Assay and Imaging.
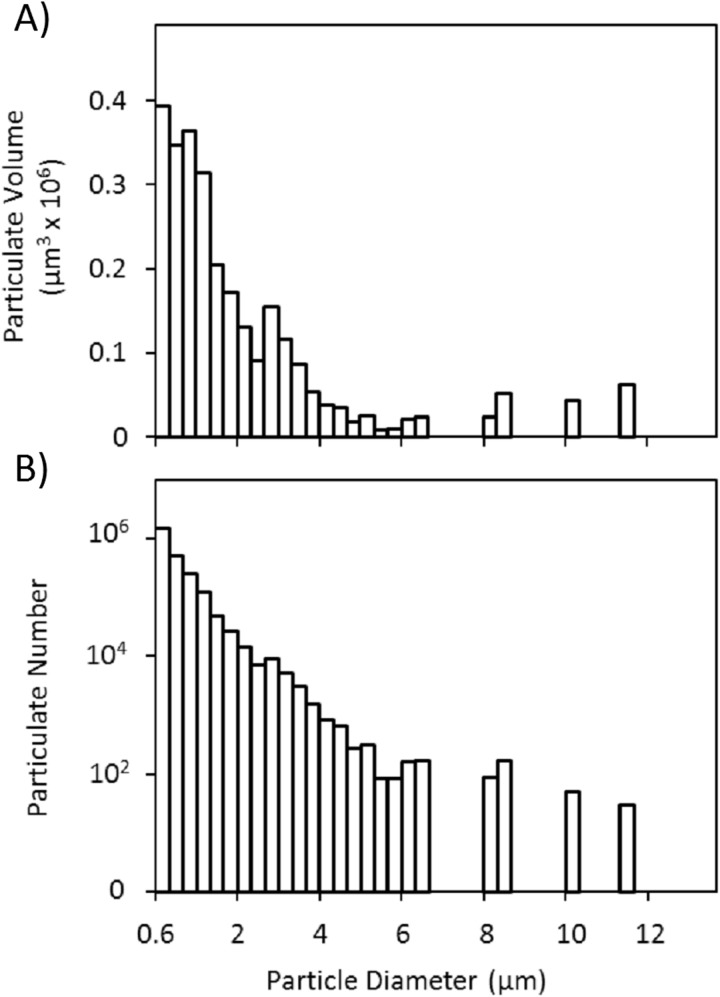
No differences were detected between TEST, CTRL, ENVR, and BASE solutions for either GAG (p = 0.87) or collagen content (p = 0.93) (Table 1). Qualitative visualizations from confocal imaging regarding particle geometry (Fig. 3) matched size distributions obtained via particle analyzer (Figs. 4(a) and 4(b)).
Table 1.
Biochemical assay measurements showing no detected difference in either glycosaminoglycan [(a) p > 0.87] or collagen [(b) p > 0.93] content among the TEST, CTRL, ENVR, or BASE groups at any time point (n = 8 per group, 1, 2, 6, 24 h)
| Glycosaminoglycan mass (μg) | Collagen mass (μg) | ||
|---|---|---|---|
| BASE (h) | 1 | 0.79 ± 0.39 | 0.56 ± 0.32 |
| 2 | 0.95 ± 0.54 | 0.35 ± 0.22 | |
| 6 | 1.05 ± 0.40 | 0.40 ± 0.20 | |
| 24 | 0.81 ± 0.37 | 0.53 ± 0.31 | |
| ENVR (h) | 1 | 0.99 ± 0.47 | 0.42 ± 0.29 |
| 2 | 0.99 ± 0.42 | 0.44 ± 0.17 | |
| 6 | 0.52 ± 0.43 | 0.46 ± 0.24 | |
| 24 | 0.74 ± 0.44 | 0.50 ± 0.19 | |
| CTRL (h) | 1 | 0.79 ± 0.38 | 0.58 ± 0.34 |
| 2 | 0.67 ± 0.29 | 0.36 ± 0.21 | |
| 6 | 0.73 ± 0.51 | 0.55 ± 0.38 | |
| 24 | 0.52 ± 0.59 | 0.38 ± 0.19 | |
| TEST (h) | 1 | 0.79 ± 0.34 | 0.39 ± 0.25 |
| 2 | 0.88 ± 0.36 | 0.42 ± 0.12 | |
| 6 | 0.62 ± 0.43 | 0.57 ± 0.32 | |
| 24 | 0.61 ± 0.50 | 0.42 ± 0.36 | |
Fig. 3.
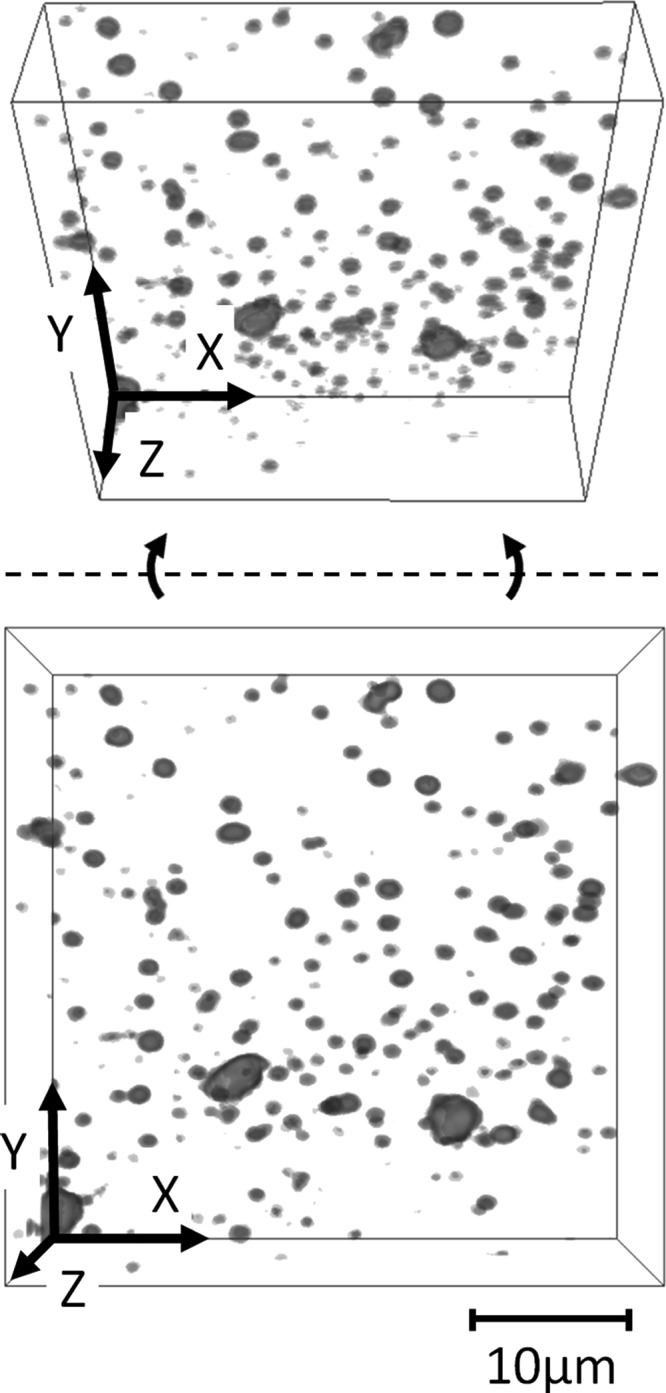
Wear particulate confocal z stack and angled z stack showing qualitative agreement between observed size distribution and particle analyzer measurements for representative 24 h TEST solution
Fig. 4.
(a) Particulate size and (b) volume distribution for representative 24 h TEST solution showing a high number of micron sized particles
Discussion
The reported measurements of this study clearly demonstrate that the cartilage samples subjected to frictional loading produce particulate content that is significantly higher than background noise and contamination levels (Figs. 2(a) and 2(b)). The experimental design of this study accounted for shedding of cartilage debris in the absence of loading, as may occur from natural enzymatic degradation or other similar mechanisms. The design also accounted for contamination from the testing environment, such as dust particles from the air or debris from the dishes and fluid handling equipment. By enforcing a clean testing environment, and minimizing enzymatic degradation using protease inhibitors, it was found that environmental contamination was negligible at all time points, in comparison to the wear produced from frictional loading. Confocal images provide direct visual evidence of the debris characterization from the particle counter (Fig. 3).
Though the amount of cartilage wear observed in the TEST group was significantly higher than background noise and contamination, it should be noted that it represented only a minute amount of loss from the cartilage sample. As noted from Fig. 2(b), the particulate volume at 24 h averaged less than 2 × 106 μm3, which is less than 0.02% of the total volume of cartilage (16 × 109 μm3). This outcome is not surprising, considering the relatively short 24 h duration of loading used in this test, when compared to the normal lifetime of cartilage. It is thus remarkable that the particle analyzer used in this study is able to detect such small but significant wear levels; otherwise not detected using standard biochemical assays (Table 1). This high sensitivity will considerably facilitate future studies of the wear response of cartilage under a variety of testing conditions, allowing investigators to test various wear-related hypotheses.
The lubrication regime achieved in the testing conditions of this study is a combination of interstitial fluid pressurization and boundary lubrication [36,37]. As shown in prior studies, upon application of the load, the interstitial fluid in the tissue pressurizes and supports most of the applied load, producing a low friction coefficient [30]. As the pressure subsides, the load becomes supported by the solid matrix; thus resulting in a boundary lubrication regime and a high friction coefficient [30]. For a 4 mm diameter plug, the characteristic time for this loss of fluid pressurization is ∼1400 s [38]. Therefore, for the experiments in this study, boundary lubrication prevailed over most of the testing duration. Furthermore, the magnitude of wear reported in this study is only representative of the specific testing conditions used here. Other experimental conditions will likely produce differing amounts of wear.
In summary, the results of this study establish unequivocally that latest-generation particle analyzers are capable of detecting very low wear levels in cartilage experiments conducted over a period no greater than 24 h.
Acknowledgment
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the U.S. National Institutes of Health under Award No. R01AR043628. The authors would like to thank Mr. Aleksander V. Navratil, and Dr. Elon J. Terrell for training and use of the optical profilometer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Sevan R. Oungoulian, e-mail: so2271@columbia.edu
Stephany Chang, Department of Mechanical Engineering, Columbia University, New York, NY 10027.
Orian Bortz, Department of Mechanical Engineering, Columbia University, New York, NY 10027.
Callen E. Willis, Department of Mechanical Engineering, Columbia University, New York, NY 10027
Clark T. Hung, Department of Biomedical Engineering, Columbia University, New York, NY 10027
Gerard A. Ateshian, Department of Mechanical Engineering, Department of Biomedical Engineering, Columbia University, New York, NY 10027
References
- [1]. Jones, E. S. , 1934, “Joint Lubrication,” Lancet, 1(5783), pp. 1426–1427. 10.1016/S0140-6736(00)56557-X [DOI] [Google Scholar]
- [2]. Mccutchen, C. W. , 1962, “The Frictional Properties of Animal Joints,” Wear, 5(1), pp. 1–17. 10.1016/0043-1648(62)90176-X [DOI] [Google Scholar]
- [3]. Stachowiak, G. W. , Batchelor, A. W. , and Griffiths, L. J. , 1994, “Friction and Wear Changes in Synovial Joints,” Wear, 171(1–2), pp. 135–142. 10.1016/0043-1648(94)90356-5 [DOI] [Google Scholar]
- [4]. Forster, H. , and Fisher, J. , 1996, “The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage,” Proc. Inst. Mech. Eng. (H), 210(2), pp. 109–119. 10.1243/PIME_PROC_1996_210_399_02 [DOI] [PubMed] [Google Scholar]
- [5]. Ateshian, G. A. , and Mow, V. C. , 2005, Lubrication and Wear of Diarthroidal Joints, Basic Orthopaedic Biomechanics & Mechano-Biology, Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- [6]. Naka, M. H. , Hattori, K. , Ohashi, T. , and Ikeuchi, K. , 2005, “Evaluation of the Effect of Collagen Network Degradation on the Frictional Characteristics of Articular Cartilage Using a Simultaneous Analysis of the Contact Condition,” Clin. Biomech., 20(10), pp. 1111–1118. 10.1016/j.clinbiomech.2005.06.009 [DOI] [PubMed] [Google Scholar]
- [7]. Jay, G. D. , Torres, J. R. , Rhee, D. K. , Helminen, H. J. , Hytinnen, M. M. , Cha, C. J. , Elsaid, K. , Kim, K. S. , Cui, Y. , and Warman, M. L. , 2007, “Association Between Friction and Wear in Diarthrodial Joints Lacking Lubricin,” Arthritis Rheum., 56(11), pp. 3662–3669. 10.1002/art.22974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Simon, W. H. , 1971, “Wear Properties of Articular Cartilage In Vitro ,” J. Biomech., 4(5), pp. 379–389. 10.1016/0021-9290(71)90058-3 [DOI] [PubMed] [Google Scholar]
- [9]. Meachim, G. , 1972, “Light Microscopy of Indian Ink Preparations of Fibrillated Cartilage,” Ann. Rheum. Dis., 31(6), pp. 457–464. 10.1136/ard.31.6.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Clarke, I. C. , Contini, R. , and Kenedi, R. M. , 1975, “Friction and Wear Studies of Articular-Cartilage—Scanning Electron-Microscope Study,” ASME J. Lubric. Tech-T, 97(3), pp. 358–368. 10.1115/1.3452600 [DOI] [Google Scholar]
- [11]. Podsiadlo, P. , and Stachowiak, G. W. , 1995, “Numerical-Analysis of Shape of Wear Particles From Arthritic and Asymptomatic Synovial Joints,” J. Orthop. Rheumatol., 8(3), pp. 155–160. [Google Scholar]
- [12]. Podsiadlo, P. , Kuster, M. , and Stachowiak, G. W. , 1997, “Numerical Analysis of Wear Particles from Non-Arthritic and Osteoarthritic Human Knee Joints,” Wear, 210(1–2), pp. 318–325. 10.1016/S0043-1648(97)00061-6 [DOI] [Google Scholar]
- [13]. Stachowiak, G. W. , and Podsiadlo, P. , 1997, “Analysis of Wear Particle Boundaries Found in Sheep Knee Joints During In Vitro Wear Tests Without Muscle Compensation,” J. Biomech., 30(4), pp. 415–419. 10.1016/S0021-9290(96)00144-3 [DOI] [PubMed] [Google Scholar]
- [14]. Ozturk, H. E. , Stoffel, K. K. , Jones, C. F. , and Stachowiak, G. W. , 2004, “The Effect of Surface-Active Phospholipids on the Lubrication of Osteoarthritic Sheep Knee Joints: Friction,” Tribol. Lett., 16(4), pp. 283–289. 10.1023/B:TRIL.0000015204.41674.d3 [DOI] [Google Scholar]
- [15]. Peng, Z. , 2007, “Osteoarthritis Diagnosis Using Wear Particle Analysis Technique: Investigation of Correlation Between Particle and Cartilage Surface in Walking Process,” Wear, 262(5–6), pp. 630–640. 10.1016/j.wear.2006.07.011 [DOI] [Google Scholar]
- [16]. Lipshitz, H. , Etheredge, R., 3rd , and Glimcher, M. J. , 1975, “ In Vitro Wear of Articular Cartilage,” J. Bone Joint Surg. Am., 57(4), pp. 527–534. [PubMed] [Google Scholar]
- [17]. Lipshitz, H. , and Glimcher, M. J. , 1979, “ In Vitro Studies of the Wear of Articular Cartilage—II. Characteristics of the Wear of Articular Cartilage When Worn Against Stainless Steel Plates Having Characterized Surfaces,” Wear, 52(2), pp. 297–339. 10.1016/0043-1648(79)90070-X [DOI] [Google Scholar]
- [18]. Marnell, P. , and White, R. K. , 1980, “Quantitative-Analysis of Joint Lubrication,” Wear, 61(2), pp. 203–218. 10.1016/0043-1648(80)90286-0 [DOI] [Google Scholar]
- [19]. Bank, R. A. , Krikken, M. , Beekman, B. , Stoop, R. , Maroudas, A. , Lafeber, F. P. , and Te Koppele, J. M. , 1997, “A Simplified Measurement of Degraded Collagen in Tissues: Application in Healthy, Fibrillated and Osteoarthritic Cartilage,” Matrix Biol., 16(5), pp. 233–243. 10.1016/S0945-053X(97)90012-3 [DOI] [PubMed] [Google Scholar]
- [20]. Verberne, G. , Merkher, Y. , Halperin, G. , Maroudas, A. , and Etsion, I. , 2009, “Techniques for Assessment of Wear between Human Cartilage Surfaces,” Wear, 266(11–12), pp. 1216–1223. 10.1016/j.wear.2009.03.042 [DOI] [Google Scholar]
- [21]. Bae, W. C. , Temple, M. A. , Amiel, D. , Coutts, R. D. , Niederauer, G. G. , and Sah, R. L. , 2003, “Indentation Testing of Human Cartilage—Sensitivity to Articular Surface Degeneration,” Arthritis Rheum., 48(12), pp. 3382–3394. 10.1002/art.11347 [DOI] [PubMed] [Google Scholar]
- [22]. Parsons, I. M. T. , Millett, P. J. , and Warner, J. J. , 2004, “Glenoid Wear After Shoulder Hemiarthroplasty: Quantitative Radiographic Analysis,” Clin. Orthop., 421, pp. 120–125. 10.1097/01.blo.0000119249.61696.f1 [DOI] [PubMed] [Google Scholar]
- [23]. Temple-Wong, M. M. , Bae, W. C. , Chen, M. Q. , Bugbee, W. D. , Amiel, D. , Coutts, R. D. , Lotz, M. , and Sah, R. L. , 2009, “Biomechanical, Structural, and Biochemical Indices of Degenerative and Osteoarthritic Deterioration of Adult Human Articular Cartilage of the Femoral Condyle,” Osteoarthr. Cartil., 17(11), pp. 1469–1476. 10.1016/j.joca.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Forster, H. , and Fisher, J. , 1999, “The Influence of Continuous Sliding and Subsequent Surface Wear on the Friction of Articular Cartilage,” Proc. Inst. Mech. Eng. (H), 213(4), pp. 329–345. 10.1243/0954411991535167 [DOI] [PubMed] [Google Scholar]
- [25]. Graindorge, S. L. , and Stachowiak, G. W. , 2000, “Changes Occurring in the Surface Morphology of Articular Cartilage During Wear,” Wear, 241(2), pp. 143–150. 10.1016/S0043-1648(00)00386-0 [DOI] [Google Scholar]
- [26]. Northwood, E. , Fisher, J. , and Kowalski, R. , 2007, “Investigation of the Friction and Surface Degradation of Innovative Chondroplasty Materials Against Articular Cartilage,” Proc. Inst. Mech. Eng. (H), 221(H3), pp. 263–279. 10.1243/09544119JEIM178 [DOI] [PubMed] [Google Scholar]
- [27]. Katta, J. , Jin, Z. , Ingham, E. , and Fisher, J. , 2009, “Effect of Nominal Stress on the Long Term Friction, Deformation and Wear of Native and Glycosaminoglycan Deficient Articular Cartilage,” Osteoarthr. Cartil., 17(5), pp. 662–668. 10.1016/j.joca.2008.10.008 [DOI] [PubMed] [Google Scholar]
- [28]. Li, L. , Patil, S. , Steklov, N. , Bae, W. , Temple-Wong, M. , D'lima, D. D. , Sah, R. L. , and Fregly, B. J. , 2011, “Computational Wear Simulation of Patellofemoral Articular Cartilage During In Vitro Testing,” J. Biomech., 44(8), pp. 1507–1513. 10.1016/j.jbiomech.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Maloney, W. J. , Smith, R. L. , Schmalzried, T. P. , Chiba, J. , Huene, D. , and Rubash, H. , 1995, “Isolation and Characterization of Wear Particles Generated in Patients Who Have Had Failure of a Hip-Arthroplasty Without Cement,” J. Bone Joint Surg. Am., 77(9), pp. 1301–1310. [DOI] [PubMed] [Google Scholar]
- [30]. Krishnan, R. , Kopacz, M. , and Ateshian, G. A. , 2004, “Experimental Verification of the Role of Interstitial Fluid Pressurization in Cartilage Lubrication,” J. Orthop. Res., 22(3), pp. 565–570. 10.1016/j.orthres.2003.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Ateshian, G. A. , 2009, “The Role of Interstitial Fluid Pressurization in Articular Cartilage Lubrication,” J. Biomech., 42(9), pp. 1163–1176. 10.1016/j.jbiomech.2009.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Farndale, R. W. , Buttle, D. J. , and Barrett, A. J. , 1986, “Improved Quantitation and Discrimination of Sulphated Glycosaminoglycans by Use of Dimethylmethylene Blue,” Biochim. Biophys. Acta, 883(2), pp. 173–177. 10.1016/0304-4165(86)90306-5 [DOI] [PubMed] [Google Scholar]
- [33]. Stegemann, H. , and Stalder, K. , 1967, “Determination of Hydroxyproline,” Clin. Chim. Acta, 18(2), pp. 267–273. 10.1016/0009-8981(67)90167-2 [DOI] [PubMed] [Google Scholar]
- [34]. Hollander, A. P. , Heathfield, T. F. , Webber, C. , Iwata, Y. , Bourne, R. , Rorabeck, C. , and Poole, A. R. , 1994, “Increased Damage to Type II Collagen in Osteoarthritic Articular Cartilage Detected by a New Immunoassay,” J. Clin. Invest., 93(4), pp. 1722–1732. 10.1172/JCI117156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Krahn, K. N. , Bouten, C. V. , Van Tuijl, S. , Van Zandvoort, M. A. , and Merkx, M. , 2006, “Fluorescently Labeled Collagen Binding Proteins Allow Specific Visualization of Collagen in Tissues and Live Cell Culture,” Anal. Biochem., 350(2), pp. 177–185. 10.1016/j.ab.2006.01.013 [DOI] [PubMed] [Google Scholar]
- [36]. Gleghorn, J. P. , and Bonassar, L. J. , 2008, “Lubrication Mode Analysis of Articular Cartilage Using Stribeck Surfaces,” J. Biomech., 41(9), pp. 1910–1918. 10.1016/j.jbiomech.2008.03.043 [DOI] [PubMed] [Google Scholar]
- [37]. Gleghorn, J. P. , Doty, S. B. , Warren, R. F. , Wright, T. M. , Maher, S. A. , and Bonassar, L. J. , 2010, “Analysis of Frictional Behavior and Changes in Morphology Resulting From Cartilage Articulation With Porous Polyurethane Foams,” J. Orthop. Res., 28(10), pp. 1292–1299. 10.1002/jor.21136 [DOI] [PubMed] [Google Scholar]
- [38]. Carter, M. J. , Basalo, I. M. , and Ateshian, G. A. , 2007, “The Temporal Response of the Friction Coefficient of Articular Cartilage Depends on the Contact Area,” J. Biomech., 40(14), pp. 3257–3260. 10.1016/j.jbiomech.2007.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]