Abstract
Conium maculatum Linn. (Umbelliferae) has been traditionally used in the treatment of spasmodic disorders, and to relieve nervous excitation, rheumatic pains in the old and feeble, pain in stomach, pain of gastric ulcer, nervousness and restlessness. Alkaloids have long been considered as bioactive group of constituents present in C. maculatum. Despite a long tradition of use, C. maculatum has not been evaluated pharmacologically to validate its traditional claims for analgesic and antiinflammatory activities. Thus, the present investigations were undertaken with an objective to evaluate alkaloidal fraction of C. maculatum aerial parts for analgesic and antiinflammatory activities. Test doses (100 or 200 mg/kg, p.o.) of alkaloidal fraction were evaluated for analgesic activity using tail flick test and antiinflammatory activity using carrageenan-induced paw oedema test in rats. Morphine (5 mg/kg, p.o.) and indomethacin (5 mg/kg, p.o.) were used as standard analgesic and antiinflammatory drugs, respectively. Alkaloidal fraction of the plant exhibited significant analgesic activity at a dose of 200 mg/kg as it showed significant increase in tail flicking reaction time with respect to the control during 2 h intervals of observation. It also exhibited significant antiinflammatory activity at a dose of 200 mg/kg as it inhibited paw oedema in rats to 71% and reduced the paw volume one-fourth to the control during 1st h of the study. The present investigations suggest that alkaloids are responsible for analgesic and antiinflammatory activities of C. maculatum.
Keywords: Alkaloids, analgesic, antiinflammatory, Conium maculatum, Umbelliferae
Conium maculatum Linn. (Umbelliferae), commonly known as Dog's poison, has been used in the treatment of spasmodic and inflammatory disorders[1], and to relieve nervous excitation[2,3], rheumatic pain in the old and feeble, pain in stomach, pain of gastric ulcer, nervousness and restlessness. C. maculatum has been traditionally used externally to treat herpes, erysipelas, breast tumours, and as an antispasmodic, a sedative or an analgesic[4]. The dried leaf and juice of the plant were listed in pharmacopoeias of London and Edinburgh from 1864 to 1898 and the last official medicinal recognition appeared in the British Pharmaceutical Codex of 1934 in Great Britain[5].
Phytochemically, C. maculatum has been reported to contain piperidine alkaloids viz., coniine[6–8], 2-phenyl-3,4,5,6-tetrahydropyridine and 5-hydroxy-2- pentylpiperidine[9]; fatty constituents[10,11] viz., palmitic, stearic, oleic, petroselinic, linolenic and arachidic acids, and unsaponifiable matter containing β-sitosterol and lupeol, flavonoids[12], coumarins, polyacetylenes, volatile and nonvolatile oils. Piperidine alkaloids from C. maculatum have been reported to exhibit teratogenic[13,14] and abortifacient[15] activities. In a clinical trial, ‘Prasham ghanavati’, an Ayurvedic preparation containing C. maculatum as one of the herbs, when given to the students for 110-117 days, showed improvement in memory and power of concentration of the students[16].
Despite a long tradition of use, C. maculatum has not been evaluated pharmacologically to validate its traditional claims for analgesic and antiinflammatory activities. Further, alkaloids have long been considered as bioactive group of constituents present in C. maculatum aerial parts. Therefore, it was envisaged to evaluate total alkaloidal fraction of C. maculatum aerial parts for analgesic and antiinflammatory activities.
Conium maculatum aerial parts were procured from Himalaya Herbs Store, Saharanpur, UP, India. Identity of the plant was confirmed from Raw Materials Herbarium and Museum, National Institute of Science Communication and Information Resources, New Delhi (Ref. No. NISCAIR/RHMD/Consult/2008-09/1170/202).
The alkaloidal fraction was obtained from aerial parts of C. maculatum. Aerial parts (5 Kg) of C. maculatum were treated with calcium oxide, and then soxhlet extracted with chloroform (Ranbaxy Fine Chemicals, New Delhi, India). The chloroform extract was concentrated to one-fourth of its original volume under reduced pressure. It was then partitioned in a separator using 5×50 ml of 2% acidified water (HCl-water). The aqueous fraction was made basic using 20% sodium hydroxide solution to pH 8-9 followed by partitioning with chloroform (5×50 ml). The chloroform fraction was rich in alkaloids (0.143% w/w).
Albino rats of Wistar strain (150-200 g) of either sex were used in the entire study. The animals were fed standard pellet diet (Ashirwad Industries, Chandigarh, India) and water ad libitum. They were housed in standard polypropylene cages and kept under controlled room temperature (24±2°; relative humidity 60-70%) in a 12 h light-dark cycle. Groups of five rats were used in all sets of experiments. The animals were fasted for 12 h before use. The approval from the Institutional Animal Ethical Committee was taken before carrying out biological studies.
Indomethacin (Triko Pharmaceuticals, Rohtak, Haryana, India) and morphine (Pharma-Chemico Laboratories, Solan, India) were used as standard antiinflammatory and analgesic drugs, respectively. The standard and test drugs were suspended in distilled water using tween 80 (5%) as emulsifying agent. These were administered to rats by per oral route.
The tail flick latency was assessed by the analgesiometer (Inco, India). The strength of the current passing through the naked nicrome wire was kept constant at 6 amps[17]. The distance between the heat source and tail skin was 1.5 cm. The site of application of the radiant heat in the tail was maintained at 2.5 cm, measured from the root of the tail. The cut off reaction time was fixed at 10 s to avoid tissue damage. Three basal reaction times for each mouse at a gap of 5 min were taken to confirm normal behaviour of the mice. The reaction time was recorded for 2 h at intervals of 30 min, 1 h and 2 h after the treatment with control, standard drug and test doses of C. maculatum alkaloidal fraction. The percentage maximum possible effect (% MPE) was calculated from the formula as[18], %MPE=(actual time-basal time)/(cut off time-basal time)×100.
Acute inflammation was produced by subplantar injection of 0.1 ml of 1% suspension of carrageenan in distilled water in the right hind paw of the rats, 1 h after oral administration of drugs[18]. The paw volume was measured for 3 h at intervals of 1, 2 and 3 h after the carrageenan injection using plethysmometer. The difference between the two readings was taken as the volume of oedema and the percentage antiinflammatory activity was calculated.
The results have been expressed as mean±standard deviation (SD). The test doses were compared with standard and control by two way analysis of variance (ANOVA) followed by Student Newman Keul's test[19].
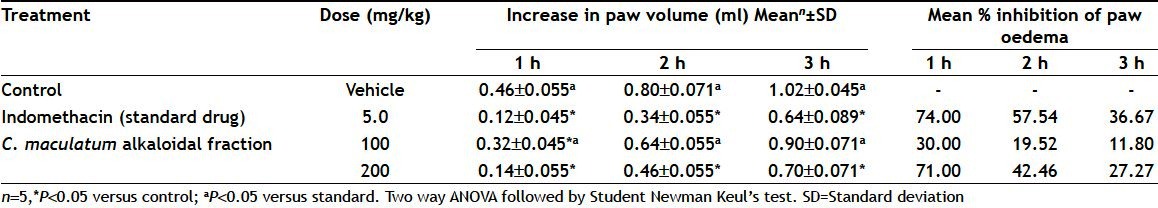
The alkaloidal fraction of C. maculatum was evaluated for analgesic and anti-inflammatory activities using tail flick test and carrageenan-induced paw oedema test, respectively, at the doses of 100 or 200 mg/kg, p.o. Table 1 shows average tail flick reaction time and % MPE using tail flick test in rats after oral administration of C. maculatum alkaloidal fraction (100 or 200 mg/kg). Table 2 shows mean increase in paw volume and percent inhibition of paw oedema using carrageenan-induced paw oedema in rats after oral administration of C. maculatum alkaloidal fraction (100 or 200 mg/kg).
TABLE 1.
ANALGESIC ACTIVITY OF CONIUM MACULATUM ALKALOIDAL FRACTION
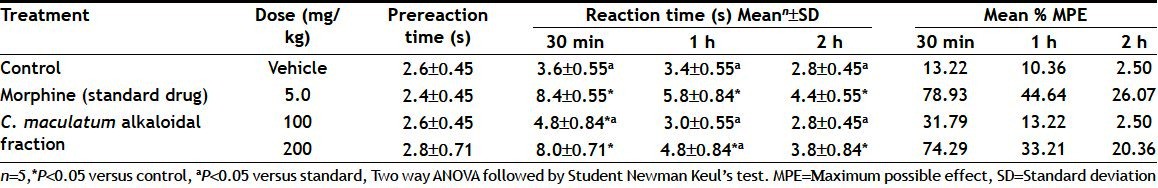
TABLE 2.
ANTI-INFLAMMATORY ACTIVITY OF CONIUM MACULATUM ALKALOIDAL FRACTION
In the tail flick model, the test drug in different doses increased the pain threshold significantly during the period of observation and this indicates the involvement of a higher center. Morphine (5 mg/kg, p.o.) was taken as standard drug to test analgesic activity of C. maculatum alkaloidal fraction. Mean tail flicking reaction time and % MPE were taken as parameters for assessing analgesic activity. Alkaloidal fraction of C. maculatum exhibited significant analgesic activity at a dose of 200 mg/kg as it showed significant tail flicking reaction time with respect to the standard and control during 2 h intervals of observation. The maximum activity was observed after 30 min of observation. The analgesic activity of alkaloidal fraction of C. maculaum (200 mg/kg) was decreased during 1 or 2 h of observations in the similar manner as observed in the standard group.
Carrageenan-induced hind paw oedema is the standard experimental model of acute inflammation. Carrageenan is the phlogistic agent of choice for testing antiinflammatory drugs as it is nonantigenic and devoid of apparent systemic effects. Moreover, the experimental model exhibits a higher degree of reproducibility[20]. Carrageenan-induced oedema is a biphasic response. The first phase is mediated through the release of histamine, serotonin and kinins whereas second phase is related to the release of prostaglandins and slow reacting substances which peak at 3 h.
In the present study, indomethacin (5 mg/kg) was taken as a standard drug to test antiinflammatory activity of C. maculatum alkaloidal fraction. Mean increase in paw volume and percent inhibition of carrageenan induced paw oedema were taken as parameters for assessing antiinflammatory activity of the test drug. Alkaloidal fraction of C. maculatum exhibited significant antiinflammatory activity at a dose of 200 mg/kg as it inhibited paw oedema in rats to 71% in comparison to standard drug which inhibited to 74%. The present investigations suggest that alkaloids are responsible for analgesic and antiinflammatory activities of C. maculatum aerial parts.
Footnotes
Madaan and Kumar: Analgesic and Antiinflammatory Activity of Conium maculatum
REFERENCES
- 1.Manjunath BL. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products. New Delhi: Council of Scientific and Industrial Research; 1948. Conium maculatum; p. 29. [Google Scholar]
- 2.Felter HW, Lloyd JU. King's American Dispensatory. Portland: Eclectic Medical Publications; 1898. [Last accessed on 2012 Mar 13]. Conium maculatum. Available from: http://www.henriettesherbal.com/eclectic/kings/conium.html . [Google Scholar]
- 3.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: Council of Scientific and Industrial Research; 1956. Conium maculatum; p. 75. [Google Scholar]
- 4.Holm L. World Weeds, Natural Histories and Distribution. New York: Wiley Publishers; 1997. Conium maculatum; p. 1129. [Google Scholar]
- 5.Bowman C, Sanghvi S. Pharmacological actions of hemlock (Conium maculatum) alkaloids. J Pharm Pharmacol. 1963;15:1–25. doi: 10.1111/j.2042-7158.1963.tb12738.x. [DOI] [PubMed] [Google Scholar]
- 6.Seeger R, Neumann HG. Connin-piperidine alkaloid of Conium and Aloe. Dtsch Apoth Ztg (DAZ) 1991;131:720–3. [Google Scholar]
- 7.Mauror B. Alkaloids, bases and essential oils. Perfum Flavor. 1994;19:19–27. [Google Scholar]
- 8.Holstege DM, Galey FD, Booth MC. Development and validation of a multiresidue alkaloid screen. In: Garland T, Barr AC, editors. Toxic Plants and Natural Toxicants, Proceedings of 5th International Symposium on Poisonous Plants held at San Angelo, Texas, May 19-23, 1997. Wallingford, Oxon, UK: CAB International Publishing; 1998. pp. 233–8. [Google Scholar]
- 9.Lang DJ, Smith RA. Two new alkaloids of Conium maculaum and evidence for tautomeric form for ?-coniceine. In: Garland T, Barr AC, editors. Toxic Plants and Natural Toxicants, Proceedings of 5th International Symposium on Poisonous Plants held at San Angelo, Texas, May 19-23, 1997. Wallingford, Oxon, UK: CAB International Publishing; 1998. pp. 412–9. [Google Scholar]
- 10.Kaul VK, Banerjee A, Nigam SS. Chemical investigations of the seed fat of Conium maculatum L. Herb Hung. 1985;24:177–81. [Google Scholar]
- 11.Lotti G, Paradossi C, Marchini F. Analytical characterization of new seed oils. Rev Soc Ital Sci Ailment. 1985;14:263–70. [Google Scholar]
- 12.Zdanevich EV, Belodubrovskaya GA. Conium maculatum L: chemical composition, useful and harmful properties. Rastit Resur. 1997;33:108–13. [Google Scholar]
- 13.Panter KE, Gardner DR, Shea RE, Molyneux RJ, James LF. Toxic and teratogenic piperidine alkaloids from Lupinus Conium and Nicotiana species. In: Garland T, Barr AC, editors. Toxic Plants and Natural Toxicants, Proceedings of 5th International Symposium on Poisonous Plants held at San Angelo, Texas, May 19-23, 1997. Wallingford, Oxon, UK: CAB International Publishing; 1998. pp. 345–50. [Google Scholar]
- 14.Panter KE, James LF, Gardner DR, Molyneux RJ. The effects of poisonous plants on embryonic and fetal development in livestock. In: Colegate SM, Dorling PR, editors. Plant Association of Toxins, Proceedings of 4th International Symposium on Poisonous Plants held at Fremantle, Western Australia, Australia, September 26-October 1, 1993. Tucson, Arizona, USA: CAB International; 1994. pp. 325–32. [Google Scholar]
- 15.Merzouki A, Ed-derfoufi F, Mesa JM. Hemp (Cannabis sativa L.) and abortion. J Ethnopharmacol. 2000;73:501–3. doi: 10.1016/s0378-8741(00)00323-8. [DOI] [PubMed] [Google Scholar]
- 16.Surn PA, Sardeshmukh SP. Kulkarni PH, editor. To assess the effect of Prasham as Medhya Rasayan. Ayurved Research Papers II. 1995:192–4. [Google Scholar]
- 17.Chakraborty A, Devi RK, Rita S, Sharatchandra K, Singh TI. Preliminary studies on anti-inflammatory and analgesic activities of Spilanthes acmella in experimental models. Indian J Pharmacol. 2004;36:148–50. [Google Scholar]
- 18.Kanaan SA, Faabe NE, Haddab JJ, Abdelnoor AM, Atweh SF. Endotoxin induced local inflammation and hyperalgesia in rats and mice: A new model for inflammatory pain. Pain. 1999;66:373–9. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- 19.Scheffer WC. Statistics for the Biological Sciences. Philippines: Addison-Wesley Publishing Company, Inc.; 1980. Analysis of Variance; pp. 121–41. [Google Scholar]
- 20.Winter CA, Orahovats PD, Flataker L, Lehman EG, Lehman JT. Studies on the pharmacology of N-allylnormorphine. J Pharmacol Exp Ther. 1954;111:152–60. [PubMed] [Google Scholar]


