Abstract
Purpose/Objectives
Abdominal and pelvic radiotherapy is limited by the radiosensitivity of the small and large intestine. PHY906, a state-of-the-art adaptation of a traditional Chinese medicine, decreased intestinal injury from chemotherapy in preclinical studies and is in clinical trials with chemotherapy. This project assessed whether PHY906 would also reduce intestinal injury from whole-abdomen irradiation in mice.
Materials/Methods
BALB/c mice received whole-abdomen irradiation (2 Gy/day) ± PHY906 by oral gavage twice daily for 4 days. Intestinal injury was assayed by physiological observations and histological studies. Effects of PHY906 on tumor radiation response were assayed in tumor growth studies.
Results
PHY906 decreased the toxicity of fractionated abdominal irradiation. Radiation alone produced marked blunting and loss of villi, crypt loss, crypt hyperplasia and irregular crypt morphology, which were reduced by PHY906. The radiation-induced reduction in viable crypt counts was also mitigated by PHY906. PHY906 did not alter radiation-induced weight loss, but resulted in more rapid recovery. PHY906 did not alter growth, local invasion or metastatic spread of EMT6 mouse mammary tumors or protect tumors from growth delays produced by single-dose and fractionated irradiation.
Conclusion
In this mouse model system, PHY906 decreased the toxicity of abdominal irradiation, without protecting tumors, thereby increasing the therapeutic ratio.
Introduction
Several relatively common malignancies are treated with radiotherapy to the pelvis or abdomen, including carcinomas of the cervix, colon, rectum, prostate and pancreas. Over 440,000 people in the USA will be diagnosed with one of these cancers in 2012 (American Cancer Society, 2012). Radiation therapy is of value both in the curative treatment of these newly diagnosed malignancies and in the palliation of symptoms associated with advanced recurrent or residual disease; the inherent radiosensitivity of the small and large intestine is a limiting factor in these treatments (Hauer-Jensen et al. 2003, Benson et al. 2004, Sonis et al. 2004, Andreyev 2005, Randall et al., 2006, Baglan et al. 2002). Symptoms of early radiation injury to the intestines range from mild diarrhea and cramping at low doses to severe diarrhea that can produce life-threatening electrolyte imbalances, dehydration, renal insufficiency, severe pain and sepsis at high doses. Therapies that protect against early radiation-induced injuries or ameliorate their symptoms would be of great value, not only because they would improve quality of life for patients during radiotherapy, but also because gastrointestinal side effects can lead to treatment interruptions, which lengthen overall treatment time and may reduce the efficacy of therapy. Moreover, patients with severe side effects may refuse to continue treatment, with fatal consequences.
Pelvic and abdominal irradiation also produce late injuries, which develop months to years after irradiation. These include chronic inflammatory and fibrotic changes, chronic diarrhea, rectal bleeding, malabsorption, ulcers, fistulae, adhesions and obstructions, which can range from mild to life threatening and can be very difficult to manage (Hauer-Jensen et al. 2003, Benson et al. 2004, Sonis et al. 2004, Andreyev 2005, Randall et al. 2006, Abayomi et al. 2009). The risk of serious late gastrointestinal (GI) side effects, which increases with the radiation dose and the volume of bowel irradiated, limit many radiotherapy regimens. While it was once thought that late injuries reflected damage to the vascular bed and stromal elements, it is now thought that these injuries can also reflect late sequellae of the early parenchymal injuries (Trott and Hermann 1991, Booth and Potten 2001, Denham et al. 2001, Potten 2004, Wang et al. 2007, Wedlake et al. 2009). Interventions that reduce early injuries therefore may decrease late toxicities as well (Dent et al. 2003, McBride et al. 2004, Yeoh et al. 2007). The 5-year overall survival rates for cancer patients have been increasing progressively for several decades; 68% of all cancer patients now survive at least 5 years after diagnosis (American Cancer Society, 2012). As long-term survivals improve, more patients are at risk of developing late toxicities. The high and improving 5-year survival rates and cure rates for cancers treated with pelvic radiotherapy highlight the need to identify agents that ameliorate the severity and incidence of chronic gastrointestinal sequellae associated with this treatment.
The studies reported here examine a new approach to reducing intestinal injuries from radiation therapy, by using PHY906, a novel formulated Chinese medicine prepared from four herbs (Scutellariae baicalensis, Paeonia lactiflora, Ziziphus jujuba and Glycyrrhiza uralensis with a relative weight ratio of 3:2:2:2) (Ng et al. 2007, Ye et al. 2007, Tilton et al. 2010, Lam et al. 2010, Farrell and Kummar 2003, Yen et al. 2009, Saif et al. 2010, Zhang et al. 2010). PHY906 was developed on the basis of a traditional Chinese medicine formula, Huang-Qin-Tang, which has been used for over 1800 years for treatment of a variety of gastrointestinal ailments, including diarrhea, vomiting, nausea, stomach cramps and fever that have been induced by infectious agents or by ingested and environmental toxins. Because the symptoms treated with PHY906 are similar to those induced by cancer chemotherapy, PHY906 recently has been evaluated extensively in laboratory and clinical studies as a possible approach to ameliorating the gastrointestinal side effects of chemotherapy (Farrell and Kummar 2003, Saif et al. 2010, Wang et al. 2011, Eng et al. 2011, Kummar et al. 2011).
A unique aspect of the development of PHY906 has been the attention given to characterizing and standardizing this complex natural product and to developing quality control procedures to ensure the safety and batch-to-batch consistency of the product (Ye et al. 2007, Tilton et al. 2010, Zhang et al. 2010, Kummar et al. 2011). These include extensive liquid chromatography/mass spectrometry (LC/MS) analyses to identify the chemical species present in PHY906 as well as to develop LC/MS profiles that allow comparison of different batches of the formulation. These chemical fingerprints are combined with standardized biological tests, including individual target bioassays and comprehensive genomic bioresponse profiling, to ensure the consistency of the product.
In studies of PHY906 as a potential adjunct to chemotherapy, PHY906 was given to mice bearing various transplanted and xenografted tumors during the period of administration of capecitabine, irinotecan, etoposide, and β-l-Dioxolane-cytidine (Lam et al. 2010, Kummar et al. 2011). Gastrointestinal toxicities, assessed by measuring transient drug-induced weight loss and by histologic analyses of tissue specimens, were decreased significantly by treatment with PHY906. In the case of irinotecan, for which severe delayed-onset diarrhea is the major dose-limiting toxicity, PHY906 also reduced drug-induced mortality in mice. Importantly, PHY906 did not reduce the efficacy of the drugs in inhibiting tumor growth, but instead increased drug-induced tumor growth delays (Lam et al. 2010, Kummar et al. 2011).
Past studies with irinotecan have provided insights into the mechanisms by which PHY906 exerts its effects (Lam et al. 2010, Kummar et al. 2011, Wang et al.2011). In histological studies, irenotecan caused destruction of the jejunum mucosal architecture; PHY906 did not alter the changes induced by irenotecan 2 days after drug treatment. However, the injury in mice receiving drug alone became more severe on day 4 in mice treated with irenotecan alone, while the group receiving PHY906 plus irenotecan had regained a normal jejunal architecture at this time. This suggests that PHY906 did not alter production of the initial injury, but instead promoted recovery from the injury. In support of this concept, PHY906 did not prevent the initial DNA damage induced by this drug as assayed by TUNEL staining. Instead, PHY906 accelerated the disappearance of TUNEL-positive cells, decreased the number of apoptotic cells (positive for cleaved caspase-3), and increased cell proliferation in mice treated with irenotecan. The mechanism underlying the effects of PHY906 on recovery from irenotecan-induced gastrointestinal injury is thought to involve potentiation of the Wnt/b-catenin signalling pathway and inhibition of cytokine cascades, possibly mediated by downregulation of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), iNOS (inducible nitric oxide synthase) and cox-2 (cyclooxygenase 2) activity (Lam et al. 2011). The mechanism by which PHY906 increased the tumor growth delays induced by irenotecan in colon 38 tumors was also examined in detail (Wang et al. 2011) and is thought to reflect a change in the microenvironment within irenotecan-treated tumors by PHY906, which induces pro-inflammatory and pro-apoptotic pathways that may favor tumor rejection. The molecular mechanisms underlying this appear to include upregulation of IRF-5 (Interferon regulatory factor 5), which enhances inflammation, and suppression of IRF-1(Interferon regulatory factor 1), which blocks anti-apoptotic pathways activated in the tumor cells (Wang et al. 2011).
The fact that PHY906 increased the therapeutic ratio for treatment with several clinically-used anticancer drugs in several preclinical model systems supported the development of clinical trials testing PHY906 as an adjunct to chemotherapy. Initial clinical trials (Farrell and Kummar 2003, Saif et al. 2010, Eng 2010, Kummar et al. 2011) have shown the safety and tolerability of PHY906 as an adjuvant to irinotecan and capecitabine based chemotherapy, and provided the basis for ongoing multicenter trials examining the efficacy of PHY906 in reducing the toxicities of these chemotherapy regimens. These studies provided the basis for the studies reported here, which began to assess whether PHY906 could also ameliorate the toxicities of whole abdomen irradiation in mice, and therefore might have value as an adjunct to clinical radiotherapy regimens that irradiate portions of the intestine to doses producing significant injury.
Materials and Methods
Animals, cells and tumors
All in vivo experiments were performed using BALB/c Rw mice 2.5–3.5 months of age that were bred and raised in our production colony. Mice in the production and experimental colonies were housed under barrier conditions in “full-service” individually ventilated microisolator caging, with autoclaved cages, food, water and bedding, and serviced in a clean-air hood as described previously (Duan et al. 2012). These colonies are managed by the Yale Animal Resources Center, which delivers all husbandry services and selects, purchases and provides all equipment and supplies. These specific-pathogen-free mice are screened routinely for microbiology status by the Veterinary Preventive Medicine Group of the Section of Comparative Medicine at Yale as described in detail previously (Duan et al. 2012) and care was taken to ensure the microbiological status of the mice throughout these studies reported here. Each mouse was used in only one experiment and all mice were ear-tagged to allow monitoring of individual animals. Tumor measurements, weight monitoring, and other experimental manipulations were performed in a biological safety cabinet within the animal room and irradiations were performed using an irradiator located within this barrier facility. Protocols used with experimental animals were reviewed and approved by the Yale Institutional Animal Care and Use Committee and experiments were performed in compliance with the policies of the government, Yale University and the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and with the principles outlined in the Guide for the Care and Use of Laboratory Animals.
Cell culture experiments were performed using EMT6 mouse mammary tumor cells, which were maintained as monolayer cultures in Waymouth’s medium (Invitrogen, Carlsbad CA, USA) supplemented with 15% serum (Gemini Bioproducts, West Sacramento CA, USA) and antibiotics (Invitrogen, Carlsbad CA, USA) at 37°C in an atmosphere of 95% air / 5% CO2. EMT6 cells have been used extensively in our past studies and their characteristics are well defined (Rockwell 1977, Rockwell et al. 2012, Duan et al. 2012). Cell cultures for experiments were plated in standard 60 mm cell culture dishes using the media described above and treated in mid exponential growth under aerobic conditions. Cultures were treated with PHY906 and/or radiation as described below and the monolayers were then washed and trypsinized to form single cell suspensions, which were assayed for cell survival using a colony formation assay, as described previously (Rockwell 1977, Rockwell 2010, Rockwell et al. 2012). Surviving fractions for treated cultures were calculated relative to the plating efficiencies of untreated control cultures plated in the same experiments.
Tumors were produced by inoculating 2 × 105 EMT6 cells, harvested from exponentially growing cell cultures, intradermally into the flank as described previously (Rockwell 1977, Rockwell et al. 2012, Duan et al. 2012). The three diameters of each tumor were measured three times per week with vernier calipers from the time the tumors became measurable, and tumor volumes were calculated using the formula for the volume of a hemiellipsoid (Rockwell et al. 2012, Duan et al. 2012). When the tumors reached an average volume of 100 mm3, they were stratified by volume into control and treatment groups, which were treated with radiation and/or PHY906 as described below. Tumor volumes were measured until each tumor reached a volume of 1000 mm3 and the times needed for control and treated tumors to grow from the initial treatment volumes to four times the treatment volumes were calculated and compared (Begg 1987, Moulder and Rockwell 1987, Rockwell et al. 2012, Duan et al. 2012). At the time of euthanasia, mice were necropsied and the lungs (the most common site for metastases in this tumor line) were removed, rinsed in saline and fixed in Bouin solution (Ricca Chemical Co., Arlington, TX) and the numbers of metastases on the surfaces of the lungs were counted under a dissecting microscope as described previously (Rockwell et al. 2012, Duan et al. 2012).
PHY906
PHY906 was provided for these studies without cost as a sterile powder by PhytoCeutica (New Haven CT); the processes used in the production and fingerprinting of PHY906 are described in detail elsewhere (Tilton et al. 2010). PHY906 was reconstituted in our laboratory in sterile distilled water on the day of use. A dose of 500 mg/kg/fraction was administered by oral gavage twice daily, at approximately 8:30 AM and 4 PM during the 4 days of irradiation. The AM dose was given 30 min before irradiation. In some experiments, mice were given two 4-day courses of PHY906 separated by a 3-day rest period. These regimens model those used in clinical trials with PHY906 and used traditionally with Huang-Qin-Tang. In preliminary experiments, mice were sham-treated with an analogous regimen using sterile water and in some cases sham irradiated to test for any effects of the experimental manipulations on the mice and tumors; none were observed. For cell culture studies, PHY906 was prepared in the same manner as for animals, and the sterile solution was added to the cell culture medium.
Irradiation
Cell cultures were irradiated with 320 kV X-rays produced by an XRAD (Precision X-ray, Branford CT, US) at 12.5 mA, 2 mm Al filtration, and a dose rate of 2.6 Gy/min. Mice in experiments examining the effects of whole-abdomen irradiation were anesthetized with ketamine/xylazine and positioned on their backs with the thorax, head and long bones shielded with lead, and the whole abdomen was irradiated with 250 kV X-rays produced by a Siemens Stabilipan (Malvern, PA, US) at 15 mA, 2 mm Al filtration and a dose rate of 1.1 Gy/minute. Two mice (generally one treated with radiation alone and one treated with PHY906 plus radiation) were irradiated simultaneously. Radiation was given between 9 AM and 10 AM to control for the circadian rhythms in the proliferation and radiosensitivity of the intestine. Mice received 4 daily fractions of 2 Gy/fraction. Sham-irradiated mice were handled analogously, but were not exposed to radiation. In tumor growth studies, mice with 100 mm3 tumors that had been implanted as described above were anesthetized with ketamine/xylazine and positioned with the body shielded. The tumors were then irradiated locally with 250 kV X-rays produced by a Siemens Stabilipan (Malvern, PA, US) at 15 mA, 2 mm Al filtration and a dose rate of 6.4 Gy/min. Tumors received a single dose of 10 Gy or 4 daily fractions of 2.5 Gy/fraction. Because the X-ray doses received by the intestines, bone marrow and other critical normal tissues of these mice were less than 5% of the tumor dose, these mice did not exhibit significant systemic injuries from the radiation. Sham-irradiated mice were treated analogously, but not exposed to radiation.
Studies of whole-abdomen irradiation
Mice in studies examining the effects of PHY906 on mice receiving whole-abdomen irradiation were randomized into four groups: control mice, mice treated with fractionated whole-abdomen irradiation (4 × 2 Gy), mice treated with PHY906, and mice treated with radiation plus PHY906. In studies of weight loss and symptoms, mice were weighed and examined 3 times per week and their overall physical status was scored using a standard rodent condition scale that rates general body appearance and condition, fur (including grooming), mucous membrane coloration, posture, activity, response to handling, neuro/musculoskeletal function, evidence of dehydration, and body temperature. In addition, the anal region was checked for fecal or blood staining and fecal output and appearance was monitored for any evidence of diarrhea or intestinal bleeding.
For histologic analyses, mice in each treatment group were selected at random to be assayed at various times from 2 days to 2 weeks after the first treatment and encoded to blind subsequent observers. Mice were euthanized, necropsied, and the entire small intestine was immersion-fixed in Bouin solution (Ricca Chemical Co., Arlington, TX) overnight at room temperature. Six random 3-4 mm sagittal “donut” sections of jejunum (duodenum and ileum excluded) were collected for crypt counts. The tissues were processed, embedded in paraffin, sectioned at 5 microns, and stained with hematoxylin and eosin (HE) by routine methods. Digital light microscopic images were acquired using a Zeiss Axio Imager A1 microscope, an AxioCam MRc Camera, and cAxioVision 4.7.1 imaging software (Carl Zeiss MicroImaging, Inc., Thornwood, NY). The resulting images were optimized using Adobe Photoshop CS (San Jose, CA). The total number of crypts for each section was counted (Withers and Elkind 1970) and the histopathological features of the intestines were characterized by a veterinarian with expertise in mouse pathology, who was blinded to the experimental manipulations.
Statistical analyses
Data are shown as means ± SEM; geometric means are used where appropriate. The significance of differences between groups was assessed by Mann Whitney U tests and curves were compared by ANOVA. Significance was defined as p < 0.05.
Results
Cell culture studies
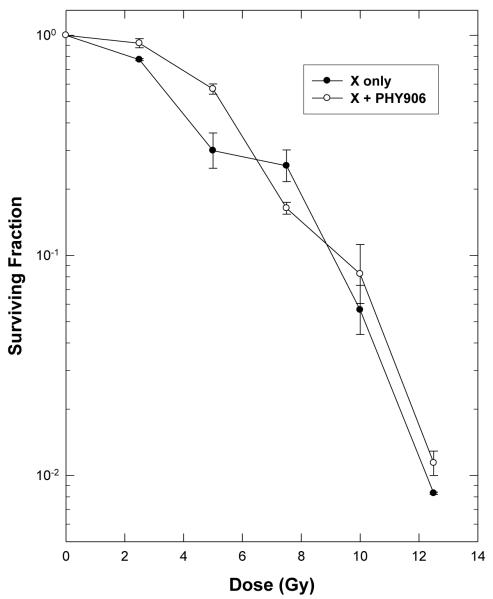
Initial cell culture studies were performed to test for any direct cytotoxic or radiosensitizing effects of PHY906 on tumor cells. Sterile solutions of PHY906 were added to the medium overlying exponentially-growing EMT6 monolayers and cell survival was assayed 24 hr later, using a clonogenic assay. Minimal cytotoxicities were observed only at PHY906 doses above the tissue concentrations expected in vivo. Treatment for 24 h with 2 mg/ml PHY906 reduced the surviving fraction of EMT6 cells in vitro to 0.45 ± 0.04. The same concentration of PHY906 that had been irradiated with 12.5 Gy reduced the surviving fraction to 0.59 ± 0.05, showing that irradiation of PHY906 did not produce cytotoxic products. To ascertain whether PHY906 had direct radiosensitizing or radioprotective activity, 2 mg/ml PHY906 was added to the culture medium 4 h before cells were irradiated with graded doses of X-rays; cultures were incubated for an additional 20 hr before being suspended, counted and plated to assay cell viability. This 24 hr exposure to high doses of PHY906 before, during and after irradiation did not alter the cellular radiosensitivity (Figure 1). This lack of effect of PHY906 on the radiation dose-response curve indicates that the components of this herbal formulation did not act as radiosensitizers or radioprotectors and did not alter cellular radiosensitivity by altering the post-irradiation repair of radiation damage. This finding suggests that any effects on the radiation responses during the in vivo studies would reflect tissue-level effects of PHY906, rather than a direct effect of PHY906 on cellular radiation damage and repair.
Figure 1.
PHY906 does not alter the radiosensitivity of EMT6 tumor cells in cell culture. Survival curves for EMT6 mouse mammary tumor cells in exponential growth in cell culture treated with radiation alone or in combination with 2 mg/ml of PHY906 present for 4 h before, during and 20 h after irradiation. The surviving fractions for cells treated with PHY906 plus radiation was normalized to the survivals for cells with PHY906 alone. Points are means ± SEM of data from 3 independent experiments.
Effects of PHY906 on mice receiving whole-abdomen irradiation
The effect of PHY906 on the response of mice to abdominal irradiation was examined using mice irradiated with 4 daily 2 Gy fractions of X-rays. This radiation regimen produced changes in the behavior, appearance and weight of the mice. Irradiated mice were somewhat more lethargic than controls and showed less response to handling; they also groomed less vigorously and effectively than control mice. Activity, behavior and appearance normalized more rapidly in the irradiated, PHY906-treated mice than in mice receiving radiation alone. Unirradiated mice treated with PHY906 were indistinguishable from untreated control mice in weight, behavior and appearance throughout the duration of the experiments. Although diarrhea is a common side effect of abdominal irradiation in human patients and in rodents irradiated at higher doses, no evidence of diarrhea (e.g. peri-anal staining, changes in the number or appearance of stools) was observed in the irradiated mice during the studies reported here.
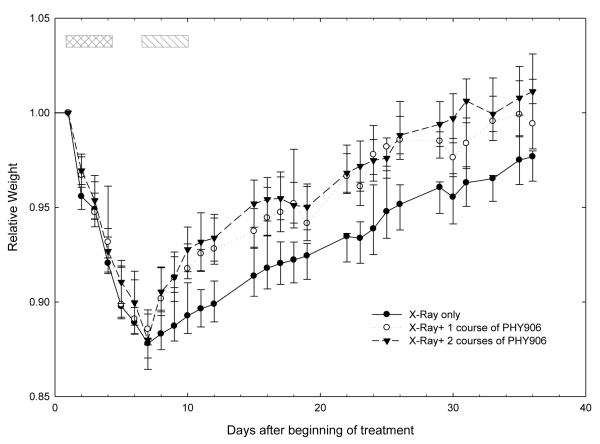
Irradiation produced a steady weight loss beginning 1 day after the first irradiation, continuing throughout and after the 4-day irradiation period and reaching a nadir at 88% of baseline weight 8 days after the first radiation fraction (Figure 2); this nadir was followed by a steady increase in weight over the remainder of the 5-week observation period. Two regimens of treatment with PHY906 were tested in the studies shown on Figure 2: a short regimen in which mice received PHY906 twice daily for 4 days, at ~8:30 AM (30 minutes before irradiation) and again at ~4:00 PM, and also a longer regimen in which mice received two 4-day series of PHY906 treatments, on this same schedule, separated by a 3-day rest period. These regimens were used to model traditional and current PHY906 treatment regimens in patients, which use this compound in regimens alternating 4-day treatment periods with 3-day rest periods (Kummar et al. 2011). Treatment with PHY906 did not have a statistically significant effect during the period of the initial weight loss, but did have a significant effect on recovery, resulting in a more rapid return to the initial weight. The pattern of recovery was similar in mice receiving 1 and 2 series of PHY906 treatments.
Figure 2.
Effect of PHY906 on the weight of mice receiving fractionated whole-abdomen irradiation. Mice were randomized to receive whole abdomen irradiation (4 daily 2 Gy fractions, days 1-4) alone or in combination with PHY906 treatment (~8:30 AM and ~4PM daily by oral gavage; 500 mg/kg/dose) for 4 days. Mice received one 4-day course of PHY906 or two 4-day courses of PHY906 separated by a 3-day interval. Crosshatched box: radiation and PHY906. Single hatched box: second course of PHY906. The weight for each mouse was normalized to the pretreatment weight of that animal. Data are means ± SEM from three replicate experiments, each with 6 mice per group.
Histologic findings
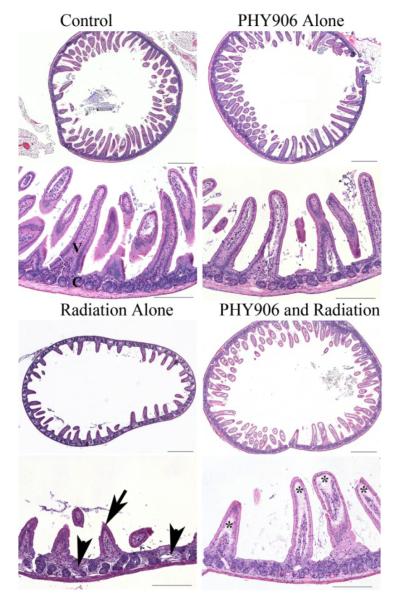
Untreated control mice and mice treated with the 4-day radiation and/or PHY906 regimens described above were euthanized at various times during and after the treatment regimens, necropsied and tissue samples were taken for histopathologic examination by a blinded investigator. Representative hemotoxylin and eosin (HE) sections of the mid jejunum from control mice, mice treated with PHY906 alone, radiation alone, and radiation plus PHY906 1 day and 2 weeks after the last irradiation are shown at low and high power magnification in Figures 3 and 4, respectively. The overall morphology of villi and crypts in control and unirradiated, PHY906-treated mice were similar, with numerous long villus tips (V) and small regular crypts (C) within the lamina propria of the intestinal mucosa. Mice receiving radiation alone had marked blunting and loss of villi (arrow), crypt loss, and crypt hyperplasia with irregular crypt morphology (arrowheads). The intestines of mice receiving PHY906 along with irradiation were morphologically very different from those of mice receiving radiation alone. Sections from these mice were more similar in crypt number and morphology to control mice and mice treated with PHY906 alone than they were to the irradiated mice, although some sections showed minor loss of villi and edema within the villus lamina.
Figure 3.
Histopathology reveals decreased intestinal damage from radiation in mice treated with PHY906. Representative HE-stained sections of the jejunum, collected 1 day after the final irradiation from control mice, mice treated with PHY906 alone, radiation alone, and radiation plus PHY906. The overall morphology of villi and crypts in control and PHY906 treated mice were similar, with numerous long villus tips (V) and small regular crypts (C) within the lamina propria of the intestinal mucosa. Mice receiving radiation alone had marked blunting and loss of villi (arrow), crypt loss, and crypt hyperplasia with irregular crypt morphology (arrow heads). Overall, mice receiving radiation and PHY906 were histologically similar to control mice and mice treated with PHY906 alone in crypt number and morphology. However, there were sections with minor loss of villi and with edema (*) within the villus lamina. Upper panels: scale bar = 500 microns, lower panels: scale bar = 200 microns.
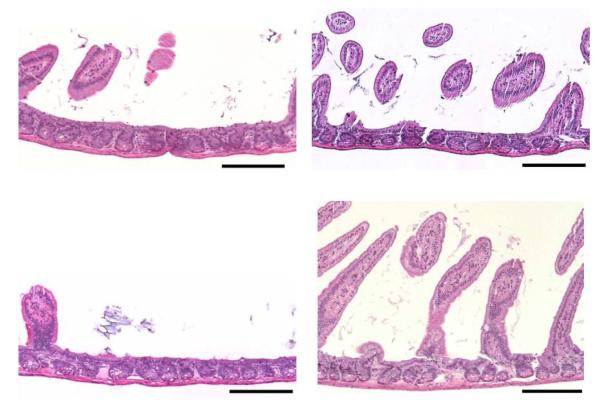
Figure 4.
Representative HE sections of small intestine from mice receiving radiation alone (left panels), or radiation plus PHY906 (right panels) 2 weeks earlier. At this time, there was still mild to marked villus blunting and villus loss in irradiated mice, which was variable in different sections. Crypt numbers also remained lower than normal. In mice treated with PHY906 along with radiation, there was preservation of villus length and distribution, as well as preservation of crypt numbers. Scale Bars = 200 microns.
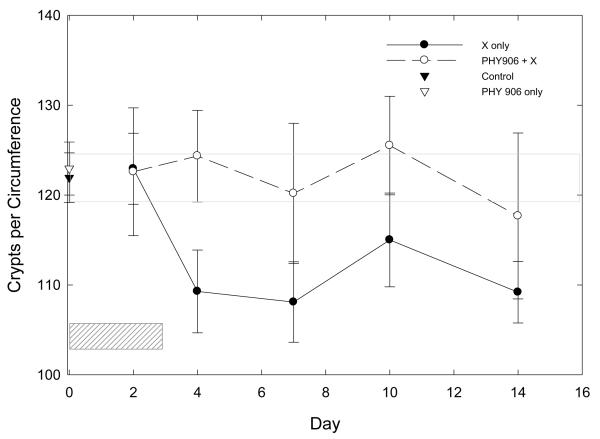
Quantitative counts of viable crypts/circumference, also made by an observer blinded to the treatment received by the animal, confirmed these qualitative observations (Figure 5). The mean number of crypts/circumference was reduced in irradiated mice, beginning the day after the last irradiation (Day 4), and remained below that in control mice throughout the 2-week observation period. Crypt numbers for untreated control mice and for mice treated with PHY906 alone were similar. The crypt counts in mice treated with PHY906 during irradiation were above those of mice receiving radiation alone, and these counts did not differ significantly from those in unirradiated control mice.
Figure 5.
Number of Crypts per circumference in the mid jejunum of mice given fractionated whole abdomen irradiation (4 × 2 Gy) alone or in combination with PHY906 (500 mg/kg, twice daily). Hatched box shows days of treatment. Open gray box shows SEM of crypt counts in control mice. Points are means ± SEM.
Effect of PHY906 on tumor growth
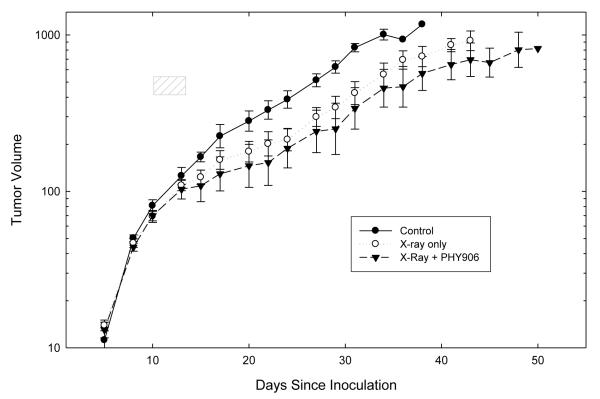
To be useful as an adjunct to radiotherapy, an agent that mitigates the side effects of radiation must produce its mitigating effect without stimulating tumor growth and without decreasing the antineoplastic effects of radiation. We therefore examined the effects of PHY906 on the growth of EMT6 tumors and on the growth of tumors receiving concomitant treatment with either a single large dose of radiation (10 Gy given 30 minutes after the first dose of PHY906) or 4 daily 2.5 Gy fractions (given on the same days as PHY906). Table I summarizes the results of four experiments, showing the mean times needed for treated and control tumors to grow from the treatment volume of ~100 mm3 to 4 times the treatment volume. Figure 6 shows more detailed tumor growth data from a representative experiment. PHY906 alone did not produce significant changes in the growth of the unirradiated tumors (Table I). As expected, irradiation with single or multiple doses of X-rays inhibited tumor growth. Treatment with PHY906 did not alter the growth of irradiated tumors significantly.
Table I.
Effect of PHY906 on tumor growth delays induced by irradiation in four independent experiments. Tumors were inoculated, randomized and treated with irradiation alone or in combination with PH906. The times for the tumors in each group to grow from the volume at the time of randomization to 4 times that volume are shown, with their SEM
| Radiation Regimen |
Time to 4X | ||||
|---|---|---|---|---|---|
| Control | Sham | PHY906 only | X-ray Only | PHY906 + X-ray | |
| 10 Gy | 22.4 ± 1.1 | 21.8 ± 0.9 | 21.0 ± 1.4 | 27.4 ± 1.1* | 29.0 ± 1.2* |
| 10 Gy | 13.5 ± 1.4 | 11.3 ± 0.6 | 13.6 ± 1.3 | 21.0 ± 1.1* | 20.8 ± 0.9* |
| 4 X 2.5 Gy | 3.8 ± 0.8 | 18.3 ± 1.4* | 19.4 ± 1.3* | ||
| 4 X 2.5 Gy | 15.6 ± 1.4 | 18.4 ± 1.0 | 20.0 ± 0.7* | ||
Significantly different from control (p <0.05). PHY906 did not produce statistically significant changes in the time to 4X for either unirradiated or irradiated tumors (p.>0.05). The tumor growth curves for a representative experiment (row 3) are shown on Figure 6; tumor growth curves for other experiments are not show.
Figure 6.
Effect of PHY906 on the tumor growth delay induced by fractionated irradiation. Tumors were stratified by volume on day 10 into groups (6 mice/group) which were randomly assigned to receive no treatment, radiation alone, or radiation + PHY906. PHY906 was given twice daily by oral gavage at a dose of 500 mg/kg/treatment. Tumors were irradiated locally with 4 daily fractions of 2.5 Gy per fraction, given 30 min after the morning PHY906 treatment. Points are means ± SEM. This graph shows tumor growth curves from a representative experiment. Analyses of the growth delays from this experiment are shown in row 3 of the Table. Similar results were found in a replicate experiment using this same regimen, and also in two experiments examining tumor growth after a single dose of 10 Gy. Growth delay data from all 4 tumor growth experiments are summarized on the Table.
The behavior and weight of the mice in these 4 tumor growth experiments was monitored throughout tumor growth; no differences between the PHY906-treated and non-PHY906-treated mice were observed. In addition, when each tumor reached a volume of 1000 mm3 the mouse was euthanized and necropsied; no differences in local invasion or lymphatic metastases were seen in PHY906-treated and non-treated groups. At necropsy, the lungs were fixed and the metastases on the lungs were counted. As anticipated, mice receiving localized tumor irradiation had more visible lung metastases than unirradiated mice, because the longer survival times of mice with irradiated tumors allowed more time for metastases to grow to visible sizes. PHY906 treatment did not alter numbers of metastases significantly in either unirradiated mice or mice receiving tumor irradiation (data not shown).
Overall, PHY906 did not alter the growth, local invasion, or metastasis of unirradiated EMT6 tumors and did not protect these tumors from the antineoplastic effects of radiation. Treatment with PHY906 therefore mitigated the toxic intestinal effects of radiation without altering the growth or radiation responses of the tumors, thereby increasing the therapeutic ratio and producing therapeutic gain.
Discussion
The studies presented here reflect a radical new paradigm for the use of Traditional Chinese Medicine, developed in the Cheng laboratory at Yale, which combines principles and agents from Traditional Chinese Medicine with state-of-the-art Western Medicine (Tilton et al. 2010, Lam et al. 2011). A unique aspect of the development of PHY906 has been the attention given to characterizing and standardizing this complex natural product and to developing quality control procedures to ensure the safety and batch-to-batch consistency of the product (Ye et al. 2007, Ng et al. 2007, Tilton et al. 2010, Zhang et al. 2011). These include extensive liquid chromatography/ mass spectrometry analyses to identify the chemical species present in PHY906 and to allow comparison of different batches. These chemical fingerprints are combined with standardized biological tests, including individual target bioassays and comprehensive genomic bioresponse profiling, to ensure the consistency of the biological activities of PHY906. These procedures were developed at PhytoCeutica, a biotechnology company that was founded by members of the Pharmacology Department at Yale to foster the development of Traditional Chinese Medicines into FDA-approved prescription drugs for use in the treatment of cancer.
The availability of PHY906, a well characterized, standardized state-of-the-art version of a traditional Chinese herbal medicine (Huang-Qin-Tang) provided a unique resource for this and other preclinical projects, as well as for clinical trials. Studies with herbal medicines and other complementary and alternative medicines (CAM) are generally complicated and compromised by inconsistency in the available products (Tilton et al. 2010, Wong et al. 2001, Okada 1996). Many herbal products are essentially undefined. The components present in any given herb will vary with the exact strain of the plant, and with the location and conditions of cultivation, the age of the plant, the season of harvest, the portion of the plant harvested, and other factors related to the growth of the herbs. Variations in handling of the herbs during and after harvesting and in the methods used to prepare and store the final preparation result in additional variability (Hsu et al. 1980, Tang and Eisenbrand 1992, Chinese Botany). As a result, the source-to-source and batch-to-batch variability in herbal preparations makes rigorous evaluation of their effects in experimental models or in human subjects problematic. PHY906 therefore represents an innovative and novel reagent: a “traditional herbal medicine” that is prepared from a defined combination of 4 herbs through a standardized procedure that is monitored from selection of crops to preparation of the final product, followed by characterization of that product using modern chemical and biological fingerprinting techniques to ensure a uniform and well-defined therapeutic agent.
This paper presents the first report examining PHY906 in combination with radiation. To the best of our knowledge, the effect of the traditional version of this medicine, Huang-Qin-Tang, on radiation injury has not been examined previously. The studies reported here showed that treatment with PHY906 mitigated the intestinal injuries of fractionated whole-abdomen irradiation as assessed by qualitative and quantitative histologic analyses and improved recovery from radiation injury as assessed by recovery of body weight and observations of animal condition. In contrast, PHY906 did not alter the response of EMT6 tumors to irradiation with a single dose of 10 Gy or fractionated irradiation with 4 daily 2.5 Gy fractions. The studies reported here did not examine the potential mechanisms underlying the effects of PHY906. Our data are compatible with the conclusions drawn from the experiments in the Cheng laboratory, described above, which are elucidating the mechanisms by which PHY906 altered GI injury and tumor growth after treatment with anticancer drugs (Lam et al. 2010, Kummar et al. 2011, Wang et al. 2011). Those data showed that PHY906 did not alter the initial injury from the drug. Our data confirm this finding for radiation: the in vitro tumor cell survival curves (Figure 1) showed no effect of concomitant treatment with PHY906 on radiation cytotoxicity and our studies of weight changes in mice receiving whole-abdomen irradiation (Figure 2) showed no effect of PHY906 on the initial weight loss in irradiated mice. In studies with anticancer drugs, PHY906 appeared to improve the recovery from radiation-induced GI injury. Our studies suggest that this is also true for radiation. PHY906 treatment resulted in a more rapid recovery from radiation-induced weight loss, and a more rapid return to normal behavior and appearance in the irradiated mice. It therefore seems probable that the mechanisms by which PHY906 ameliorates radiation-induced gastrointestinal injury are similar to those by which it ameliorates injury from chemotherapy. PHY906 increased the effects of some chemotherapeutic agents on some tumors (Lam et al. 2010, Kummar et al. 2011, Wang et al. 2011). Our experiments did not show a statistically significant effect of PHY906 on the radiation-induced growth delays in EMT6 tumors, although as shown on Table 1 and Figure 6 data from 3 of our 4 experiments were compatible with increased effects. Our data are therefore compatible with the concept that PHY906 could increase the response of tumors to radiation. Because the mechanisms by which PHY906 increases the response of colon 38 tumors to irenotecan (summarized above and detailed in Wang et al. 2011) appears to involve modulation of drug-induced changes in innate host immune responses and inflammation, and modulation of apoptotic pathways in the tumor cells, these effects would be expected to vary with the treatment agent, the hosts, and the tumor characteristics. Further work would be needed to assess whether PHY906 could improve tumor radiation responses with other radiation regimens in EMT6 tumors or would produce greater effects in tumor systems where apoptosis was a more prevalent mode of tumor cell death after irradiation.
This paper presents the first report examining PHY906, or the traditional version of this herbal medicine, Huang-Qin-Tang, in combination with radiation. More generally, rigorous scientific evaluations of well defined CAM in combination with radiation and with other conventional cancer therapies are still rare and novel, despite the very high rate of use of CAM by cancer patients during and after treatment. A recent survey of cancer patients in our department showed that 72% of our patients used complementary programs such as visualization or Reiki and 66% used oral or topical CAM during their course of radiation therapy (Moran et al. 2012). The effects (beneficial or harmful) of most of the ingested agents used by the radiotherapy patients on the efficacy and toxicities of cancer therapy are unknown. The studies reported here were part of an ongoing effort in our group designed to use cell culture and animal models to examine the effects of specific, well defined CAM and nutritional supplements in combination with radiotherapy and chemotherapy (Redlich et al. 1998, Rockwell et al. 2005; Rockwell et al. 2011) and to assess their potential for producing benefit or harm in this setting. If our laboratory studies suggest that these agents may have merit as adjuncts to radiotherapy and warrant testing in rigorous clinical trials, our goal is to facilitate translation of the findings into clinical trials that improve the treatment of cancer patients. The data shown in this report suggest that PHY906 could have value as an adjunct to radiotherapy and support both the performance of additional preclinical studies examining the interactions of PHY906 and radiation and also translation of these findings to design clinical trials examining the potential value of PHY906 as an adjunct to radiotherapy in patients receiving abdominal and/or pelvic irradiation.
Acknowledgements
This project was supported by a pilot grant from the Yale Comprehensive Cancer Center, and by core resources provided by the Yale Comprehensive Cancer Center (NCI center grant 16359), P01CA154295 from the NCI, the Yale Center for Clinical Investigation (CTSA grant NCRR UL1 RR024139), the Department of Therapeutic Radiology, and the Section of Comparative Medicine.
Footnotes
Declaration of Potential Conflicts of Interest: Yung-Chi Cheng is the coinventor of PHY906 as an adjuvant to cancer therapy and a scientific founder and a shareholder of PhytoCeutica. None of the other authors have conflicts of interest related to the work reported in this paper.
References
- Abayomi J, Kirwan J, Hackett A. The prevalence of chronic radiation enteritis following radiotherapy for cervical or endometrial cancer and its impact on quality of life. European Journal of Oncology Nursing. 2009;13:262–267. doi: 10.1016/j.ejon.2009.02.007. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer Facts and Figures. American Cancer Society; Atlanta: 2012. 2012. [Google Scholar]
- Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut. 2005;54:1051–1054. doi: 10.1136/gut.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-Fu-based chemotherapy and radiation therapy for rectal cancer. International J Radiation Oncology Biology Physics. 2002;52:176–183. doi: 10.1016/s0360-3016(01)01820-x. [DOI] [PubMed] [Google Scholar]
- Begg AC. Principles and practices of the tumor growth delay assay. In: Kallman RF, editor. Rodent tumor models in experimental cancer therapy. Plenum Press; New York: 1987. pp. 114–121. [Google Scholar]
- Benson AB, 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr, McCallum R, Mitchell EP, O’Dorisio TM, Vokes EE, Wadler S. Recommended guidelines for the treatment of cancer-treatment induced diarrhea. Journal of Clinical Oncology. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- Booth D, Potten CS. Protection against mucosal injury by growth factors and cytokines. Journal of the National Cancer Institute Monographs. 2001;29:16–20. doi: 10.1093/oxfordjournals.jncimonographs.a003433. [DOI] [PubMed] [Google Scholar]
- Chinese Botany. 1st ed Vol. 7. Shanghai Science and Technology Publishing Company; Shanghai, China: 1999. [Google Scholar]
- Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? International Journal of Radiation Oncology Biology Physics. 2001;50:1105–1106. doi: 10.1016/s0360-3016(01)01556-5. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiation Research. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Duan Q, Liu Y, Booth C, Rockwell S. Use of Fenbendazole-containing therapeutic diets for mice being used in experimental cancer therapy studies. Journal of American Association Laboratory Animal Science. 2012;51:224–230. [PMC free article] [PubMed] [Google Scholar]
- Eng C. Are herbal medicines ripe for the cancer clinic? Science Translational Medicine. 2010;2(45):45ps41. doi: 10.1126/scitranslmed.3001517. [DOI] [PubMed] [Google Scholar]
- Farrell MP, Kummar S. Phase I/IIA randomized study of PHY906, a novel herbal agent, as a modulator of chemotherapy in patients with advanced colorectal cancer. Clinical Colorectal Cancer. 2003;2:253–256. doi: 10.3816/CCC.2003.n.007. [DOI] [PubMed] [Google Scholar]
- Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Seminars in Radiation Oncology. 2003;13:357–371. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Hsu CS. Commonly used Chinese herb formulas with illustrations. Oriental Healing Art Institute; Los Angeles, CA: 1980. [Google Scholar]
- Kummar S, Copur MS, Rose M, Wadler S, Stephenson J, O’Rourke M, Brenckman W, Tilton R, Liu SH, Jiang Z, Su T, Cheng YC, Chu E. A phase I study of the Chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clinical Colorectal Cancer. 2011;10:85–96. doi: 10.1016/j.clcc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Lam W, Bussom S, Guan F, Jiang Z, Zhang W, Gullen EA, Liu SH, Cheng YC. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Science Translational Medicine. 2010;18:45–59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- McBride WH, Chiang C-S, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. A sense of danger from radiation. Radiation Research. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- Moran MS, Yang IJ, Ma S, et al. A prospective, multi-center study assessing the use of complementary and alternative medicine practices by patients undergoing definitive radiation therapy for breast cancer. International Journal of Radiation Oncology Biology Physics. 2012 in press. [Google Scholar]
- Moulder JE, Rockwell S. Comparison of tumor assay methods. In: Kallman RF, editor. Rodent tumors models in experimental cancer therapy. Plenum Press; New York: 1987. pp. 272–278. [Google Scholar]
- Ng K-M, Liang Z, Lu W, Tang HW, Zhao Z, Che CM, Cheng YC. In vitro analysis and spatial profiling of phytochemicals in herbal tissue, by matrix-assisted laser desorption/ionization mass spectrometry. Analytical Chemistry. 2007;79:2745–2755. doi: 10.1021/ac062129i. [DOI] [PubMed] [Google Scholar]
- Okada F. Kampo medicine, a source of drugs waiting to be exploited. Lancet. 1996;348:5–6. doi: 10.1016/s0140-6736(05)64351-6. [DOI] [PubMed] [Google Scholar]
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiation Research. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A gynecologic oncology group study. Journal of Clinical Oncology. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Rockwell S, Chung JS, Sikora AG, Kelley M, Mayne ST. Vitamin A inhibition of radiation-induced pneumonitis in the rat. Journal of Nutrition. 1998;128:1661–1164. doi: 10.1093/jn/128.10.1661. [DOI] [PubMed] [Google Scholar]
- Rockwell S. In vivo-in vitro tumor systems: New models for studying the response of tumors to therapy. Laboratory Animal Science. 1977;27:831–851. [PubMed] [Google Scholar]
- Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine, black cohosh. Breast Cancer Research and Treatment. 2005;90:233–239. doi: 10.1007/s10549-004-4260-x. [DOI] [PubMed] [Google Scholar]
- Rockwell S. Tumor cell survival. In: Teicher B, editor. Tumor Models in Cancer Research. 2nd ed Humana Press Inc; New York: 2010. pp. 607–624. [Google Scholar]
- Rockwell S, Liu Y, Mayne ST, Redlich CA. Subclinical vitamin A deficiency does not increase development of tumors in irradiated or unirradiated lungs. Experimental Biology Medicine. 2011;236:1173–1189. doi: 10.1258/ebm.2011.011082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell S, Liu Y, Seow HA, Ishiguro K, Baumann RP, Penketh PG, Shyam K, Akintujoye OM, Glazer PM, Sartorelli AC. Preclinical evaluation of Laromustine for use in combination with radiation therapy in the treatment of solid tumors. International Journal of Radiation Biology. 2012;88:277–285. doi: 10.3109/09553002.2012.638359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif MW, Lansigan F, Ruta S, Lamb L, Mezes M, Elligers K, Grant N, Jiang ZL, Liu SH, Cheng YC. Phase I study of the biological formulation PHY906 and capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomedicine. 2010;17:161–169. doi: 10.1016/j.phymed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Sonis T, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- Tang W, Eisenbrand G. Chinese drugs of plant origin: chemistry, pharmacology and use in traditional and modern medicine. 1st ed Springer-Verlag Press; New York: 1992. [Google Scholar]
- Tilton R, Paiva AA, Guan JQ, Marathe R, Jiang Z, van Eyndhoven W, Bjoraker J, Prusoff Z, Wang H, Liu SH, Cheng YC. A comprehensive platform for quality control of botanical drugs (Phytomics QC): A case study of Huangqin Tang (HQT) and PHY906. Chinese Medicine. 2010;5:30. doi: 10.1186/1749-8546-5-30. Available from: http://www.cmjournal.org/content/5/1/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott K-R, Hermann T. Radiation effects on abdominal organs. In: Scherer E, Streffer C, Trott KR, editors. Radiopathology of organs and tissues. Springer-Verlag; New York: 1991. pp. 313–346. [Google Scholar]
- Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World Journal Gastroenterology. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Bussom S, Chen J, Quinn C, Bedognetti D, Lam W, Fulan G, Jiang Z, Mark Y, Zhao Y, Stroneck DF, White J, Marincola FM, Cheng Y-C. Interaction of a traditional Chinese Medicine (PHY906) and CPT-11 on the inflammatory process in the tumor microenvironment. BMC Medical Genomics. 2011;4:38. doi: 10.1186/1755-8794-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlake LJ, Thomas K, Lalji A, Blake P, Khoo VS, Tait D, Andreyev HJ. Predicting late effects of pelvic radiotherapy: is there a better approach? International Radiation Oncology Biology Physics. 2010;78:1163–1170. doi: 10.1016/j.ijrobp.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Withers HR, Elkind MM. Micro-colony survival assay for cells of the mouse intestinal mucosa exposed to radiation. International Journal of Radiation Biology. 1970;17:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Wong R, Saga CM, Sagar SM. Integration of Chinese medicine into supportive cancer care: a modern role for an ancient tradition. Cancer Treatment Reviews. 2001;27:235–246. doi: 10.1053/ctrv.2001.0227. [DOI] [PubMed] [Google Scholar]
- Ye M, Liu S-H, Jiang Z, Lee Y, Tilton R, Cheng YC. Liquid chromatography/mass spectrometry analysis of PHY906, a Chinese medicine formulation for cancer therapy. Rapid Communications in Mass Spectrometry. 2007;21:3593–3607. doi: 10.1002/rcm.2832. [DOI] [PubMed] [Google Scholar]
- Yen Y, So S, Rose M, Saif MW, Chu E, Liu SH, Foo A, Jiang Z, Su T, Cheng YC. Phase I/II study of PHY906/Capecitabine in advanced hepatocellular carcinoma. Anticancer Research. 2009;29:4083–4092. [PubMed] [Google Scholar]
- Yeoh ASJ, Gibson RJ, Yeoh EE, Bowen JM, Stringer AM, Giam KA, Keefe DM. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-κB, COX-1, and COX-2. Molecular Cancer Therapeutics. 2007;6:2319–2327. doi: 10.1158/1535-7163.MCT-07-0113. [DOI] [PubMed] [Google Scholar]
- Zhang W, Saif MW, Dutschman GE, Li X, Lam W, Bussom S, Jiang Z, Ye M, Chu E, Cheng YC. Identification of chemicals and their metabolites from PHY906, a Chinese medicine formulation, in the plasma of a patient treated with irinotecan and PHY906 using liquid chromotography/tandem mass spectrophotometry. (Lo/Ms/Ms) Journal of Chromotography A. 2010;1217:5758–5793. doi: 10.1016/j.chroma.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]