Abstract
Chronic stress contributes to vulnerability for depression and drug addiction. The function of the serotonergic system has been found to be modified by chronic stress and these changes may play an important role in stress-related relapses to drug craving. The 5-HT1B receptor is expressed in nucleus accumbens projection neurons and modulates drug reward mechanisms and there is evidence suggesting that stress alters the regulation and function of these receptors. To examine the role of these receptors in integrating the effects of stress on reward mechanisms, we examined whether chronic or acute social defeat stress (SDS) regulates 5-HT1B mRNA in dorsal and ventral striatum, regions that are critical for integrating the effects of environmental stresses on reward motivated behavior. In a third experiment, 5-HT1B mRNA regulation in response to another acute stressor, inescapable tailshock, was measured. Our results indicate that intermittent and daily SDS procedures attenuated body weight gain, induced adrenal hypertrophy, and reduced the preference for saccharin, a sweet solution preferred by normal rats. There was a trend for daily, but not intermittent SDS to increase 5-HT1B receptor mRNA levels in nucleus accumbens. Therefore, in the next experiment, we examined daily SDS in greater detail and found that it increased 5-HT1B receptor mRNA expression in rostral nucleus accumbens shell, an area especially associated with reward functions. Neither acute SDS, nor acute tailshock stress had a significant impact on 5-HT1B mRNA expression in the striatum. Since increased 5-HT1B receptor expression in nucleus accumbens shell neurons can facilitate cocaine and alcohol reward mechanisms, this adaptation in endogenous 5-HT1B mRNA may be involved in the SDS-associated increase in vulnerability for developing addiction.
Keywords: chronic stress, serotonin, mesocorticolimbic pathway, 5-HT1B receptor, reward
1. Introduction
Certain types of chronic stress can exacerbate the course of mood disorders [1] and contribute to the vulnerability to initiate drug abuse or trigger relapse [2, 3]. Altered hedonic response to natural rewards is a core feature of both mood disorders and drug addiction, and may be an important interface between stress response and reward mechanisms in the brain [4]. The mesocorticolimbic reward pathway is critical in assessing reward (but see [5]), and plasticity in this circuit may contribute to stress-induced anhedonia as well as adaptations related to drug use [4]. Hedonic deficits induced by chronic stress can be detected in the form of decreased preference for a sweet solution [6, 7]; this index of anhedonia can be reversed by antidepressant treatment [8–10].
The mesocorticolimbic circuit for assessing and responding to reward includes the nucleus accumbens (NAc) in the ventral striatum, the dorsal striatum, the ventral tegmental area (VTA) and the medial prefrontal cortex. These regions have also been implicated in certain types of stress, the symptomatology of depression [4], and drug abuse [11]; manipulations of the NAc can produce both pro-depressive and antidepressant effects [4, 12, 13]. Serotonin is a key modulator of stress responses and reward function [14, 15], and several lines of evidence implicate the serotonin-1B (5-HT1B) receptor in NAc neurons as an important mediator of drug and stress adaptations in reward-related behavior. In the NAc shell (NAcSh), 5-HT1B receptors are expressed in medium spiny neurons and are located on axon terminals in targets such as the VTA where they inhibit GABA release [16–18]. These projections to VTA provide inhibitory feedback that reduces subsequent release of dopamine from VTA projections back to NAc [19, 20]; therefore, activation of these 5-HT1B receptors disinhibits dopaminergic function. Available evidence suggests that acute cocaine exposure (both contingent and noncontingent) regulates 5-HT1B mRNA in these neurons, and increased 5-HT1B expression in these neurons enhances a variety of behavioral responses to cocaine, alcohol, and amphetamine [21–24]. There is also evidence that stress regulates these 5-HT1B receptors, but the evidence is less clear [22, 25, 26].
Given the link between hedonic state, reward, stress, and serotonin, it is possible that 5-HT1B receptors in the mesocorticolimbic reward pathway are critical modulators of hedonic state that are regulated by stress and drug experiences and in turn modulate hedonic and drug reward mechanisms. To begin to tease apart the different aspects of these relationships, we have examined the impact of two well-established stress models in rats, social defeat and inescapable tail shock, on 5-HT1B mRNA expression and hedonic behavior. Therefore, we wanted to examine the effect of stress in the absence of cocaine exposure to assess its role in regulating 5-HT1B mRNA expression in the striatum. We used the social defeat model as a chronic and acute stressor, as it is a well-established and reliable method of examining the effects of chronic stress on hedonic and drug addiction mechanisms. For example, it induces anhedonic behavior in the sucrose preference test [7], increases sensitization to psychostimulants, and escalates cocaine self-administration [27, 28]. Additionally, we used acute inescapable tail shock because it has been shown to be a reliable and reproducible method of eliciting stress-induced anhedonia (i.e., decreased consumption of sucrose solution) [29], increases immediate early gene expression in serotonergic neurons [30], and has been shown to facilitate drug – induced dopamine efflux in the NAcSh [31]. Our over-riding hypothesis is that stress exposure increases 5-HT1B mRNA expression in the ventral striatum as a compensatory adaptation to hedonic challenges, but that this increases the rewarding effects of drugs and thereby accelerates the progression of addiction. The hypothesis for the current set of studies is that chronic stress increases expression of 5-HT1B mRNA in the ventral striatum. We will investigate 5-HT1B mRNA regulation along the full extent of the striatum. Our rationale for a rostral-caudal investigation is supported by previous studies that have shown that activating GABA receptors along the rostral-caudal gradient of the NAc elicits behavioral outcomes, in terms of hedonic response [32]. Studies investigating behavioral drug response have also implicated rostral-caudal differences in drug mechanisms [33–35].
2. Methods and Materials
2.1 Subjects
Male Sprague Dawley rats (250–275g) were two months old at the time of delivery into the animal facility and were used in experiments after a one week acclimation period. Male (375–425g) and female (8 weeks old) Long Evans rats were used as stimulus animals (i.e. resident pairs) only; no dependent measures were collected from these animals. All rats were procured from Harlan Laboratories (Indianapolis, IN). Experimental animals (Sprague Dawley) were individually housed; resident stimulus animals (Long Evans) were pair-housed in a male-female configuration, in a temperature and humidity controlled vivarium, on a 12-12h light-dark cycle, with lights-on at 0600. Food and water were available ad libitum. In Experiment 2, food was available ad libitum in food hoppers and was weighed daily. All procedures were approved by the Institutional Animal Care and Use Committee. This research was conducted according to the requirements of all applicable local, national, and international standards for the care and use of laboratory animals [36].
2.2 Social Defeat Stress
2.2.1 Residents
Male Long Evans rats were housed for at least four breeding cycles with tubally ligated females (females were tubally ligated to prevent pregnancy while maintaining hormonal tone and sexual receptivity). Males were then screened for aggression [37]. Only those that consistently displayed aggressive behavior toward intruder stimulus animals (which were not used in the following experiments) were included in the study.
2.2.2 Social Defeat
Social defeat procedures were similar to those described by Covington, et. al. [38] and took place between the hours of 0730 and 1100. Briefly, females were removed from residents’ cages; an intruder was placed in the resident’s cage under a mesh protective enclosure for 10 minutes, resulting in the instigation of aggressive behavior from the resident. The mesh enclosure was then removed, and the animals were allowed to interact. Defeat was characterized by the resident pinning the intruder in a supine position for at least 5 consecutive seconds or the resident biting the intruder five times in a row. Time to defeat averaged between 10 and 60 sec; if there was no defeat by five minutes, the interaction was ended. After defeat, the intruder was placed under the mesh enclosure and remained in the resident’s cage for an additional 30 minutes. Intruders were then returned to their home cages and females returned to the respective resident’s cage. All animals were monitored for health during the experiment, including assessment of coat condition, body weight, and visual inspection for wounds. Any animal that had a wound that required stitches was removed from the study (two animals met that criterion).
2.3 Saccharin Testing
Animals were presented with two 50mL sipper bottles of liquid for one hour of access between the hours of 1630 and 1800. One bottle contained tap water and the other had 0.1% saccharin in tap water. In Experiment 2, animals had one drink session exposure to establish baseline drinking and one exposure after the last defeat episode to determine the effects of stress on consumption of the sweet liquid. In Experiment 3, animals had two drink session exposures on two consecutive days, and then testing on 4, 7, and 10 days after the onset of stress to determine the time course of stress-induced saccharin drinking deficits. Baseline drinking in Experiment 3 was an average of the two exposure days. The saccharin testing protocol was developed with data from pilot studies that determined the best time of day and length of exposure for detecting stress-induced deficits in intake. Animals that drank less than 2 mL of liquid during the baseline exposures were excluded from the study (approximately 5%).
2.4 Experimental Design
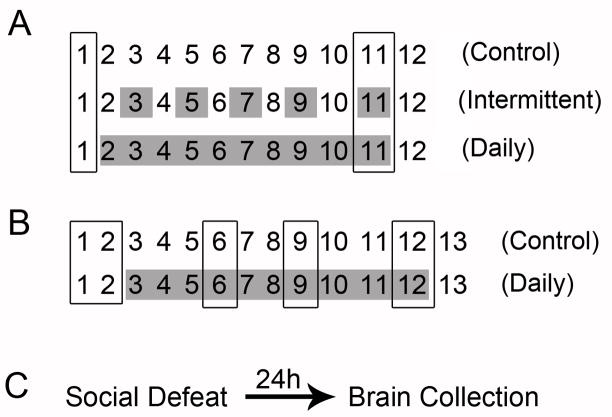
Figure 1 depicts the experimental designs. In Experiment 1, animals were exposed to either daily social defeat stress (10 days), intermittent (every other day for 10 days; 5 days total) stress, or remained in home cages as controls. Before the onset of stress, these animals were exposed to a 0.1% saccharin solution; their preference for the solution was then tested 8 hours after the last defeat episode (Figure 1A). Experiment 2 was a follow-up to Experiment 1, and was designed to measure NAc 5-HT1B mRNA along the entire rostral-caudal axis. Therefore, in Experiment 2, animals were either socially defeated daily for 10 days or remained in home cages as controls. These groups were exposed to the 0.1% saccharin solution on two separate days before the onset of stress, which reduced baseline variability, and then after 4, 7, and 10 days of stress. Saccharin preference testing took place 8 hours after defeat (Figure 1B) to allow the time to recover from the stress procedure. Since acute exposure to cocaine, a rewarding substance, has been shown to increase 5-HT1B mRNA in the NAcSh, but extended saccharin access does not [26], saccharin testing was restricted to only the chronic stress groups. Additionally, animals in Experiments 1 and 2 were sacrificed 24 hours after the last social defeat, to avoid possible effects of saccharin on 5-HT1B mRNA expression. In Experiment 3, animals were exposed to a single social defeat. They were sacrificed 24 hours later and brains were processed for in situ hybridization (Figure 1C). Information on the tailshock stress experiment can be found in the Supplemental methods and materials section.
Figure 1. Schematic Diagrams of Experimental Designs.
A) In Experiment 1, animals were drink trained and then defeated every other day (intermittent), every day (daily), or remained in their cages as controls. The effect of chronic stress on saccharin preference was assessed 8 hours after the last defeat episode. B) In Experiment 2, animals were defeated daily or remained in their cages as controls. Saccharin preference was assessed on two separate days before stress to determine baseline, and then at 4, 7, and 10 days after the onset of social defeat stress. Boxed days represent saccharin testing; shaded days represent social defeat. C) In Experiment 3, animals received either one social defeat stress, or remained in their cages as controls. They were then sacrificed 24 hours later. See supplemental material and methods section for tailshock stressor experimental design information.
2.5 Tissue collection
Four hours after the end of the tailshock session or 24 hours after the last social defeat episode, animals were sacrificed via CO2 inhalation. Brains were extracted and flash frozen in isopentane cooled on dry ice and then stored at −70°C until processing. Tissue was sliced at 20μm on a Leica CM3000 frozen cryostat (Bannockburn, IL) and mounted on Fisherbrand Superfrost glass microscope slides (Pittsburgh, PA). Slides were stored in boxes with desiccator pellets at −70°C until the in situ hybridization assay was performed. Adrenal and thymus glands were collected into saline filled 12- or 24-well plates on wet ice (Experiment 2). Organs were cleaned and weighed on the day of processing.
2.7 in situ hybridization histochemistry
in situ hybridization was performed as previously described [39, 40]; we probed for corticotrophin releasing hormone (CRH) mRNA in the hypothalamus and 5-HT1B mRNA in striatum. Briefly, tissue was fixed, deaminated, delipidated, and dehydrated. For 5-HT1B mRNA, oligonucleotide probes (Sigma Aldrich, St. Louis, Mo) were labeled with 33P α-dATP (MP Biomedicals, Solon, OH) using terminal deoxytransferase (Fermentas, Glen Burnie, Maryland). For CRH mRNA, riboprobes were transcribed with 33P α-dUTP (Amersham) constituting 65% of total UTP and T7 RNA polymerase. Tissue was hybridized in a humidified chamber overnight at 37°C and then washed in 2X sodium chloride/sodium citrate solution for one hour at 55°C and then one hour at room temperature, dehydrated, and exposed to Phosphor Screens (Perkin Elmer). Phosphor screens were developed 24 hours later with the Cyclone storage phosphor scanner (Perkin Elmer, Meridien, CT). In situ hybridization signal was quantified using computer-based densitometry software (MCID Analysis 7.0). Background measures were taken from white matter and subtracted from regional measurements to yield integrated gray levels. Measurements were taken from 2–4 sections per animal at each rostral-caudal level studied, and then averaged.
2.8 Statistics
Food, body weight gain, and saccharin consumption were compared using two factor repeated measures ANOVA. Adrenal and thymus weights were compared using a single factor ANOVA. 5-HT1B mRNA levels in Experiment 1 were analyzed with a single factor ANOVA. In addition to a single factor ANOVA to determine differences between the three groups, we made one a priori planned comparison in which we decided to compare only control and daily groups for NAcSh 5-HT1B expression with a Student’s t-test. Our rationale for this specific comparison was that if intermittent stress had an intermediate effect on 5-HT1B mRNA expression, then it may wash out meaningful differences between the control and daily stress groups. 5-HT1B mRNA levels in Experiments 2 and 3 were analyzed using Student’s t-test for each individual coordinate along the rostral-caudal axis. Kruskal Wallis ANOVA on Ranks was used when data violated the standard assumptions of ANOVA. Correlation was assessed with Pearson’s Product Moment analysis.
3.Results
3.1 Experiment 1
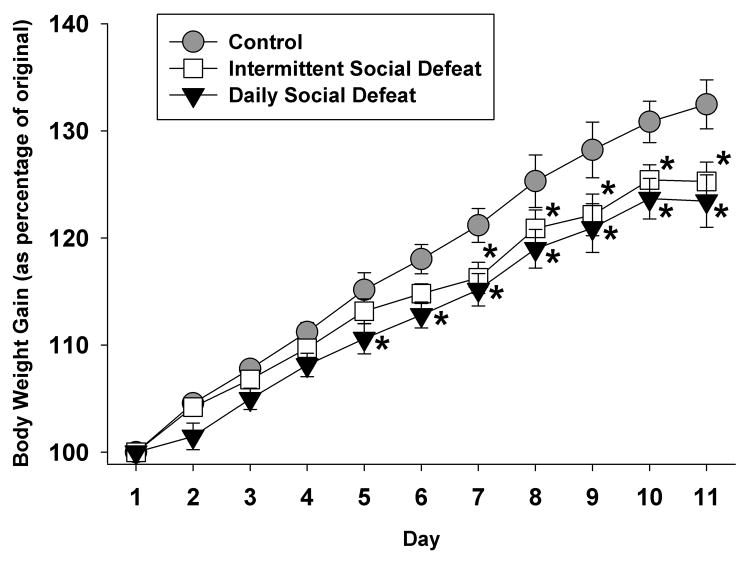
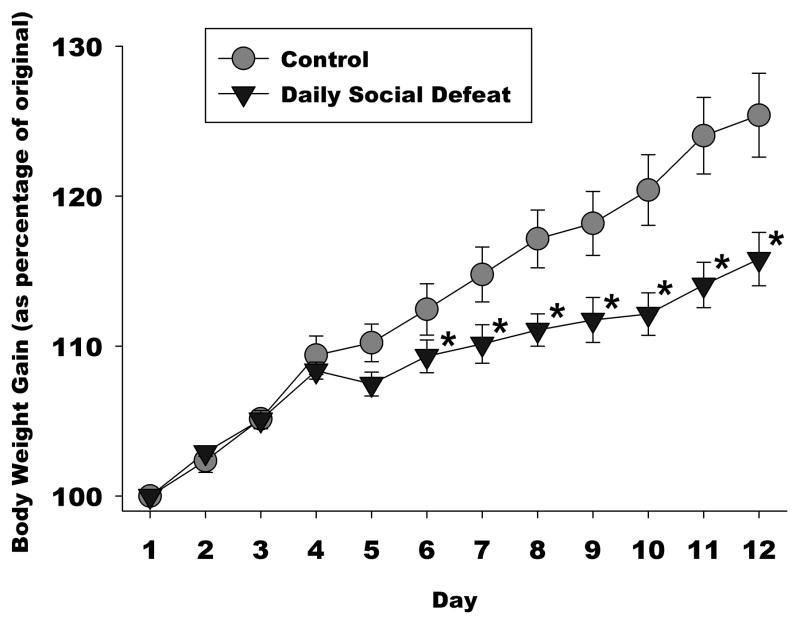
The purpose of this experiment was to determine the impact of social defeat stress frequency on hedonic state and the expression of 5-HT1B receptors in the mesolimbic reward pathway. Both chronic and intermittent social defeat decreased the rate of body weight gain (Figure 2), consistent with other chronic stress paradigms [42–44]. There was a main effect of group (F 2, 208 = 4.46, p<0.05), day (F 10, 208 = 332.38, p<0.001), and an interaction between group and day (F 20, 208 = 2.75, p<0.001) on body weight gain. Post hoc tests revealed no differences between groups on days 1–4, but a significant difference between daily social defeat and controls on days 5–11, and a significant difference between intermittent social defeat and controls that manifested on days 7–11. There were no significant differences between daily and intermittent stress duration on body weight gain.
Figure 2. Stress Effects on Body Weight Gain.
Both chronic and intermittent social defeat attenuated body weight gain. The daily defeated group gained significantly less body weight compared to homecage controls beginning at day 5, while intermittently defeated animals’ body weight gain deficits manifested at 7 days after the onset of defeat. *=p<0.05, compared to control group.
Food was available ad libitum in hoppers located inside the cages and was monitored by weighing hoppers daily. Table 1 lists food intake during social defeat. While there was no main effect of group (F 2, 179 = 2.11, p=0.15), there was a main effect of day (F 9, 179 = 4.81, p<0.001), as well as a group by day interaction (F 18, 179 = 1.72, p<0.05). Post hoc tests revealed a significant difference between the control and daily social defeat groups on the first day of defeat (p=0.001), and the intermittent group was significantly different than both control and daily defeat groups on day 4 (p=0.02 and 0.03, respectively). Table 2 lists organ weights. Neither chronic, nor intermittent stress decreased raw thymus weight (F 2, 17 = 0.16, p=0.85) or thymus weight adjusted as a percentage of body weight (adjusted weight; F 2, 17 = 0.68, p=0.52). Additionally, there were no significant group differences in raw adrenal weight (F 2, 17 = 0.20, p=0.82). Adjusted adrenal weight failed the assumptions of standard ANOVA; a Kruskal-Wallis test on Ranks revealed a significant group effect (p<0.05) with post hoc analysis showing a significant difference between control and daily groups (p<0.01), suggesting adrenal hypertrophy in the daily defeated group.
Table 1.
Food intake during chronic social defeat stress.
| Control | Intermittent | Daily | |
|---|---|---|---|
| Day 1 | 23.6 ± 1.23 | 20.9 ± 0.74 | 17.7 ± 2.11* |
| Day 2 | 23.5 ± 0.22 | 26.3 ± 0.84 | 23.5 ± 1.28 |
| Day 3 | 25.1 ± 1.33 | 22.1 ± 0.96 | 23.8 ± 1.01 |
| Day 4 | 23.3 ± 0.42 | 27.7 ± 0.97*,# | 23.8 ± 0.87 |
| Day 5 | 27.0 ± 0.82 | 23.1 ± 0.44 | 23.7 ± 0.40 |
| Day 6 | 25.5 ± 0.76 | 27.3 ± 1.36 | 26.5 ± 3.15 |
| Day 7 | 25.6 ± 0.67 | 24.9 ± 2.67 | 24.0 ± 1.34 |
| Day 8 | 26.5 ± 0.43 | 23.3 ± 0.78 | 23.3 ± 1.05 |
| Day 9 | 25.6 ± 0.92 | 23.4 ± 1.25 | 23.5 ± 1.06 |
| Day 10 | 25.6 ± 0.61 | 24.5 ± 1.19 | 23.5 ± 1.02 |
Data are listed as ±SEM.
p<0.05 vs. control;
p<0.05 vs. daily stress.
Table 2.
Organ weights after intermittent and chronic social defeat stress.
| Control | Intermittent | Daily | |
|---|---|---|---|
| Adrenal gland | |||
| mg of raw weight | 45.06 ± 1.78 | 45.01 ± 2.05 | 46.42 ± 1.35 |
| mg per 100g body weight | 14.81 ± 0.45 | 15.31 ± 0.71 | 16.50 ± 0.22* |
| Thymus gland | |||
| mg of raw weight | 532 ± 44.5 | 559 ± 28.3 | 549 ± 28.5 |
| mg per 100g body weight | 175 ± 15.4 | 194 ± 12.1 | 193 ± 8.6 |
Data are listed as ±SEM.
p<0.05 vs. control.
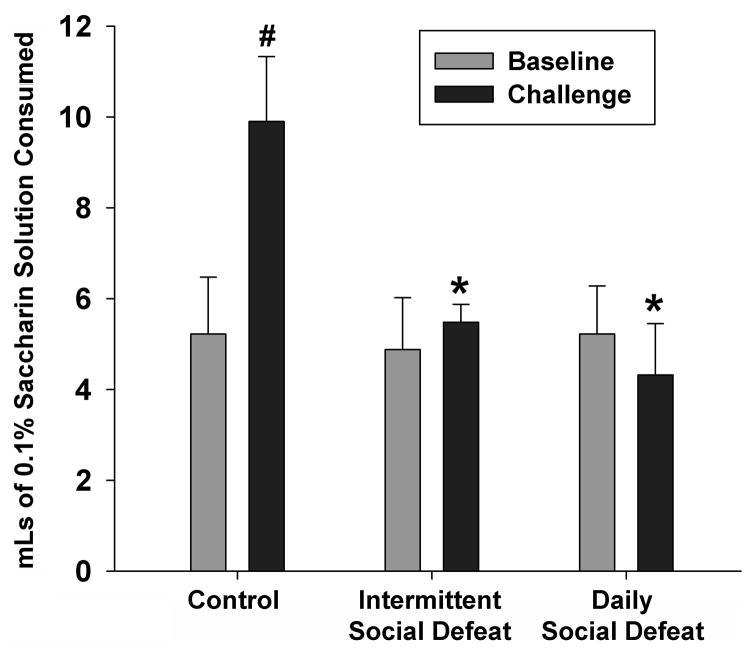
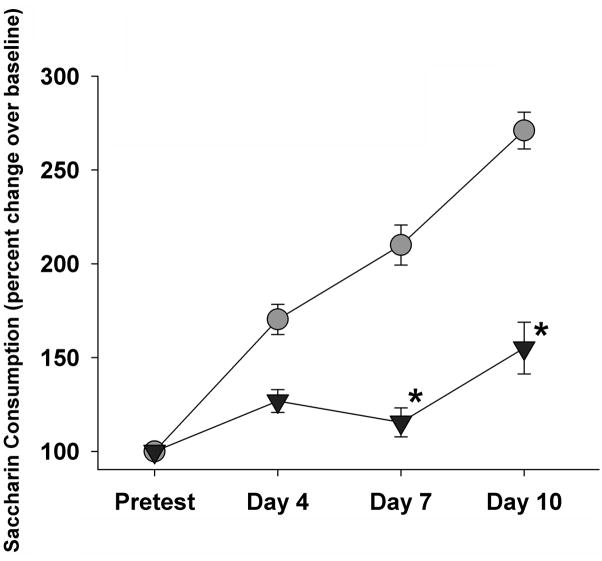
Both daily and intermittent stress decreased consumption of the saccharin solution, compared to controls (Figure 3). There was a main effect of time (F 1, 31 = 5.82, p<0.05) and an interaction between group and time (F 2, 31 = 8.39, p<0.05), but no main effect of group (F 1, 31 = 2.19, p=0.15). More specifically, in the challenge drinking test, control animals significantly increased saccharin intake over baseline (p<0.001), while neither the intermittent, nor the daily stressed groups altered drinking behavior over time. Finally, control animals drank significantly more saccharin during the challenge test than intermittent or daily stress groups (P=0.002 and 0.014, respectively).
Figure 3. Stress Effects on Saccharin Preference.
Both daily and intermittent stress induced hedonic deficits in the form of decreased saccharin preference. There were significant within-group effects, with the control group drinking more saccharin at the challenge, as well as a main effect of group, with both intermittent and daily stress groups drinking less saccharin than controls on the challenge test. #=p<0.05 vs. within group compared to baseline; *=p<0.05 vs. controls on challenge test.
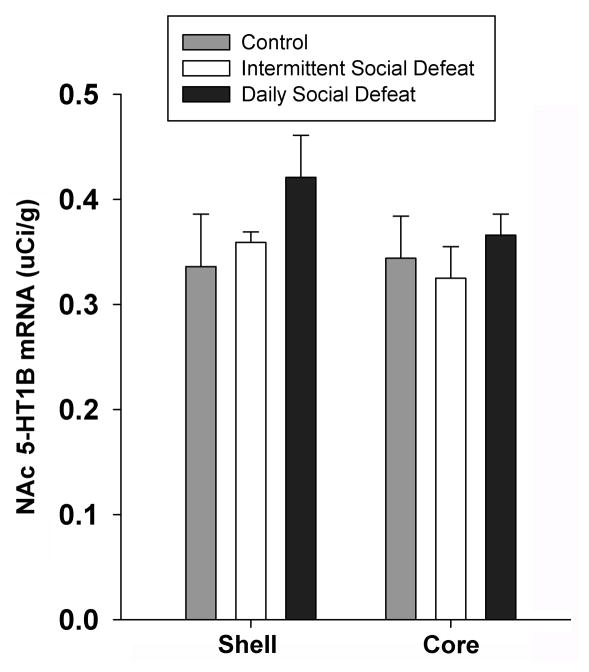
Figure 4 shows the results of an in situ hybridization for 5-HT1B receptor mRNA in ventral striatum for control, intermittent stress, and daily stress groups. 5-HT1B mRNA signal was measured in NAc, in both the core and shell, at approximately 1.2 to 1.0 mm anterior to bregma. A one way ANOVA revealed no group differences in the NAcSh (F 2, 18 = 1.17, p<0.34) or NAc core (F2, 17 = 0.44, p<0.65), although there was indication of a trend for increased 5-HT1B mRNA in NAcSh in the animals that received daily social defeat, so we investigated this further in Experiment 3, in which we examined 5-HT1B mRNA expression along the rostral to caudal extent of striatum.
Figure 4. 5-HT1B mRNA Regulation After Intermittnet and Chronic Social Stress.
While a single factor ANOVA revealed no significant group effects on 5-HT1B mRNA expression levels in either the NAcSh or NAc core, an a priori direct comparison revealed a trend toward increased mRNA levels in the NAcSh.
3.2 Experiment 2
The purpose of this study was to determine whether 5-HT1B mRNA throughout the striatum was altered after daily social defeat stress. Figure 5 depicts body weight gain over the course of the experiment. There was a main effect of group (F 1, 230 = 6.27, p<0.05) and day (10, 230 = 93.11, p<0.001), and an interaction between group and day (F 10, 230= 7.93, p<0.001). More specifically, defeated animals had attenuated body weight gain on days 6–11 compared to controls, consistent with chronic stress.
Figure 5. Chronic Social Stress Effects on Body Weight Gain.
There was a main effect of group and day, as well as an interaction between group and day on body weight gain. Chronically stressed animals had attenuated body weight gain on days 6–11 compared to controls. *=p<0.05.
To determine the time course of chronic stress effects on hedonic deficits, baseline saccharin consumption was measured (the two trials were averaged) and then saccharin preference was tested 4, 7, and 10 days after the onset of daily social defeat stress (Figure 6). There was a main effect of group (F 1, 82 = 4.45, p<0.05) and day (F 3, 82 = 11.56, p<0.001), as well as an interaction between group and day (F 3, 82 = 3.67, p<0.05). Specifically, socially defeated animals consumed significantly less saccharin solution after 7 (p<0.05) and 10 days (p<0.01) of daily defeat, compared to baseline measurements. Additionally, within group comparisons revealed that control animals drank significantly more saccharin solution over time, while there were no differences between days in the defeated group.
Figure 6. Chronic Stress Induces Hedonic Deficits.
Chronic social defeat stress significantly induced hedonic deficits in the form of decreased saccharin preference 7 and 10 days after social defeat. *=p<0.05.
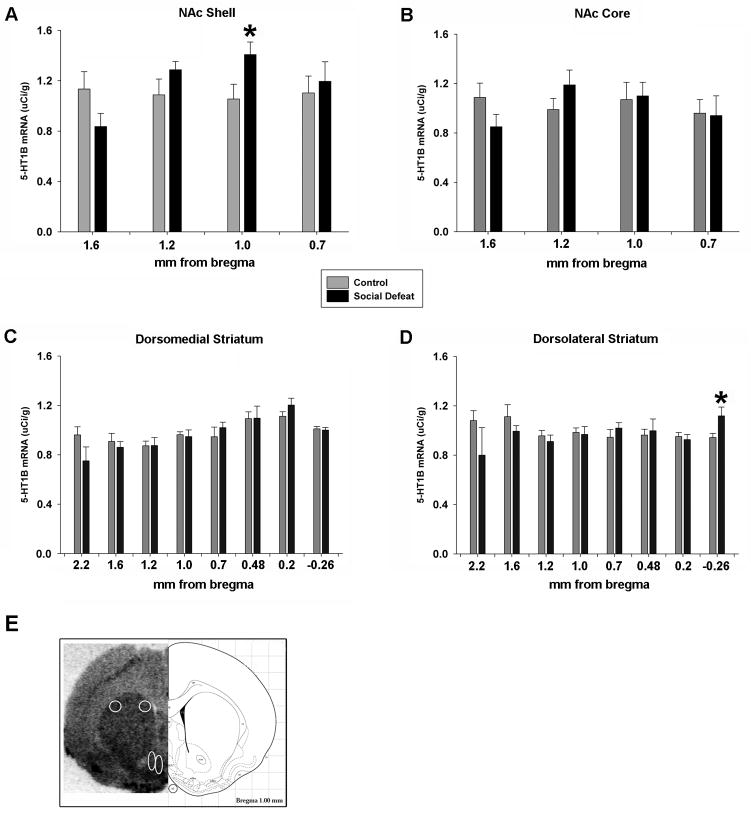
To determine the extent of rostral to caudal expression changes in 5-HT1B mRNA levels in response to chronic stress, 5-HT1B mRNA was measured in the NAc at four levels: 1.6, 1.2, 1.0, and 0.7 mm anterior to bregma. Student’s t-test revealed no significant differences in 5-HT1B mRNA expression in the NAcSh at 0.7 (p=0.66), 1.2 (p=0.18), or 1.6 (p=0.08) mm rostral to bregma (Figure 7A). However, chronic social defeat significantly increased 5-HT1B mRNA in the NAcSh at 1.0 mm rostral to bregma (p<0.05). In the NAc core, there were no significant group differences at any level assayed; p-values ranged from 0.14 to 0.97 (Figure 7B). Dorsal striatum was analyzed from the rostral-most tip at 2.7 mm anterior to bregma to −0.26 mm caudal to bregma. There were no group effects on rostral-most striatal 5-HT1B mRNA expression (level 2.7 mm rostral to bregma, data not shown; p=0.65). Additionally, there were no differences in 5-HT1B mRNA expression in the dorsomedial striatum at any level (p-values ranged from 0.17 to 0.99; Figure 7C). In the dorsolateral striatum, at the level of −0.26 mm caudal to bregma, daily defeated animals had significantly increased 5-HT1B receptor mRNA (p=0.04). There were no significant group effects at any other level (p-values ranged from 0.17 to 0.83; Figure 7D). Figure 7E shows a representative picture of 5-HT1B mRNA hybridization signal from a chronically defeated animal. Striatal regions that were quantitated have been circled and include dorsolateral striatum, dorsomedial striatum, NAc core, and NAcSh.
Figure 7. 5-HT1B mRNA Regulation After Chronic Stress.
A) Ten consecutive days of social defeat stress significantly increased 5-HT1B mRNA signal in the NAcSh at 1.0 mm anterior to bregma. There were no differences in mRNA expression in the NAc core (B) or in the dorsomedial striatum (C). D) At −0.26 mm posterior to bregma, chronic stress increased 5-HT1B mRNA expression in the dorsolateral striatum. E) A representative picture of 5-HT1B mRNA hybridization signal from a chronially defeated animal is shown beside a panel adapted from the Paxinos and Watson rat brain atlas [64] (1.0 rostral to bregma is depicted). Striatal regions that were quantitated have been circled and include dorsolateral striatum, dorsomedial striatum, NAc core, and NAcSh. *=p<0.05.
3.3 Experiment 3
The purpose of this experiment was to determine whether an acute social defeat stress regulated striatal 5-HT1B receptor mRNA, and whether the regulation was specific to regional rostral-caudal location. In the dorsal striatum, there were no differences between control and acutely stressed groups at any level in either the dorsomedial striatum (p-values ranged from 0.42 to 0.98) or the dorsolateral striatum (p-values ranged from 0.40 to 0.98). Integrated gray levels are listed in Table 3. In addition, there were no significant group differences at any level in either the NAc core (p-values ranged from 0.17 to 0.80) or the NAcSh (p-values ranged from 0.31 to 0.76).
Table 3.
5-HT1B mRNA signal after acute social stress, by brain region.
| Region | mm From Bregma | Control | Social Defeat Stress |
|---|---|---|---|
| Nucleus Accumbens Shell | |||
| 1.6 | 0.40 ± 0.07 | 0.36 ± 0.05 | |
| 1.2 | 0.42 ± 0.07 | 0.35 ± 0.05 | |
| 1.0 | 0.38 ± 0.07 | 0.40 ± 0.06 | |
| 0.7 | 0.46 ± 0.06 | 0.41 ± 0.05 | |
| Nucleus Accumbens Core | |||
| 1.6 | 0.43 ± 0.10 | 0.32 ± 0.05 | |
| 1.2 | 0.46 ± 0.08 | 0.35 ± 0.04 | |
| 1.0 | 0.40 ± 0.08 | 0.38 ± 0.05 | |
| 0.7 | 0.40 ± 0.07 | 0.38 ± 0.05 | |
| Dorsolateral Striatum | |||
| 1.6 | 0.57 ± 0.08 | 0.57 ± 0.05 | |
| 1.2 | 0.51 ± 0.09 | 0.43 ± 0.05 | |
| 1.0 | 0.49 ± 0.07 | 0.51 ± 0.06 | |
| 0.7 | 0.53 ± 0.09 | 0.45 ± 0.05 | |
| Dorsomedial Striatum | |||
| 1.6 | 0.48 ± 0.08 | 0.48 ± 0.05 | |
| 1.2 | 0.43 ± 0.07 | 0.40 ± 0.03 | |
| 1.0 | 0.53 ± 0.07 | 0.47 ± 0.06 | |
| 0.7 | 0.50 ± 0.09 | 0.42 ± 0.05 | |
Data are listed as ±SEM.
p<0.05 vs. control.
4. Discussion
Social defeat is a potent and ethologically relevant stressor that can interfere with normal hedonic state and increase susceptibility to the rewarding effects of drugs of abuse [6]. Since previous data from this and other laboratories suggest that certain types of stressors, such as needle poke, social stress, and cocaine withdrawal may modulate 5-HT1B receptor expression in NAc [22, 25, 26], and activation of 5-HT1B receptors in the mesocorticolimbic circuit increases the rewarding effects of cocaine [20, 21, 24] and alcohol [23, 45], we investigated whether social defeat stress regulated 5-HT1B mRNA levels in dorsal and ventral striatum. The results of these experiments indicate a relationship between stress duration and 5-HT1B mRNA expression in the striatum, such that chronic social stress regulated 5-HT1B mRNA transcript in the NAcSh and dorsolateral striatum at anatomically specific levels on the rostral-caudal axis, while an acute social stress had no effect. Although exposure to an acute tailshock stress (see supplementary material) had no significant effects on 5-HT1B mRNA expression in the NAc, it did increase CRH mRNA expression in the PVN four hours after stress, indicating that the procedure induced a physiological stress response. Interestingly, there were significant correlations between CRH mRNA levels and 5-HT1B mRNA expression at specific levels in both subregions of NAc, suggesting some interaction between tail shock stress, CRH mRNA, and 5-HT1B expression that could be examined in more detail in future studies. It is also possible that repeated tail shock sessions or mRNA measurement at a different time point might have revealed an effect of this stress procedure on 5-HT1B mRNA.
We examined chronic social defeat since it has an especially strong impact on NAc function and gene expression [46–48]. The effects of repeated social defeat on physiological and behavioral indices of chronic stress and 5-HT1B mRNA regulation were tested Experiment 1. The results indicate that both intermittent and daily social defeat decreased body weight gain and induced adrenal hypertrophy, effects that have been previously described for social defeat and other chronic stress models [42, 49–52]; therefore, it appears that the social defeat procedure used in these experiments was an effective stress procedure. In addition, we observed that both intermittent and daily social defeat reduced the preference for saccharin, a non-nutritive sweet tastant that has been previously used as an index of the hedonic state of a subject [6, 7]. Additionally, we determined whether the frequency or duration of stress exposure is associated with changes in 5-HT1B mRNA expression. We found that neither intermittent nor daily stress regulated receptor expression in the NAc core, but observed a trend (p=0.08) toward an increase in the NAcSh of animals stressed daily. The final experiment investigated whether an acute social stress (or tailshock, see supplemental data) would regulate 5-HT1B transcript levels and found no social stress effect and a very modest effect in the tailshock group in the dorsomedial striatum at +1.2 mm anterior to bregma.
The NAc is an area that is involved in assessing the emotional valence of incoming stimuli, and may be important in mediating stress and fear responses. In addition to being divided into the core and shell subregions, which subserve distinct roles in behaviors such as drug seeking [53, 54], reactivation of extinguished conditioned place preference [55], and appetitive behavior [56], there is also a somatotopic organization of inputs and outputs and functional roles across the rostral-caudal extent of the NAc [57–60]. For example, Reynolds and Berridge showed that injection of muscimol, a GABAA agonist, into rostral NAcSh facilitated food reward but induced negative affect and fear behavior when infused into caudal NAcSh [32]. Therefore, anatomical precision is valuable in assessing the regulation of gene expression and behavioral impact across this gradient. We designed the third experiment to take these rostral-caudal differences in function into consideration. After chronic social defeat, we measured 5-HT1B mRNA across a wide rostral-caudal span (from 1.6 to 0.7 mm rostral to bregma) and found that in the NAcSh at the +1.0 mm level, chronic social stress dramatically upregulated 5-HT1B receptor mRNA.
Although rostral NAcSh at around +1.6 mm from bregma had the greatest positive hedonic impact in the Reynolds and Berridge study, we did not detect differences in 5-HT1B mRNA expression in this specific area, consistent with attenuated saccharin consumption in stressed groups. On the other hand, Reynolds and Berridge observed increased fear behavior with muscimol injection into NAcSh at 1.0 mm anterior to bregma; in the present study, chronic social stress significantly increased 5-HT1B receptor mRNA expression at this locus. Since increased 5-HT1B heteroreceptor activity in these neurons should result in reduced GABA release in VTA and disinhibition of dopaminergic afferents back to NAc, we would predict that increased 5-HT1B expression in these neurons would also tend to inhibit their activity (similar to muscimol) and might enhance the sensitivity of these neurons to serotonin release, which is frequently elevated during aversive experiences [61]. Indeed, we observed that both rewarding and aversive responses to cocaine were enhanced by increased 5-HT1B expression, depending on the timing of conditioning using the place conditioning procedure [21], although we did not examine the precise location of gene transfer along the rostral-caudal gradient in that study.
Two functional gradients may be relevant to these results. First, a dorsolateral to ventromedial gradient has been proposed to organize the striatum in regard to inputs and functional outputs; numerous subtle distinctions between core and shell have been observed [62]. The NAcSh, and especially the medial subregion that we examined here, is considered to be part of the “extended amygdala” and receives projections from limbic regions associated with stress reactivity, whereas NAc core receives more sensorimotor and “associational” inputs. For example, NAcSh receives inputs from ventral hippocampus and NAc core from dorsal hippocampus, regions of hippocampus that are associated with emotional and motivational vs. cognitive processing, respectively [63]. A second gradient from rostral to caudal has been hypothesized to contribute to rewarding vs. aversive functions, respectively. However, the somatotopic organization of inputs to rostral vs. caudal NAcSh has not been examined in great detail. In control animals, 5-HT1B mRNA was relatively similar across the rostral-caudal continuum of NAc core, whereas there was a shift toward decreased 5-HT1B mRNA in rostral shell and increased 5-HT1B mRNA in caudal shell. In principal, if 5-HT1B receptors in the NAcSh are increased, the mid-caudal striatum would be more sensitive to serotonergic input and modulation of emotional state in the face of environmental challenges. This is in agreement with the Berridge and Reynolds study that showed that the caudal NAcSh was involved in fear behaviors, whereas anterior parts were not [32].
The NAcSh is also heavily involved in modulating behaviors associated with drugs of abuse. Previous work from our group using viral mediated gene transfer to increase 5-HT1B receptor levels in the NAcSh resulted in increased ethanol consumption [23, 45], changes in the pattern of consumption during both acquisition and maintenance of ethanol drinking [23], and enhancement of cocaine reward behavior that is dose dependent [21]. In addition to enhancing susceptibility to drugs of abuse, 5-HT1B receptors play a role in the interaction between stress and drugs. For example, increased 5-HT1B receptor expression in the NAcSh paired with a mild stressor results in facilitation of both the psychomotor activating effects of amphetamine, as well as amphetamine sensitization [22]. 5-HT1B receptor regulation also occurs in response to forced cocaine withdrawal [26]. It is clear that this receptor plays an important role in drug abuse, and the current study suggests that the 5-HT1B receptor in the NAcSh is modulated by stress in a regionally specific manner. Many studies demonstrate an intimate link between drug abuse/relapse and stress. Importantly, this receptor population is not responsive to acute stress, but is regulated by chronic stress exposure, which in turn negatively affects hedonic state. Taken together, these studies suggest that 5-HT1B receptors in the mesolimbic reward pathway contribute to the hedonic and emotional impact of the environment on the organism, as well as the organism’s subsequent behavior toward other environmental challenges.
In summary, these results suggest that chronic social stress increases the expression of 5-HT1B receptor mRNA in the caudal NAcSh. The present results suggest the possibility that there may be a stress-associated gradient within NAcSh in the rostral-caudal dimension that further refines the role of serotonin in stress-related behavior. This same region and receptor plays a role in increased susceptibility to the rewarding effects of psychostimulants such as cocaine and amphetamine. Upregulation of this receptor in this pathway may tend to increase stress-related susceptibility to the rewarding and addictive aspects of drugs of abuse; thus, this receptor may serve as a valuable target for manipulating drug reward mechanisms in stressed individuals.
Supplementary Material
Research Highlights.
Chronic social defeat induces hedonic deficits.
Chronic social defeat upregulates 5-HT1B receptor mRNA expression in the NAcSh.
5-HT1B mRNA is regulated by stress along a rostral-caudal gradient.
Acknowledgments
This work was supported by grants from NIDA 5F32DA026265 (ARF) and 5R01DA016432 (JFN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Calabrese F, et al. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S208–16. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16(3):387–94. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 4.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–36. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 6.Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120(2):102–28. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rygula R, et al. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162(1):127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Rygula R, et al. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174(1):188–92. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Von Frijtag JC, Van den Bos R, Spruijt BM. Imipramine restores the long-term impairment of appetitive behavior in socially stressed rats. Psychopharmacology (Berl) 2002;162(3):232–8. doi: 10.1007/s00213-002-1093-3. [DOI] [PubMed] [Google Scholar]
- 10.Bekris S, et al. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161(1):45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 12.Bewernick BH, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Eisch AJ, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54(10):994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19(14):1417–22. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa H, et al. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol. 2000;58(6):1271–8. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12(5):2000–6. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14(11 Pt 1):6763–7. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan QS, Yan SE. Serotonin-1B receptor-mediated inhibition of [(3)H]GABA release from rat ventral tegmental area slices. J Neurochem. 2001;79(4):914–22. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- 20.O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311(2):711–9. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- 21.Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25(10):3125–31. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson SM, Sandygren NA, Neumaier JF. Pairing mild stress with increased serotonin-1B receptor expression in the nucleus accumbens increases susceptibility to amphetamine. Eur J Neurosci. 2009;30(8):1576–84. doi: 10.1111/j.1460-9568.2009.06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furay AR, et al. Overexpression of 5-HT(1B) mRNA in nucleus accumbens shell projection neurons differentially affects microarchitecture of initiation and maintenance of ethanol consumption. Alcohol. 2010 doi: 10.1016/j.alcohol.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumaier JF, et al. Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22(24):10856–63. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoplight BJ, Vincow ES, Neumaier JF. Cocaine increases 5-HT1B mRNA in rat nucleus accumbens shell neurons. Neuropharmacology. 2007;52(2):444–9. doi: 10.1016/j.neuropharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Neumaier JF, et al. Acquisition of and withdrawal from cocaine self-administration regulates 5-HT mRNA expression in rat striatum. J Neurochem. 2009;111(1):217–27. doi: 10.1111/j.1471-4159.2009.06313.x. [DOI] [PubMed] [Google Scholar]
- 27.Miczek KA, et al. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27(8):787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130(3):203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- 29.Christianson JP, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193(1):87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grahn RE, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826(1):35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 31.Bland ST, et al. Stress potentiation of morphine-induced dopamine efflux in the nucleus accumbens shell is dependent upon stressor uncontrollability and is mediated by the dorsal raphe nucleus. Neuroscience. 2004;126(3):705–15. doi: 10.1016/j.neuroscience.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22(16):7308–20. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranaldi R, Beninger RJ. Rostral-caudal differences in effects of nucleus accumbens amphetamine on VTA ICSS. Brain Res. 1994;642(1–2):251–8. doi: 10.1016/0006-8993(94)90929-6. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann P, Privou C, Huston JP. Differential sensitivity of the caudal and rostral nucleus accumbens to the rewarding effects of a H1-histaminergic receptor blocker as measured with place-preference and self-stimulation behavior. Neuroscience. 1999;94(1):93–103. doi: 10.1016/s0306-4522(99)00309-7. [DOI] [PubMed] [Google Scholar]
- 35.Essman WD, McGonigle P, Lucki I. Anatomical differentiation within the nucleus accumbens of the locomotor stimulatory actions of selective dopamine agonists and d-amphetamine. Psychopharmacology (Berl) 1993;112(2–3):233–41. doi: 10.1007/BF02244916. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Laboratory Animal Research, C.o.L.S., National Research Council, . Guide for the Care and Use of Laboratory Animals. NATIONAL ACADEMY PRESS; 1996. [Google Scholar]
- 37.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60(3):253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 38.Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183(3):331–40. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- 39.Neumaier JF, et al. Serotonergic lesioning differentially affects presynaptic and postsynaptic 5-HT1B receptor mRNA levels in rat brain. Brain Res. 1996;722(1–2):50–8. doi: 10.1016/0006-8993(96)00178-3. [DOI] [PubMed] [Google Scholar]
- 40.Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498(5):611–23. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- 41.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–35. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich-Lai YM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148(4):1823–34. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang SS, et al. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci Bull. 2010;26(4):297–303. doi: 10.1007/s12264-010-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin KJ, et al. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38(2):73–9. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Nikulina EM, et al. Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur J Neurosci. 2008;27(9):2272–84. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vialou V, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13(6):745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Choi DC, et al. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149(2):818–26. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi DC, et al. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33(5):659–69. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon MB, et al. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2010;22(1):13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- 52.Ulrich-Lai YM, et al. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291(5):E965–73. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 53.Ghitza UE, et al. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminative-stimulus task. J Neurophysiol. 2004;92(3):1608–14. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- 54.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7(4):389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, et al. The nucleus accumbens core has a more important role in resisting reactivation of extinguished conditioned place preference in morphine-addicted rats. J Int Med Res. 2008;36(4):673–81. doi: 10.1177/147323000803600408. [DOI] [PubMed] [Google Scholar]
- 56.Cassaday HJ, Horsley RR, Norman C. Electrolytic lesions to nucleus accumbens core and shell have dissociable effects on conditioning to discrete and contextual cues in aversive and appetitive procedures respectively. Behav Brain Res. 2005;160(2):222–35. doi: 10.1016/j.bbr.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994;59(3):609–23. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 58.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797(1):73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 59.Haber SN, et al. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293(2):282–98. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- 60.Nauta WJ, et al. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3(4–5):385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 61.Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682(1–2):189–96. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- 62.Voorn P, et al. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–74. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paxinos GWC. The Rat Brain In Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.