Abstract
The current study was undertaken to study the effect of a macerated extract of Nigella sativa seeds in normal as well as in tumour bearing mice against gamma radiation-induced cellular damage to normal tissues. This was done to mimic the clinical setting where in, normal tissues of cancer patients undergoing radiotherapy are exposed to the deleterious effects of radiation. The protection of cellular DNA was analysed in peripheral blood leucocytes of whole body irradiated mice following pretreatment with macerated extract of Nigella sativa seeds (100 mg/kg), using alkaline comet assay, and also estimating biochemical and blood parameters such as levels of antioxidant enzymes superoxide dismutase and catalase, thiobarbituric acid reactive substances and protein oxidation in organs such as spleen, liver, brain and intestine haemoglobin and total leucocyte count, respectively. The results showed that the macerated extract of Nigella sativa seeds protected the liver, spleen, brain and intestines both in normal as well as tumour bearing mice. This study concludes that macerated extract of Nigella sativa seeds has protective effects against radiation-induced damage and biochemical alterations which could be attributed to the ability to scavenge free radicals and its antioxidant properties. Hence macerated extract of Nigella sativa seeds, could be used in combination with radiation to protect against oxidative stress in normal tissues and improving the quality of life of cancer patients by mitigating unwanted side effects of radiation in normal tissues.
Keywords: Antioxidants, fibrosarcoma, macerated extract of Nigella sativa, radioprotection
Radiation therapy (RT) or radiotherapy is a prime modality to treat solid tumours[1]. One of the major hurdles with respect to RT, is the preservation of normal tissue while still ensuring the effective killing of tumour cells[2]. The free radical mediated oxidative injury occurring after exposure of ionizing radiation (IR), damages both tumour as well as the normal cells, limiting the total radiation dose that can be administered[3]. For obtaining better tumour control therefore, it is of utmost importance to protect normal tissues against radiation-induced injury, particularly when higher doses of radiation are used[4]. Further, since tumours are known to physically distort normal tissue architecture, it results in adding to the damage already produced by RT[5]. It is therefore, important to develop an agent that can improve the tolerance of normal tissues to radiation, which in turn will translate to an improved quality for life for cancer patients[4].
There is a considerable interest in radiation medicine to develop and design strategies capable of protecting normal host tissues from the lethal actions of radiation, without compromising with its anticancer activity[5], radioprotectors being one such. Radioprotectors, might be useful adjuncts in radiotherapy should it be able to increase the tolerance of normal tissues without affecting or by even increasing the sensitivity of tumours, thereby enabling higher radiation doses to be delivered[6]. Although a large number of compounds have demonstrated activity as radioprotectors in laboratory studies, most of them have failed even before reaching the preclinical stage because of severe toxicity and side effects[7]. Other synthetic agents tested, also known to have promising effect, have failed during clinical use as they were expensive, displayed toxicity on repeated administration and also had a nondifferential effect in tumour and normal tissues.
Therefore, there is a surge of interest to find new agents particularly derived from natural sources like those obtained from edible or medicinal plants/herbs, which have been consumed by human beings. These compounds are often regarded as nontoxic even at higher concentrations, having the added advantage of low cost and easily accessibility[8]. Considerable research done over the years show that, phytochemicals plays an important role as in vivo radioprotective agents, many of which are known to be antioxidants found in small quantities in our diet[8]. Some examples include tocols (tocopherols and tocotrienols), soy-isoflavones, vitamin A, β-carotene, selenium (organic and inorganic), zinc, copper, the enzyme superoxide dismutase (SOD) and its mimetics[9].
Nigella sativa L.(NS) belonging to the family Ranunculaceae is an annual herbaceous plant growing in countries bordering the Mediterranean Sea, Pakistan and India. It is used as a spice, food preservative and protective in health remedies in traditional folk medicine for the treatment of numerous disorders by Asian and Far Eastern countries[10,11]. The seeds of this plant (commonly known as black seed, black caraway, black cumin or kalonji) have a rich and diverse chemical composition and are known to promote health and treat diseases. In recent years, the seed composition has been extensively studied for both phytochemical and pharmacological properties[12]. It is now known that the black seed contains a variety of actives some of them being amino acids, proteins, carbohydrates, both fixed oils (84% fatty acids, including linolenic and oleic) and volatile oils, alkaloids, saponins, crude fiber, as well as minerals, such as calcium, iron, sodium and potassium. Among the other active ingredients, the quinone constituents present in the volatile oil, of which thymoquinone (TQ), constitutes about 27-57%, has been attributed to be the most important active present in the whole seeds or their extracts[13,14]. Dithymoquinone, thymohydroquinone and thymol are the other pharmacologically active constituents, which have been identified by high performance liquid chromatography (HPLC)[15]. The advantage of this seed and oil, demonstrated by numerous studies is a very low degree of toxicity[14].
Therefore, the current study was aimed at demonstrating the radiation-modifying ability of macerated extract of Nigella sativa (MNS). Maceration was chosen as both the volatile and nonvolatile components could be extracted besides the active phytoconstituent; TQ, being thermolabile would be stable by the chosen cold extraction technique. Further, since herbal medicines are complex mixtures of different compounds that often exert a synergistic effect and exert their full beneficial effect as whole extracts[16]. Qualitative and quantitative phytochemical tests were performed on the extract for the presence of different phytoconstituents. Further, since the action of radiation-modifiers is mediated primarily through scavenging the primary free radicals and subsequently prevention in generation of other reactive oxygen species (ROS), we first investigated the radical scavenging property of MNS in a cell-free system. The study was extended to investigate the influence of MNS in modification of cellular oxidative damage (e.g. DNA damage, alterations in antioxidant enzymes) induced by IR in vivo in organs of normal and tumour bearing mice. Since, the aim was in vivo radioprotection, mice were whole body irradiated (WBI) with a single dose of radiation of 4 Gy, as if we had opted for only tumour irradiation per se, we would not have been able to quantify the damaging effects of IR in the normal organs which is the main aim in this study.
MATERIALS AND METHODS
All experiments were carried out on random bred female Swiss albino mice, weighing 25-30 g and obtained from the animal house facility of the Biomedical Group, Bhabha Atomic Research Centre, Mumbai, India. The mouse colony was maintained under conditions of controlled temperature (23±2°), humidity (50±5%), and a 12 h light/dark cycle. The animals were housed in sanitized polypropylene cages containing autoclaved paddy husk as bedding with free access to standard mouse food and water. Animals were treated humanely in compliance with the national guidelines and the protocol conformed to the Institutional Animal Ethical Committee.
Plant material:
The seeds of NS were supplied by Ayurvision, Mumbai, India and were authenticated by Agharkar Institute, Pune. Herbarium samples were deposited in the herbarium of the Bombay College of Pharmacy, voucher number BCP/06/08. Sixty five grams of the seeds then macerated with 100 ml of absolute ethanol, with constant stirring at 1200 rpm, in a closed vessel. The next day, the solvent was filtered, marc was drained, and the procedure was repeated for 6 days for complete extraction of the phytochemicals. The expressed liquid was then mixed with the strained liquid. It was then filtered to get an extract that was poured into an evaporating dish and left open. The extract was then labelled as MNS and preserved in an airtight amber coloured glass container at 4° until use. The acute oral toxicity of the MNS extract was performed as per The Organisation for Economic Co-operation and Development (OECD) guidelines where MNS was administered orally at a dose of 2000 mg/kg to mice. The dose for the current study was selected based on previously conducted and reported dose studies in the laboratory wherein mice, fed orally with the different doses of ethanol extract of NS (0-100 mg/kg bw) for 5 consecutive days followed by 2 Gy WBI showed significant protection against oxidative injury to spleen and liver as measured by lipid peroxidation (LPO) and the activity of antioxidant enzymes[17].
Phytochemical investigation of macerated extract of Nigella sativa:
The preliminary qualitative screening of extract was done by HPTLC fingerprinting using the standard mobile phases to get an insight into the possible phytoconstituents present. The presence of volatile components was confirmed by GC/MS spectroscopy whereas UV/Vis spectral analysis was performed for presence of TQ. The total phenolic content in MNS was quantified by Folin-Ciocalteu procedure[18], the percentage saponins (mg/100 mg) was estimated by anisealdehyde-sulphuric acid method[19] while the quantification of TQ was done by reported HPTLC method[20]. The IC50 values obtained for MNS extracts for inhibition of free radicals was determined by free radical (2,2-diphenyl-1-picrylhydrazyl (DPPH)) scavenging activity[21] whereas the IC50 value for inhibition of superoxide radical was performed by standard reported methods[22].
Tumor transplantation:
A serially transplanted fibrosarcoma originally developed by a subcutaneous injection of 6,12-Dimethylbenzo(1,2-b:4,5-b′)dithionaphthene was used as a test system. Tumours 10-15 days old were excised, minced and single-cell suspension was prepared in a sterile environment. About 100 μl of this murine fibrosarcoma single-cell suspension (1×106 cells) in phosphate buffer saline (PBS) was then transplanted in experimental mice by subcutaneous injection on the right dorsal side of the hind limb[17]. After 7 days, solid tumours were developed in mice and the mice were grouped based on average tumour size of 100 mm3 measured using a vernier calipers discarding any outliers.
Experimental plan:
The selected mice were divided into eight groups, comprising eight animals in each group. Group 1 was denoted as normal control and received 1% carboxy methyl cellulose (CMC) p.o.; Group 2 (Normal+γ-rad) received 4 Gy of γ-rad on the 5th day; i.e. 11th day after tumour transplant; Group 3 (Normal+MNS extract) received MNS 100 mg/kg p.o.; Group 4 (Normal+MNS extract+γ-rad) received MNS 100 mg/kg p.o., 1 h prior to the 4 Gy γ-rad; Group 5 (Tumour control) received 1% CMC p.o.; Group 6 (Tumour+MNS extract) received MNS 100 mg/kg p.o.; Group 7 (Tumour+γ-rad) received 4 Gy of radiation on the fifth day i.e 11th day after tumour transplant; Group 8 (Tumour+MNS extract+γ-rad) received MNS 100 mg/kg p.o., 1 h prior to the 4 Gy γ-rad.
The MNS extract containing 100 mg/kg doses was prepared by suspending in 1% sodium CMC solution. The extract suspension was freshly prepared and administered using a mice oral feeding needle for 5 consecutive days starting from the 7th day after tumour transplantation. For WBI, 1 h after MNS extract was fed orally to the mice; animals were immobilized in specially designed well-ventilated perplex box and exposed to γ-radiation (γ-rad) in Cobalt-60 Theratron Unit at a dose of 4 Gy with a dose rate of 0.32 Gy/min at a distance of 38 cm. The required dosimetry was performed using chemical (Fricke) and farmer ion chamber dosimeter. The scheme of the experimental work is given in fig. 1.
Fig. 1.
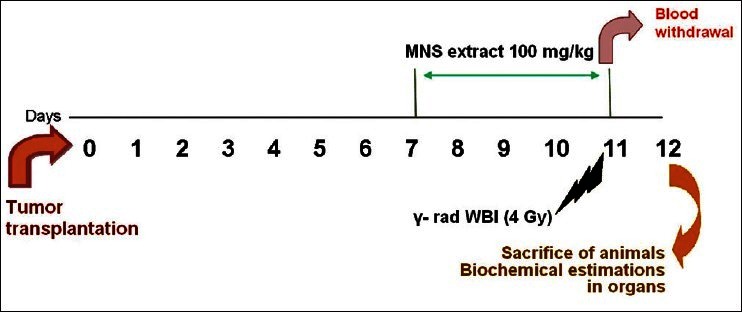
Scheme of the experimental plan
Sample collection:
One hour post-irradiation, blood was collected from the tail vein of mice and processed immediately for the alkaline comet assay, to estimate the DNA strand breaks in each group. 24 h post irradiation, blood was withdrawn for the retro orbital plexus of the mice for measurement of haemoglobin (Hb) and total leucocyte count (TLC). The animals were then sacrificed by cervical dislocation, rapidly dissected and liver, spleen, intestine and brain samples were quickly removed, washed in ice-chilled phosphate buffered saline (pH 7.4), dried on a blotting paper, weighed and kept at −20° for homogenate preparation.
Preparation of tissue homogenate:
10% homogenate of the tissues i.e. liver, spleen, intestine and brain were prepared in 10 mM ice-chilled phosphate buffered saline (pH 7.4) using a glsused to evaluate theas homogenizer. The homogenates were centrifuged at 3000 rpm for 10 min at 4° followed by two more centrifugation of supernatant at 12,000 g for 10 min at 4°. The final supernatant was used for the biochemical estimations.
Assessment of DNA damage in blood leucocytes[23]:
Assessment of DNA damage was done using the alkaline single cell gel electrophoresis (SCGE: COMET Assay), used to evaluate the γ-rad induced oxidative damage to the cellular DNA in blood leucocytes. Briefly, 100 ml of heparinized whole blood samples were mixed with 1.5 ml of 0.8% agarose solution at 42° and the mixture was poured on fully frosted slides uniformly. After solidification, the slides were immersed in lysing solution, (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid disodium salt (Na2-EDTA) with freshly added 1% Triton X-100 and 10% dimethyl sulphoxide (DMSO)), for 1 h at 4°. The slides were removed from the lysing solution, washed with alkaline electrophoresis buffer three times, and then placed on horizontal electrophoresis tank filled with freshly prepared alkaline buffer (300 mM NaOH, 1 mM Na2-EDTA, pH 13.0) at 4°. The slides were kept in the same buffer (pH 13) for 20 min to allow DNA unwinding and conversion of alkali-labile sites to single strand breaks. Electrophoresis was carried out for 20 min at 1.1 V/cm (25 V, 300 mA) using a compact power supply. After electrophoresis, the slides were washed gently to remove the alkali and detergents by placing them horizontally and flooding them slowly with 0.4 M Tris buffer at pH 7.5. The slides were stained in propidium iodide (5 mg/ml) for 1 h. After staining, the slides were rinsed with distilled water and kept on a wet paper in a closed box at 4°. Stained slides were analysed with the help of a digital imaging system using a Carl Zeiss Fluorescent microscope (Axiosokop) with bright field phase-contrast and epi-fluorescence facility. The images (100 cells/slide) were captured with high-performance JVC TK 1280E colour video camera. The integral frame grabber used in this system (Cvfb01p) is a PC-based card and it accepts colour composite video output of the camera. The quantification of the DNA strand breaks, of the stored images were done using the CASP software by which % DNA in tail, tail length, tail moment (TM) and olive tail moment (OTM) could be obtained directly. The parameter TM is calculated as the product of the tail length and percent DNA in tail while the parameter OTM is the product of the distance between the centre of gravity of the head and the centre of gravity of the tail and percent DNA in tail.
Assessment of blood parameters:
The measurement of Hb concentration was done by using kits and following the Cyanmethemoglobin method[24], the TLC count was done by haemocytometry[25] whereas the protein concentration was determined using the method of Lowry, using Bovine serum albumin as a standard.
Biochemical assays:
The antioxidant enzyme assays for SOD and catalase (CAT) activities in tissue homogenates were measured spectrophotometrically using a UV/Vis spectrophotometer (V-530, Jasco, Japan)., by the inhibition of rate of autocatalytic adrenochrome formation in a reaction buffer containing 50 mM carbonate-EDTA (pH 10.4) as adapted from the method[26] for SOD, whereas the CAT activity of the sample was determined by following the protocol of Aebi[27]. The assay is based on disappearance of H2O2 in the presence of the enzyme source. The decomposition rate of the substrate H2O2 was monitored at 240 nm. A molar absorption of 43.6 l/mol/cm was used to calculate the activity. One unit of CAT is equivalent to one micromole of H2O2 decomposed/min.
Serum LPO level was measured in terms of thiobarbituric acid reactive substances (TBARS) [28]. The formation of TBARS in mice organs from control and treated animals was determined as an index of LPO. For conducting this assay 10 mM stock solution of malondialdehyde (MDA) was prepared by adding 1 mM of 1, 1 33-tetraethoxypropane to 100 ml of 1% v/v sulphuric acid. The mixture was left at room temperature for 2 h to achieve complete hydrolysis. This stock is further diluted to about 10-100 μm. The absorbance of the solution was measured at 245 nm. A molar extinction co-efficient of 13,700 was used to calculate the concentration of MDA in the solution concentration (molar)=OD/13,700[28]. MDA solutions of different concentrations or tissue homogenates (0.1 ml) were mixed with 1 ml of 0.5% TBA reagent. The mixture was incubated at 95° for 60 min. The mixture was then cooled in an ice bath for 10 min followed by centrifugation at 12,000 rpm for 10 min. The absorbance of the supernatant was measured at 532 nm against TBA as blank. A standard curve was prepared correlating the concentration of MDA in the solution used and absorbance of the coloured complex obtained by the reaction with TBA. The concentration of MDA was read from standard calibration curve. The results were presented as nanomoles of MDA per mg of protein.
The oxidative damage to proteins was measured by the quantification of carbonyl groups given as carbonyl content (CC) based on the reaction with dinitrophenylhydrazine (DNPH) as previously described[29]. Tissue protein (200 μg in 200-300 μl) was precipitated by 20% TCA (100 μl) at ice temperature for 5 min followed by centrifugation (4000×g, 5 min., 4°). The pellet was dissolved in 200 μl of 2, 4 DNPH (10 mM prepared in 2 M HCl) and incubated at room temperature for 30 min. DNPH-reacted protein was reprecipitated by adding 20% TCA. Excess DNPH was removed by centrifuging the samples and the pellet was washed three times with 500 μl of mixture containing ethanol:acetic acid (1:1) with 15 min standing periods between each wash. Samples were redissolved in 200 μl of KH2PO4 (20 mM, pH 2.3) and absorbance was read at 370 nm. The molar extinction coefficient of DNPH is 22,000/M/cm at 370 nm used to calculate CC of protein expressed in nmoles/mg of protein.
Statistical analysis:
Experimental values are expressed as mean±SD. Comparisons of mean values between groups was performed by one way-analysis of variance (One way-ANOVA) followed by Post Hoc Tukey Test. Values of P<0.05 were considered significant. Statistics was applied using Graphpad PRISM 4 software (Graphpad Software Inc., USA).
RESULTS
The preliminary phytochemical screening of MNS extract by TLC fingerprinting indicated the presence of alkaloids, saponins, tannins and phenolic compounds, triperpenoids and flavonoids as shown in fig. 2. In addition, the GC/MS characterization of the MNS extract showed the presence of volatile compounds such as p-cymene, carvacrol, 4-terpineol and t-anethole, which was confirmed from the mass spectral library search as shown in fig. 3. The UV/Vis spectral analysis of the extract showed presence of only one main peak having maximum absorbance at around 254 nm corresponding to the peak of TQ as shown in fig. 4. The total phenolic content in MNS was 12.75 w/w quantified by Folin-Ciocalteu method while, % saponins (mg/100 mg) was determined to be 12.75. The TQ content estimated by HPTLC was found to be 0.087% whereas the alkaloid and flavonoid concentration could not be quantified because of very low concentration.
Fig. 2.
Photodocumentation for total leucocyte count chromatograms for macerated extract of Nigella sativa extracts.
Ten microlitre of extract was applied on track 1, 3, 5, 7, 9; whereas 5 μ l was applied on track 2, 4, 6, 8, 10.
Fig. 3.
GC-MS chromatograms for macerated extract of Nigella sativa extract.
Fig. 4.
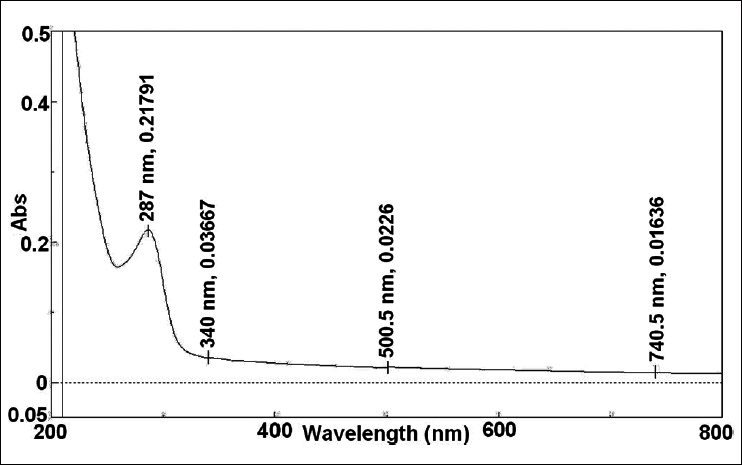
UV/Vis scan for macerated extract of Nigella sativa extract
Free radical scavenging of MNS:
Initial studies were performed in vitro using cell-free systems to assess the ability of the extract to modify the generation of free radicals by the generation of DPPH radicals and inhibition of superoxide radical. The IC50 values obtained for DPPH scavenging activity was found to be approximately 200 μg/ml whereas the IC50 value for inhibition of superoxide radical was found to be 1000 μg/ml.
Protection of blood leucocytes in vivo:
Exposure to 4 Gy γ-WBI lead to DNA strand breaks resulting into relaxation of plasmid DNA in the peripheral blood leucocytes both in normal as well as tumour bearing mice as summarized in Table 1. All the comet parameters (%DNA in tail, OTM, tail length and TM) increased from blood cells of animals subjected to radiation alone, implying formation of DNA strand breaks due to radiation exposure as evident from fig. 5. Normal as well as tumour bearing mice administered with MNS before gamma radiation reduced the levels of all comet parameters as compared to their respective radiation controls. Administration of MNS extract alone to normal mice did not cause a significant change in all the comet parameters. However, it should be noted that the tumour bearing mice had significant elevated comet parameters (P<0.05) as compared to normal values, with MNS treatment reducing the raised levels significantly (P<0.05).
TABLE 1.
MEASUREMENT OF DNA DAMAGE AFTER RADIATION DAMAGE
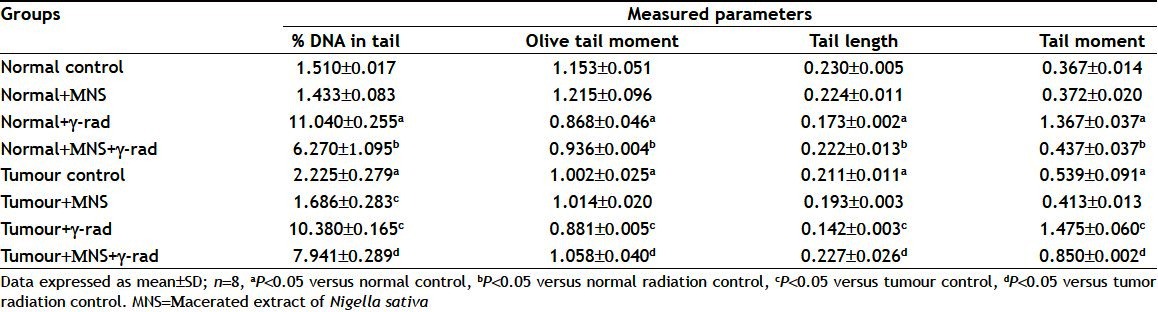
Fig. 5.
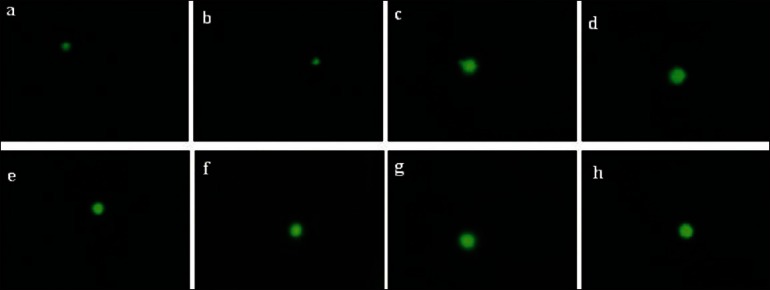
Representative photographs obtained of comet assay.
(a) Normal control; (b) Normal+MNS (100 mg/kg); (c) Normalγ-rad (4 Gy); (d) Normal+MNS (100 mg/kg)γ-rad (4 Gy); (e) Tumour control; (f) Tumour+MNS (100 mg/kg); (g) Tumourγ-rad (4 Gy); (h) Tumour+MNS (100 mg/kg)γ-rad (4Gy). Cells with higher DNA damage move out of the nucleus in radiation control group whereas MNS treated groups have prevented the DNA damge in MNS pretreated groups which is evident from figure.
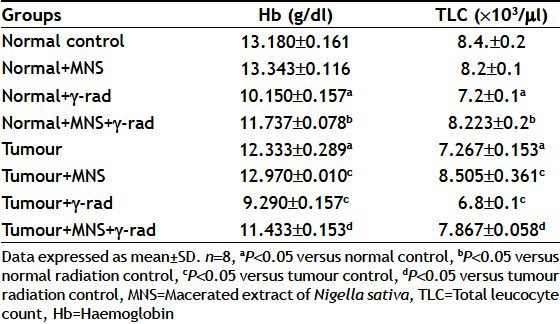
Protection of blood parameters in vivo:
Table 2, presents the effect on the Hb and TLC count and the observations indicate a significant decrease observed in the Hb and TLC count both in normal as well as tumour bearing mice upon exposure to radiation from that of untreated control. Administration of MNS 1 h prior to irradiation increased the Hb and TLC count both in normal mice. In tumour bearing animals, a significant decline in the Hb and TLC count was observed, which was restored to normal control values by treatment with the extract. Administration of MNS to normal animals did not cause a significant change in the Hb and TLC count compared to normal mice.
TABLE 2.
ESTIMATIONS OF THE PARAMETERS IN BLOOD
Protein oxidation and lipid peroxidation:
The alterations in the protein oxidation (CC) and LPO index (TBARS) corresponding to different groups of mice are shown in Table 3. The study showed that there was an increase in the protein oxidation and LPO in tissues upon exposure to WBI from that of unirradiated control in both the normal as well as tumour bearing mice. Pretreatment with MNS for 5 consecutive days decreased the levels of protein oxidation with the degree of protection offered by MNS found to vary in different tissues.
TABLE 3.
EFFECT OF MACERATED EXTRACT OF NIGELLA SATIVA AND GAMMA RADIATION ON LIPID PEROXIDATION AND CARBONYL CONTENT LEVELS IN NORMAL AND TUMOR BEARING MICE
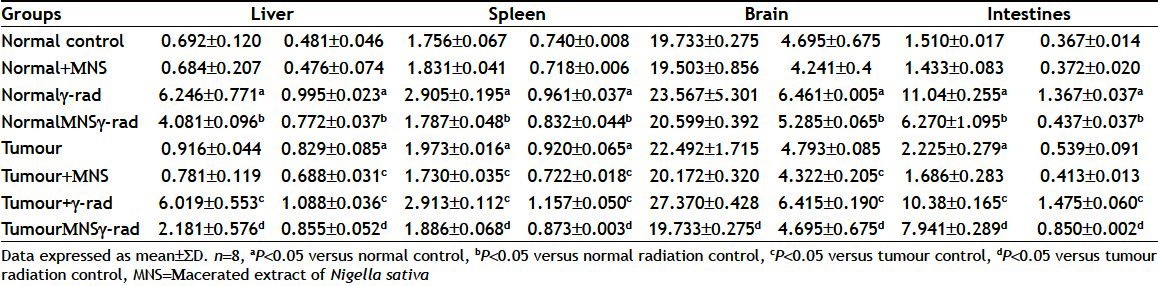
With regards to the liver, it was found that, there was an increase in the levels from 0.692±0.120 to 6.246±0.771 (P<0.05) for protein oxidation while for LPO the levels were elevated from 0.481±0.046 to 0.995±0.023 (P<0.05). Pretreatment with MNS reduced the levels to 4.081±0.096 (P<0.05) and to 0.772±0.037 (P<0.05) for protein oxidation and LPO, respectively. In tumour bearing mice, it was found that there was an increase in the levels of protein oxidation from 0.916±0.044 to 6.019±0.553 (P<0.05) pretreatment with MNS reduced the levels to 2.181±0.576 (P<0.05). In case of LPO too, similar results were obtained with WBI increasing the levels from 0.829±0.085 to 1.088±0.036 (P<0.05) and pretreatment reducing the levels to 0.855±0.052 (P<0.05).
Similarly, in case of the spleen, there was an increase in the levels of LPO and protein oxidation. The LPO values were elevated from 1.756±0.067 to 2.905±0.195 (P<0.05) which were reduced to 1.787±0.048 (P<0.05) in normal mice after pretreatment with MNS while for protein oxidation values increased from 0.740±0.008 to 0.961±0.037 (P<0.05) which were reduced to 0.832±0.044 (P<0.05) in normal mice after pretreatment with MNS. While in tumour bearing mice, the levels for protein oxidation and LPO were reduced from 2.913±0.112 (P<0.05) to 1.886±0.068 (P<0.05) and 1.157±0.050 (P<0.05) to 0.873±0.03 (P<0.05) LPO in spleen were slightly higher i.e. 1.973±0.016 and 0.920±0.065, respectively as compared to the values found in normal mice (P<0.05).
In the brain too, we observed a significant increase in the levels of protein oxidation with levels increasing from 19.733±0.275 to 23.567±0.301 (P<0.05) which were reduced to 20.599±0.392 (P<0.05) after pretreatment with MNS in normal mice. Whereas for LPO, the levels, increased from 4.695±0.675 to 6.461±0.005 (P<0.05, which were reduced to 5.285±0.065 (P<0.05) after pretreatment with MNS in normal mice. In tumour bearing mice, the protein oxidation and LPO levels were increased from 22.492±0.715 to 27.370±0.428 (P<0.05) 4.793±0.085 to 6.415±0.190 (P<0.05), respectively. Pretreatment with MNS reduced the levels to 19.733±0.275 (P<0.05) for protein oxidation and 4.695±0.675 (P<0.05) for LPO, respectively.
In the intestines, the levels for protein oxidation increased from 1.510±0.017 to 11.04±0.255 (P<0.05) which were reduced to 6.270±1.095 (P<0.05) in normal mice with pretreatment with MNS. In tumour bearing mice too, there was an increase from 2.225±0.279 to 10.38±0.165 (P<0.05) with MNS reducing the levels to 7.941±0.289 (P<0.05). In case of LPO, the levels increased from 0.367±0.014 to 1.367±0.037 (P<0.05) which were reduced to 0.437±0.037 (P<0.05) in normal mice with pretreatment with MNS. In tumour bearing mice too, there was an increase from 0.539±0.091 to 1.475±0.060 (P<0.05) with MNS reducing the levels to 0.850±0.002 (P<0.05).
Endogenous antioxidant attack:
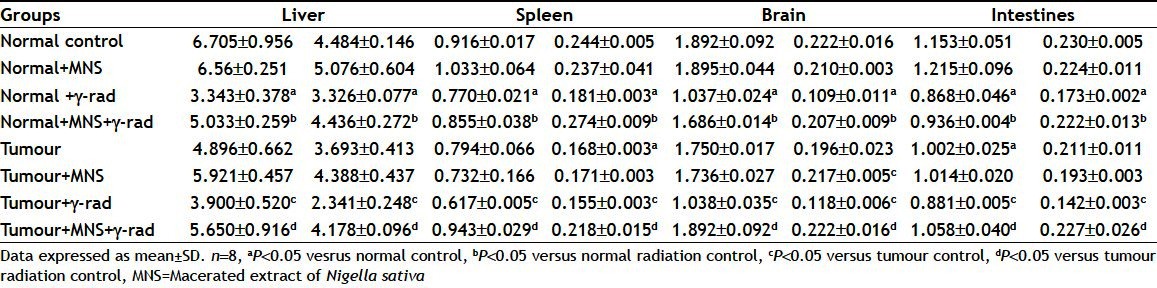
The results indicated a significant decrease in the levels of enzymatic antioxidant levels (SOD and CAT) in the tissue homogenates of liver, spleen, brain and intestine in the radiated groups both in normal as well as tumour bearing mice (Table 4). On the other hand pre-administration of the MNS extract modulated the effects of radiation by increasing the levels of antioxidant enzymes in both the groups in all the tissue homogenates. Further, MNS administered alone did not show any alterations in the levels of the antioxidant enzymes in all the above tissue homogenates in both the groups, respectively.
TABLE 4.
EFFECT OF MACERATED EXTRACT OF NIGELLA SATIVA AND GAMMA RADIATION ON SUPEROXIDE DISMUTASE AND CATALASE LEVELS IN NORMAL AND TUMOR BEARING MICE
DISCUSSION
Radiotherapy, one of the chief modalities to treat cancer, faces a major drawback as it inevitably involves exposure of normal tissues to the deleterious effects of IR. The damage to DNA and membrane lipids are the critical factors in radiation-induced cellular damage and reproductive cell death[30]. It is reported that, antioxidants having free radical scavenging property, prevent radiation-induced lesions[31]. The reason being radiation-induced cytotoxicity is mainly mediated by the generation of free radicals or ROS, like superoxide, hydroxyl radicals, singlet oxygen, and hydrogen peroxide. These ROS generated by the radiolytic decomposition of cellular water attack lipids, proteins, induces oxidation membrane damage, enzyme inactivation and DNA damage. As a consequence, of the radiation-induced alteration of intracellular redox homeostasis, it leads to oxidative stress. The highly reactive hydroxyl radicals, attacks DNA and causes single- and double-strand breaks (DSBs)[32] and oxidative damage to sugar and base residues that can later be converted to strand breaks with the DNA-DSBs being considered the most lethal events and has been found to be the main target of cell killing by radiation. Hence, selective free radical scavengers could protect normal cells during radiotherapy[30,33].
Recently, it has been reported that natural phytochemicals play an important role in radiorecovery and also protect normal tissue during exposure to radiation[32]. Due to lesser side effects as reviewed extensively by many researchers, herbal radioprotectors have been gaining prime importance in radioprotective drug discovery[30]. Earlier studies in our laboratory conducted on the ethanol extract of NS revealed good radioprotection by the extract ex vivo i.e. on irradiated splenic lymphocytes in terms of ROS and reactive nitrogen species (RNS) scavenging and levels of TBARS. The study extended to in vivo systems also revealed amelioration in terms of oxidative damage caused by γ-radiation to the cellular DNA, membrane and nucleus. The dose of 100 mg/kg was used to evaluate the protective effect in radiation-induced damage, hence, the same dose was chosen in present study[17].
Further, the results of antioxidant property of extract in vitro in cell-free system showed a significant free radical scavenging activity of MNS which was in conformity with results reported previously in essential oils obtained for NS[34]. This could be ascribed to its phenolic content as the antioxidant property of many plant extracts have been attributed to their phenolic content as their redox properties play an important role in neutralizing free radicals, quenching singlet and triplet oxygen species or decomposing peroxides[35]. It is also reported that compounds such as flavonoids, which contain hydroxyls, are responsible for the radical scavenging effects of most plants[36]. The acute toxicity data for MNS extract showed no morbidity and mortality with the 2000 mg/kg dose, which shows that the extract is safe up to 2000 mg/kg having the added advantage of being effective orally[37].
LPO is a key marker of oxidative stress and often causes extensive membrane damage, leading to cell death. It can be initiated by radiolytic products, including hydroxyl and peroxyl radicals. Hence, formation of lipid peroxides in the tissues exposed to γ-radiation was chosen as one of markers of membrane damage[38]. Biological specimens contain a mixture of TBARS, including lipid hydroperoxides and aldehydes, which increase as a result of oxidative stress and expressed in terms of MDA equivalents[39].
Different organ systems are involved in the radiation-induced damage. When animals are exposed to large acute whole body doses of radiation, death may follow due to damage to one or more of the following organ systems: the hematopoietic, gastrointestinal and central nervous system. The intestine is a highly radiosensitive and dose limiting organ with rapidly multiplying cells. IR causes acute radiation enteropathy, resulting from the death of rapidly proliferating crypt cells, disruption of the epithelial barrier, mucosal inflammation and LPO. It also involves activation of mast cells, release of TNF-α induced damage[38]. Rapidly dividing tissues such as cells of the hematopoietic system are more prone to radiation-induced damage[38].
In the present study, exposure of normal and tumour bearing mice to 4 Gy γ-radiation (sub-lethal dose) revealed significant peroxidation of lipids in all the organs compared to the untreated control. Pretreatment with MNS decreased the TBARS levels significantly in the organs such as liver, spleen, intestines and brain in normal and tumour bearing animals. Previous studies with plant extracts and phytochemicals having antioxidant properties prevented radiation-induced LPO in microsomal membranes[31]. In the present study, we have shown that presence of MNS extract during irradiation ameliorated radiation-induced oxidation of protein and peroxidation of membrane lipids (Table 3). This could be attributed to the free radical neutralizing property and antioxidant activity of MNS extract as phenolic compounds and volatile oil of NS have been reported to have antioxidant activity though it is difficult to pin point exactly which of the compounds is responsible for protecting DNA and membranes against radiation-induced damage as the extract contains various compounds.
Further, in present study the liver and spleen of tumour bearing animals demonstrated a significant increase in the levels of LPO, revealing the oxidative stress induced by the presence of tumour in the animals. These results could be correlated to those obtained from protein oxidation, another target for radiation-induced damage. The protein oxidation measured in terms of CC demonstrated higher levels after irradiation in both the normal as well as tumour bearing animals. The increased levels were more evident in spleen, liver and intestine of tumour bearing animals as compared to brain probably because of the less protein content in the brain. Pretreatment with MNS decreased the protein oxidation levels significantly in both normal as well as tumour bearing animals indicating protective effect which could be due to the antioxidant activity of MNS extract.
Radiation-induced oxidative damage to DNA encompasses several types of base damage and single or DSBs[40]. The alkaline comet assay, was chosen as it is a sensitive method by which DNA strand breaks at a single-cell level can be monitored. Using this method, we observed that pretreatment with MNS significantly decreased the comet parameters like % DNA in tail, TM, tail migration and OTM in blood leucocytes in both the irradiated normal as well as tumour bearing animals which indicates that MNS extract protected cells against radiation-induced damage. This decrease in DNA breakage seen in MNS treated, irradiated mice underlines the radioprotective efficacy of this compound in vivo, which may be attributed to its ROS scavenging activity or upregulation of DNA repair genes, which protects against radiation-induced damage by bringing error free repair of DNA damage.
Hematopoietic stem cells are highly sensitive to IR as well as chemotherapeutic drugs administered to cancer patients. In fact, myelosuppression and hematopoietic dysfunction are the most common clinical complications of IR. Therefore, an important adjunct could be to promote the recovery of hematopoiesis[41]. In our experiments, mice treated with MNS recovered from WBI as established by their increased numbers of TLC and Hb concentration in comparison with the irradiated controls that did not receive MNS in both irradiated normal as well as tumour bearing animals. The enhancement of TLC and Hb count in mice that received MNS before undergoing irradiation indicates that MNS protects and/or stimulates the proliferation of hematopoietic stem cells displaying a strong potential for clinical application and indicates that MNS stimulated the hemopoietic system, which is highly sensitive to IR owing to its high proliferation rate.
Cells normally respond to ROS by activating a diverse array of protective responses. This includes a complex range of enzymatic antioxidants such as CAT, glutathione peroxidase, SOD, manganese SOD, and nonenzymatic antioxidants such as glutathione, tocopherols and carotenoids. Oxidative repair mechanisms also exist, which include DNA, protein and lipid repair or degradation pathways[41]. ROS affects the antioxidant defence mechanisms, by reducing the intracellular concentration of GSH, and also decreasing the activity of SOD and CAT[42]. In the present study, the levels of antioxidant enzymes such as SOD and CAT decreased significantly in liver, spleen, intestine and brain, after radiation with 4 Gy in both the normal as well as tumour bearing animals which seemed to be significantly restored to the respective normal values with the MNS treatment (Table 4). The present results also indicate that MNS pretreatment protected the normal tissues against the deleterious effects inflicted by gamma radiation by protecting the normal tissues such as liver, spleen, intestines and brain by scavenging the radiation-induced ROS. Further, we observed that in terms of percent protection in tumour bearing mice, the liver was protected by 44.87 and 78.47% in SOD and CAT levels, while for CC and TBARS the percent protection was 63.77 and 21.42%, respectively. Also, in the case of spleen, the percent protection in terms of SOD, CAT, CC and TBARS was 52.84, 40.65, 35.26 and 24.55%, respectively. In the brain and intestines too, the percent protection in terms of SOD and CAT was 82.28, 88.14 and 20.09, 59.86% and 27.90, 26.81 and 23.50, 42.37 in terms of CC and TABRS, respectively. This shows that MNS significantly protected the radiosensitive organs in tumour bearing mice.
The tumour volume measurements taken on the day of sacrifice of the animals did not show a significant reduction in volume after combination treatment of radiation and MNS which could be attributed to the short duration of pretreatment with extract, the MNS dose (100 mg/kg) and a single radiation dose (4 Gy), as for therapeutic purposes the duration of both chemo as well as radio treatment usually ranges from few weeks to months. Further, in solid tumour therapy, radiation is administered to localized tumour region but in the current study WBI was chosen as we wanted to quantify the radiation effect on normal tissues which would have been difficult had it been otherwise. Hence, more studies are needed before MNS extract can be considered for clinical trials in cancer patients.
In conclusion, the present study demonstrates the significant potential of MNS to protect normal tissues from radiation-induced damage in tumour bearing mice, which is close to the situation in radiotherapy. However, further studies need to be carried out to show that the radioprotection effect of MNS is restricted to the normal tissues and not the tumour, though many studies have extensively reported of the anticancer effect of the plant. Nonetheless, the current study shows that in the presence of the tumour which has the propensity to add to the free radical mediated oxidative damage caused by IR to both normal as well the tumour tissue, the extract protected the radiation sensitive normal tissues underlying the potential application of MNS as an adjuvant in radiotherapy.
ACKNOWLEDGEMENTS
The author Reelma Velho-Pereira, is a recipient of Dr. Emidio Afonso Memorial Trust Scholarship. The authors would like to thank Mr. M. Ali and Dr. H. L. Bhilwade, RBHSD, BARC for their technical help during irradiation of the samples and for COMET assay, respectively. Authors thank to Mr. T. B. Tithe, Anchrom Enterprises (I) Pvt. Ltd., Mumbai for help in HPTLC fingerprinting of the extract, Dr. A. S. Phadke, Ayurvision for providing the seeds of Nigella sativa and Dr. A. M. Mujumdar, Head, Plant Science Division, Agharkar Research Institute, Pune for seed authentication.
Footnotes
Velho-Pereira, et al.: Radioprotection of Nigella sativa Extract in Fibrosarcoma Bearing Mice
REFERENCES
- 1.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 2.Mah LJ, Orlowski C, Ververis K, Vasireddy RS, El-Osta A, Karagiannis TC. Evaluation of the efficacy of radiation-modifying compounds using βH2AX as a molecular marker of DNA double-strand breaks. Genome Integr. 2011;2:3. doi: 10.1186/2041-9414-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: A critical review of amifostine as a cytoprotector in radiotherapy. Semin Radiat Oncol. 2003;13:62–72. doi: 10.1053/srao.2003.50006. [DOI] [PubMed] [Google Scholar]
- 4.Maurya DK, Nair CK. Preferential radioprotection to DNA of normal tissues by ferulic acid under ex vivo and in vivo conditions in tumor bearing mice. Mol Cell Biochem. 2006;285:181–90. doi: 10.1007/s11010-005-9079-1. [DOI] [PubMed] [Google Scholar]
- 5.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 6.Ping X, Junqing J, Junfeng J, Enjin J. Radioprotective effects of troxerutin against gamma irradiation in V79 cells and mice. Asian Pac J Cancer Prev. 2011;12:2593–6. [PubMed] [Google Scholar]
- 7.Singh VK, Yadav VS. Role of cytokines and growth factors in radioprotection. Exp Mol Pathol. 2005;78:156–69. doi: 10.1016/j.yexmp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Nair CK, Salvi VP. Protection of DNA from gamma-radiation induced strand breaks by Epicatechin. Mutat Res. 2008;650:48–54. doi: 10.1016/j.mrgentox.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Tiku AB, Abraham SK, Kale RK. Eugenol as an in vivo radioprotective agent. J Radiat Res. 2004;45:435–40. doi: 10.1269/jrr.45.435. [DOI] [PubMed] [Google Scholar]
- 10.Singh VK, Srinivasan V, Toles R, Karikari P, Seed T, Konstantinos AP, et al. Radiation protection by the antioxidant alpha-tocopherol succinate NATO RTG-099. 2005. [Last accessed 2011 May 18]. Available from: http://www.usuhs.mil/afrri/outreach/pdf/kumar_NATO_2005.pdf .
- 11.El-Dakhakhny M, Madi NJ, Lembert N, Ammon HP. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81:161–4. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 12.Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15:389–99. doi: 10.1097/00001813-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 15.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: A promising anti-cancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–53. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastogi L, Feroz S, Pandey BN, Jagtap A, Mishra KP. Protection against radiation-induced oxidative damage by an ethanolic extract of Nigella sativa L. Int J Radiat Biol. 2010;86:719–31. doi: 10.3109/09553002.2010.484480. [DOI] [PubMed] [Google Scholar]
- 18.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substances and antioxidants by means of Folin-Chiocalteu reagent. Methods Enzymol. 1999;99:152–78. [Google Scholar]
- 19.Uematsu Y, Hirata K, Saito K, Kudo I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J AOAC Int. 2000;83:1451–4. [PubMed] [Google Scholar]
- 20.Velho-Pereira RM, Barhate CR, Kulkarni SR, Jagtap AG. Validated high-performance thin-layer chromatographic method for the quantification of thymoquinone in Nigella sativa extracts and formulations. Phytochem Anal. 2011;22:367–73. doi: 10.1002/pca.1289. [DOI] [PubMed] [Google Scholar]
- 21.Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 22.Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 23.Chaubey RC, Bhilwade HN, Rajagopalan R, Bannur SV. Gamma ray induced DNA damage in human and mouse leucocytes measured by SCGE-Pro: A software developed for automated image analysis and data processing for Comet assay. Mutat Res. 2001;490:187–97. doi: 10.1016/s1383-5718(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 24.Anonymous. International Committee for Standardization in Haematology. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard EP 6/2: 1977) and specifications for international haemiglobincyanide reference preparation (ICSH standard EP 6/3: 1977) J Clin Pathol. 1978;31:139–43. doi: 10.1136/jcp.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochei J, Kolhatkar A, editors. Medical Laboratoy Science–Theory and Practice. 5th ed. New Delhi: Tata McGraw Hill Publishing Company; 2008. The complete blood count; pp. 275–86. [Google Scholar]
- 26.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 27.Aebi HE. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 3rd ed. Weinheim: Verlag Chemie; 1983. pp. 273–86. [Google Scholar]
- 28.Okhawa H, Ohishi N, Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 29.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 30.Veerapur VP, Prabhakar KR, Parihar VK, Kandadi MR, Ramakrishana S, Mishra B, et al. Ficus racemosa stem bark extract: A potent antioxidant and a probable natural radioprotector. Evid Based Complement Alternat Med. 2009;6:317–24. doi: 10.1093/ecam/nem119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty TK, Satav JG, Nair CK. Radiation protection of DNA and membrane in vitro by extract of Hemidesmus indicus. Phytother Res. 2005;19:387–90. doi: 10.1002/ptr.1470. [DOI] [PubMed] [Google Scholar]
- 32.Petrovic S, Leskovac A, Joksic G. Radioprotective properties of Gentiana dinarica polyphenols on human lymphocytes in vitro. Curr Sci. 2008;95:1035–41. [Google Scholar]
- 33.Maurya DK, Adhikari S, Nair CK, Devasagayam TP. DNA protective properties of vanillin against gamma-radiation under different conditions: Possible mechanisms. Mutat Res. 2007;634:69–80. doi: 10.1016/j.mrgentox.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Saradha Devi KM, Annapoorani S, Ashokkumar K. Hepatic antioxidative potential of ethyl acetate fraction of Cynodon dactylon in Balb/c mice. J Med Plant Res. 2011;5:992–6. [Google Scholar]
- 36.Aliyu AB, Musa AM, Ibrahim MA, Ibrahim H, Oyewale AO. Preliminary phytochemical screening and antioxidant activity of leave extract of Albizia Chevalieri Harms (Leguminoseae-Mimosoideae) Bayero J Pure Appl Sci. 2009;2:149–53. [Google Scholar]
- 37.OECDGuidelines for the testing of chemicals: revised draft guidelines 423. Acute oral toxicity-Acute toxic class method. 2000 Oct [Google Scholar]
- 38.Parihar VK, Prabhakar KR, Veerapur VP, Kumar MS, Reddy YR, Joshi R, et al. Effect of sesamol on radiation-induced cytotoxicity in Swiss albino mice. Mutat Res. 2006;611:9–16. doi: 10.1016/j.mrgentox.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 39.Kwon T, Watts B. Malonaldehyde in aqueous solution and its role as a measure of lipid peroxidation in food. J Food Sci. 1964;29:294–302. [Google Scholar]
- 40.Park E, Ahn GN, Lee NH, Kim JM, Yun JS, Hyun JW, et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 2008;582:925–30. doi: 10.1016/j.febslet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Jarrett SG, Boulton ME. Antioxidant up-regulation and increased nuclear DNA protection play key roles in adaptation to oxidative stress in epithelial cells. Free Radic Biol Med. 2005;38:1382–91. doi: 10.1016/j.freeradbiomed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharjee R, Sil PC. Protein isolate from the herb Phyllanthus niruri L.( Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant properties. Food Chem Toxicol. 2007;45:817–26. doi: 10.1016/j.fct.2006.10.029. [DOI] [PubMed] [Google Scholar]


