Abstract
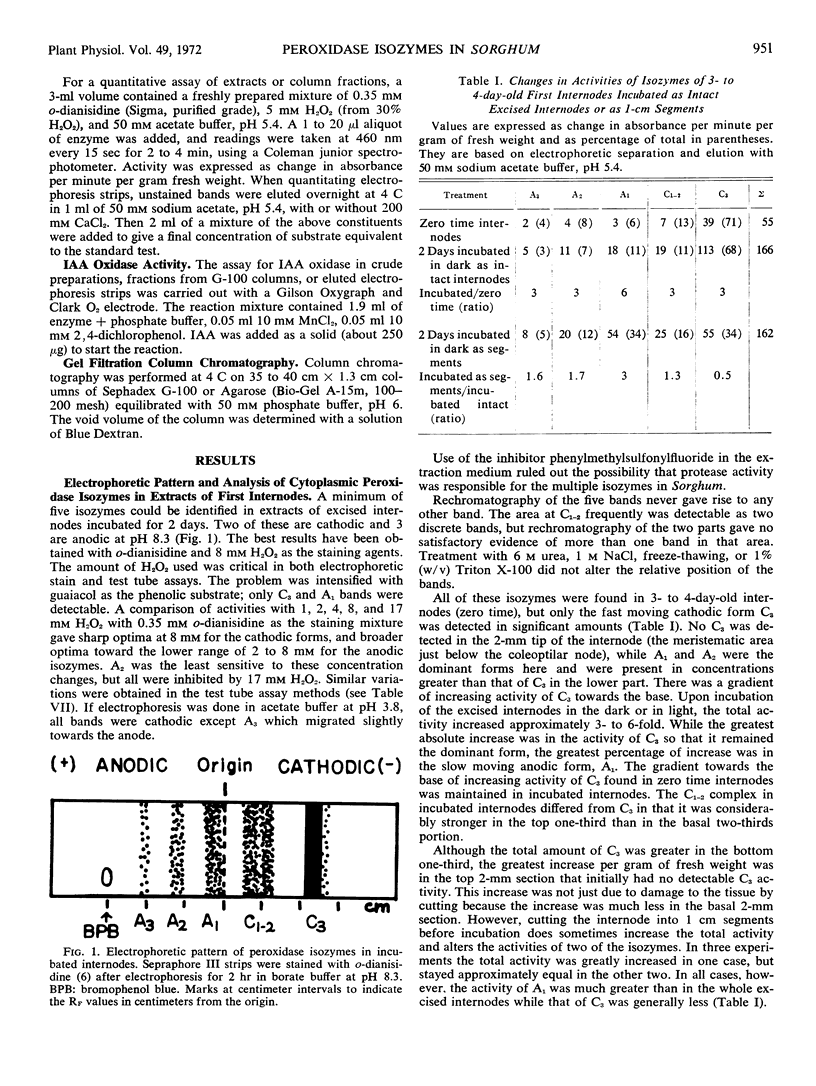
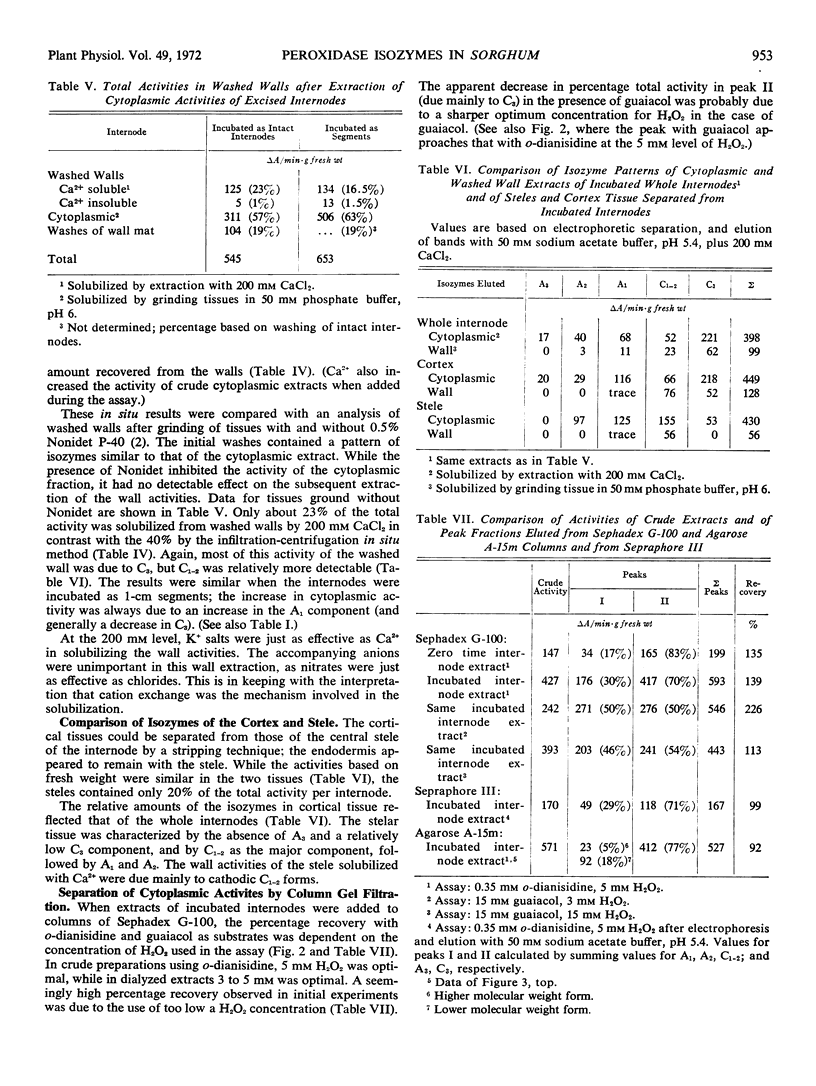
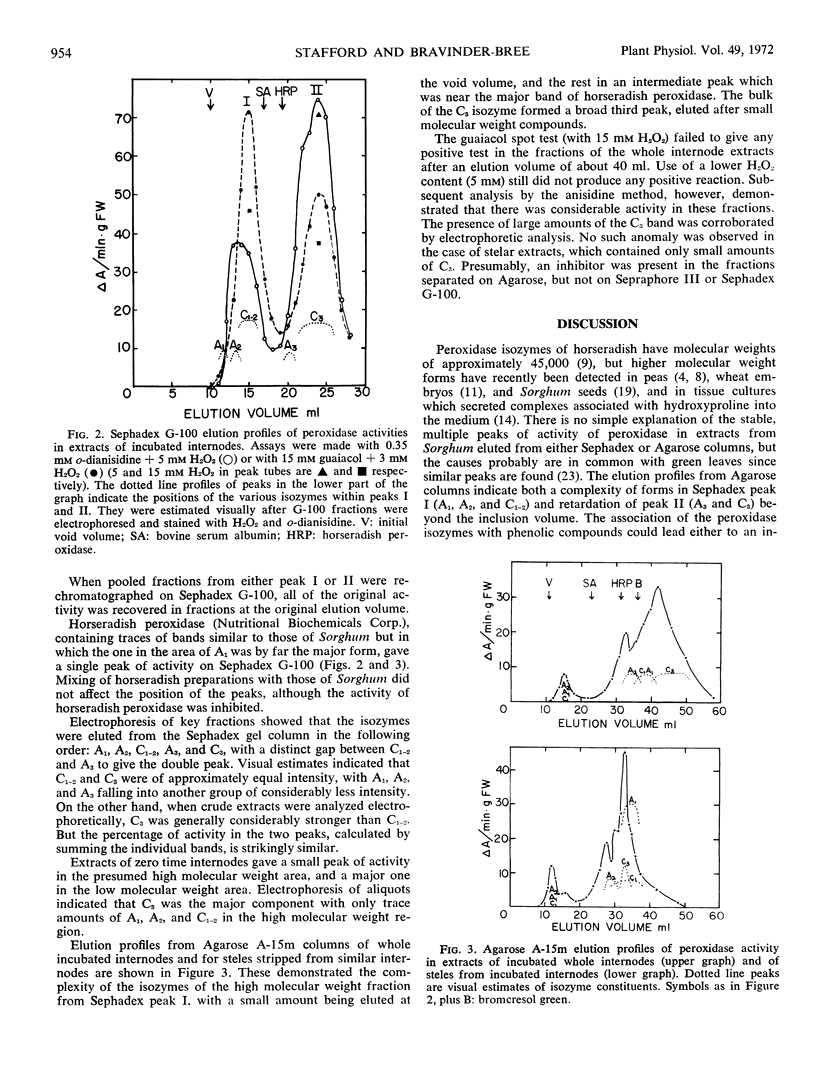
Electrophoretic analyses using Sepraphore III strips indicate the presence of a minimum of five bands of peroxidase activity detectable with o-dianisidine and H2O2 in extracts from first internodes of Sorghum vulgare var. Wheatland milo. Three of these isozymes were anodic and two were cathodic forms at pH 8.3. The relative amounts of these forms are compared in zero time and incubated excised internodes, stelar and cortical tissues of internodes, and in other parts of the plant. Localization of these isozymes with respect to walls and cytoplasm was characterized by differential centrifugation after grinding of the internodes and by an in situ extraction of walls by centrifugation after vacuum infiltration. Using the latter in situ method, 32% of the total activity of the fast moving cathodic form was exchanged from the wall after infiltration with 50 mm CaCl2. Only trace amounts of the other isozymes were localized in the walls of the cortex. The isozymes were eluted as two peaks from columns of Sephadex G-100 and three peaks from Agarose A-15m. Although such groupings may be due to asymmetric molecules and ionic interactions as well as to molecular weight differences, they may indicate associations with complexes or membranes of different cytoplasmic constituents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong D. W. An investigation of the role of plant peroxidase in cell wall development by the histochemical method. J Histochem Cytochem. 1967 Jun;15(6):335–346. doi: 10.1177/15.6.335. [DOI] [PubMed] [Google Scholar]
- Gaspar T., Hofinger M., Lacoppe J. Tamisage sur Sephadex G-100 des peroxydases, catalases, phénoloxydases et acide beta-indolylaćetique oxydases d'extraits radiculaires de Lens et de Pisum. Biochim Biophys Acta. 1969 Nov 4;191(2):463–465. doi: 10.1016/0005-2744(69)90265-4. [DOI] [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- LANZANI G. A., GALANTE E. PEROXIDASE ACTIVITIES FROM WHEAT EMBRYO RIBOSOMES. Arch Biochem Biophys. 1964 Jul 20;106:20–24. doi: 10.1016/0003-9861(64)90153-5. [DOI] [PubMed] [Google Scholar]
- Plesnicar M., Bonner W. D., Jr, Storey B. T. Peroxidase associated with higher plant mitochondria. Plant Physiol. 1967 Mar;42(3):366–370. doi: 10.1104/pp.42.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Dresler S. 4-Hydroxycinnamic Acid Hydroxylase and Polyphenolase Activities in Sorghum vulgare. Plant Physiol. 1972 Apr;49(4):590–595. doi: 10.1104/pp.49.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A. Relationships between the development of adventitious roots and the biosynthesis of anthocyanins in first internodes of sorghum. Plant Physiol. 1968 Mar;43(3):318–326. doi: 10.1104/pp.43.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber D. C., Hanna M. G., Jr Electron microscopic autoradiography of germinal center cells in mouse spleen. J Cell Biol. 1965 Jun;25(3 Suppl):109–119. doi: 10.1083/jcb.25.3.109. [DOI] [PubMed] [Google Scholar]
- Whitmore F. W. Effect of indoleacetic Acid and hydroxyproline on isoenzymes of peroxidase in wheat coleoptiles. Plant Physiol. 1971 Feb;47(2):169–171. doi: 10.1104/pp.47.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]



