Abstract
Several studies have attributed deterioration of sound localization in the horizontal (azimuth) and vertical (elevation) planes to an age-related decline in binaural processing and high-frequency hearing loss (HFHL). The latter might underlie decreased elevation performance of older adults. However, as the pinnae keep growing throughout life, we hypothesized that larger ears might enable older adults to localize sounds in elevation on the basis of lower frequencies, thus (partially) compensating their HFHL. In addition, it is not clear whether sound localization has already matured at a very young age, when the body is still growing, and the binaural and monaural sound-localization cues change accordingly. The present study investigated sound-localization performance of children (7–11 years), young adults (20–34 years), and older adults (63–80 years) under open-loop conditions in the two-dimensional frontal hemifield. We studied the effect of age-related hearing loss and ear size on localization responses to brief broadband sound bursts with different bandwidths. We found similar localization abilities in azimuth for all listeners, including the older adults with HFHL. Sound localization in elevation for the children and young adult listeners with smaller ears improved when stimuli contained frequencies above 7 kHz. Subjects with larger ears could also judge the elevation of sound sources restricted to lower frequency content. Despite increasing ear size, sound localization in elevation deteriorated in older adults with HFHL. We conclude that the binaural localization cues are successfully used well into later stages of life, but that pinna growth cannot compensate the more profound HFHL with age.
Keywords: children, directional hearing, ear morphology, head movements, older adults, transfer function
Introduction
To locate a sound source in azimuth (the horizontal plane), the auditory system relies predominantly on interaural time differences (ITDs, for frequencies up to about 1.5 kHz) and interaural level differences (ILDs, above 3 kHz; Blauert 1997). As a result of this duplex binaural encoding principle, most audible sounds can be localized in azimuth. Localization in elevation (the vertical plane) results from the direction-specific reflections of sounds by the head, torso, and pinnae. The corresponding spectral-shape cues, known as the head-related and directional transfer functions (HRTFs and DTFs, respectively, see “Methods” for explanation), are specific for an individual’s ear morphology and fall in the range of about 4–12 kHz (Batteau 1967; Middlebrooks and Green 1991). Broadband (BB) noises can be accurately localized in the vertical plane, because at changing elevations, the brain can dissociate the different DTFs. In contrast, pure tones and narrow-band noises do not provide sufficient spectral-shape information (Middlebrooks 1992).
Younger listeners
The ability to localize sounds in azimuth appears to be present already at a young age but is difficult to measure (infants 26–30 weeks: Ashmead et al. 1987; infants 8–28 weeks: Morrongiello 1988; children 4–9 years: Van Deun et al. 2009; Lovett et al. 2012). The experimental setups commonly used to test directional hearing performance of children typically present sound sources in a playful manner, for example, with a cartoon above the speaker (Van Deun et al. 2009). Typically, such tests employ a limited number of sound sources at fixed locations (often visible during or in between trials), yielding low spatial resolution of the measurements and the potential confound of memorization, rather than localization, of sounds. To avoid such limitations, we here ensured that children (7–11 years old) could only rely on acoustic information to localize sound sources in two-dimensional space. To our knowledge, such experimental conditions have so far not been applied to test the performance of children younger than 12 years of age.
Older listeners
In older adults (in our group above 63 years of age), hearing loss at higher frequencies is more pronounced than at lower frequencies. Typically, frequencies below 2 kHz are only mildly affected, while above 8 kHz, hearing thresholds for listeners above 50 years of age can increase to 45 dBHL, or poorer (Brant and Fozard 1990). As a result, older participants can hear most acoustic stimuli in their daily environment with both ears and may be able to localize sound sources in azimuth based on the audibility of low frequencies, but listeners with more severe high-frequency hearing loss (HFHL) would miss the high-frequency spectral pinna cues that discriminate different elevations.
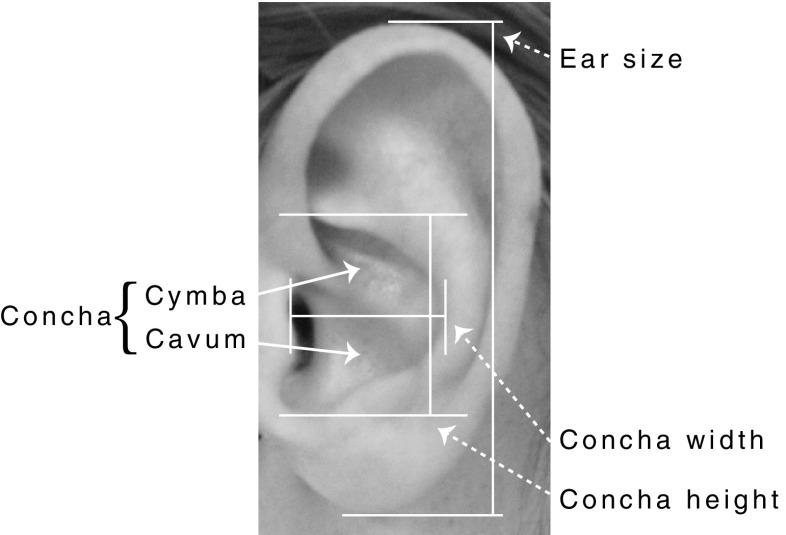
Studies using ear biometrics for subject identification indicate that pinna morphology is as unique as fingerprints (Burge and Burger 1997). Typically, the DTFs contain a prominent spectral notch between 4 and 12 kHz; the location of which depends on size and shape of the concha (Lopez-Poveda and Meddis 1996). The height of the concha cavum (Fig. 1) was reported to be the best (single) predictor for the frequency notch at 0° in elevation (Algazi et al. 2001). Interestingly, the pinna, in particular its concha height, keeps growing throughout life (Heathcote 1995; Niemitz 2007). As a result, the reflective pathways into the ear canal gradually lengthen, thereby shifting the major spectral notches to lower frequencies. If the auditory system would adapt to this slowly changing spectral-shape input, it might allow a listener with a larger concha to localize sound sources in the vertical plane by using relatively lower frequencies. Indeed, the adult human auditory system is capable to adapt to prominent changes in the DTFs in the course of days to weeks (Hofman et al. 1998; Van Wanrooij and Van Opstal 2005). As a consequence, older adults with larger ears might be able to localize sounds in elevation that subjects with smaller ears (e.g., children) cannot localize.
FIG. 1.
Human ear morphology and measurements.
Several studies have reported a decline in localization performance with advancing age, not only for vertical (Noble et al. 1994; Rakerd et al. 1998; Abel et al. 2000; Dobreva et al. 2012) but also for horizontal (Abel and Hay 1996; Dobreva et al. 2011) locations. The latter study suggested that this is caused by a central age-related deficit, resulting in an inability to use the ITDs. Several other studies have indicated an age-related decline for acoustic temporal processing (Strouse et al. 1998; Ross et al. 2007). MEG and EEG studies into the central processing of speech cues (Schneider and Pichora-Fuller 2001; Tremblay et al. 2003) have reported age-related factors affecting neural synchrony in humans, which could potentially underlie the deteriorated sound-localization abilities. However, speech cues arguably require higher processing levels in the auditory pathway than sound-source localization. It is unclear whether subcortical stages in the auditory system, which are known to process the sound-localization cues, like the superior olivary complex, dorsal cochlear nucleus, and inferior colliculus (Yin 2002; Young and Davis 2002; Zwiers et al. 2004) may be affected in older adults.
The present study investigated whether age-related HFHL and ear morphology affect sound-localization performance in azimuth and elevation. We presented four BB noises with different bandwidths (0.5–5, 0.5–7, 0.5–11, and 0.5–20 kHz) to investigate whether larger ears would result in a functional benefit. We compared listeners from three age groups: children (7–11 years of age), young adults (20–34 years old), and older adults (63–80 years of age), all exposed to the same open-loop head-movement localization paradigms.
Methods
Subjects
A group of 18 children (7–11 years; mean ± sd: 9.5 ± 1.3 years), 10 young adults (20–34 years; averaging 24.9 ± 4.9 years), and 14 older adults (63–80 years; averaging 68.4 ± 4.7 years) volunteered to participate in our experiments. Listeners were included in this study when they reported to have no hearing complaints, and did not require a hearing aid. All listeners were naïve about the purpose of the experiment and setup. The children participated because they joined a school project and could decide in which experiment(s) they wanted to participate (DTF measurement, audiogram, and/or sound-localization experiment).
Hearing loss
We measured the hearing thresholds of all young adults and older adults. Six children chose to have their hearing thresholds measured, and none reported having any hearing issues. Pure-tone audiometry was performed at 0.125, 0.25, 0.5, 1, 2, 4, 8, 9, 10, 11.2, and 12.5 kHz for each ear (in the children, up to 8 kHz). Hearing thresholds were obtained using standard 2 down–1 up procedures using an Interacoustics AC 40 clinical audiometer (Interacoustics A/S, Assens, Denmark) with either the TDH 39P headphone (0.25–8 kHz; Telephonics, Farmingdale, NY, USA) or the Koss HV1a headphone (8–16 kHz; Koss Corp., Milwaukee, WI, USA).
Ear size
To measure the ear sizes indicated in Figure 1, we digitally analyzed pictures of all subjects’ ears using Matlab. A ruler in the picture provided a 5.00-cm reference. Figure 1 shows the measures that were taken: ear size, concha width, and concha height.
Directional transfer functions
We measured the DTFs of the young adults, older adults, and five children. Following the procedure described in Bremen et al. (2010), we sampled 58 locations in the frontal median plane (from −57.5 to 85° in elevation, for both ears) three times and averaged across identical locations to compute the raw HRTFs. Subsequently, the raw HRTFs were divided by the measured transfer functions of the microphones and speakers for calibration. Spectral features that are the same for all locations are assumed to contain no important directional cues, and were therefore removed from the calibrated HRTFs. Subtracting the average HRTF across all locations eliminated the so-called common transfer function and yielded the DTFs as a function of elevation (Cheng and Wakefield 2001). Using Matlab, the location of the prominent spectral notch (see “Introduction”) was obtained by finding the frequencies, f −40 and f +40, at which maximum attenuation occurred at ±40° in elevation, and the straight line through these two points determined the typical spectral notch f 0 for E = 0° at:
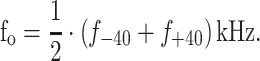 |
1 |
Apparatus
Sound-localization experiments were conducted in a completely dark room with dimensions 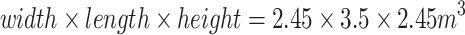 . The room was acoustically treated to absorb frequencies down to 500 Hz, and it was isolated to a background noise level of 20 dBA. Horizontal and vertical head movements were measured using the search-coil technique via a custom-made coil mounted on an adjusted lightweight plastic spectacle frame, worn by the subject (Agterberg et al. 2011). Three pairs of coils were attached to the opposing walls, floor, and ceiling to generate oscillating magnetic fields required for the search-coil technique. The subject was seated in a comfortable chair with the head in the center of the room, and could freely rotate the head. A thin, lightweight aluminum rod and laser were mounted on the spectacle frame. The laser projected a small, dim red dot onto a 1-cm2 black plate at the end of the rod at 31 cm, positioned in front of the subject’s eyes. Lining up this laser dot with a central LED on the wall defined the origin of the azimuth-elevation coordinate system for all subjects (0° in azimuth and elevation). The laser dot also provided a natural head-fixed visual pointer to direct the head at perceived sound locations during the experiment. The sound stimuli were provided by any of 58 broad-range loudspeakers (Monacor MSP-30) mounted on a motorized circular arch. The arch could be rotated to any angle in azimuth (precision better than 0.1°), whereas the different speakers on the arch provided the variation in elevation (at a spatial resolution of 2.5°; see also Van Grootel and Van Opstal 2009).
. The room was acoustically treated to absorb frequencies down to 500 Hz, and it was isolated to a background noise level of 20 dBA. Horizontal and vertical head movements were measured using the search-coil technique via a custom-made coil mounted on an adjusted lightweight plastic spectacle frame, worn by the subject (Agterberg et al. 2011). Three pairs of coils were attached to the opposing walls, floor, and ceiling to generate oscillating magnetic fields required for the search-coil technique. The subject was seated in a comfortable chair with the head in the center of the room, and could freely rotate the head. A thin, lightweight aluminum rod and laser were mounted on the spectacle frame. The laser projected a small, dim red dot onto a 1-cm2 black plate at the end of the rod at 31 cm, positioned in front of the subject’s eyes. Lining up this laser dot with a central LED on the wall defined the origin of the azimuth-elevation coordinate system for all subjects (0° in azimuth and elevation). The laser dot also provided a natural head-fixed visual pointer to direct the head at perceived sound locations during the experiment. The sound stimuli were provided by any of 58 broad-range loudspeakers (Monacor MSP-30) mounted on a motorized circular arch. The arch could be rotated to any angle in azimuth (precision better than 0.1°), whereas the different speakers on the arch provided the variation in elevation (at a spatial resolution of 2.5°; see also Van Grootel and Van Opstal 2009).
Stimuli
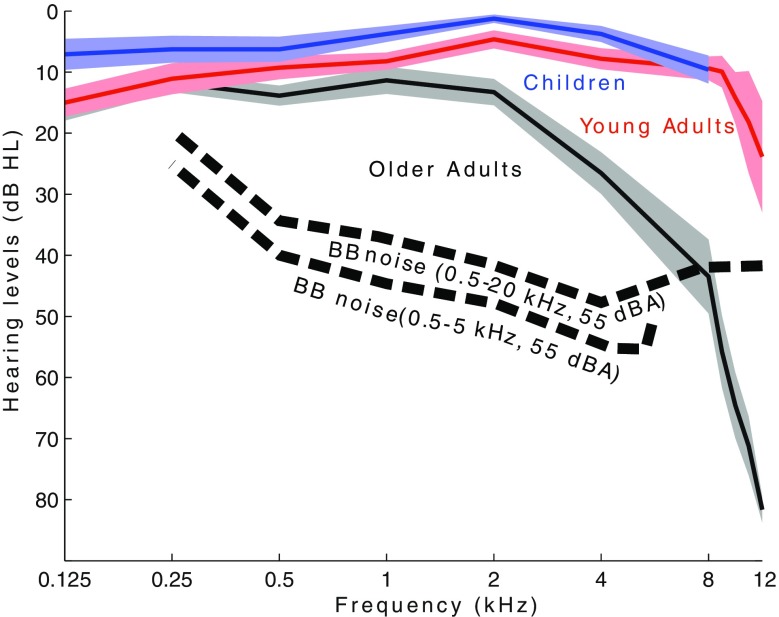
Acoustic stimuli were digitally generated in Matlab (R2012a, The Mathworks, Inc., Natick, MA, USA) using random sampling from a Gaussian distribution and were presented using Tucker-Davis RP2.1 System3 hardware (Tucker-Davis Technologies, Alachua, FL, USA), with a sampling rate of 48.828 kHz. Stimuli were stored off-line as wav-files. After attenuation by custom-built amplifiers, the stimulus was sent to the selected speaker on the arch. Four different BB Gaussian white noises were presented during the experiment. Stimuli had an overall intensity of 55 dBA (meter: Brüel & Kjær 2260, with calibrated microphone: Brüel & Kjær 4091, located at the center of the arch), were high-pass filtered at 0.5 kHz, and low-pass filtered at 5, 7, 11, or 20 kHz (zero-phase FIR filter with a Hamming window of order 50). To measure the spectrum of the noises, intensity measurements were performed at 0.25, 0.5, 1, 2, 4, 8, 10, and 12.5 kHz (in 1/3 octave bandwidths). For this purpose, noise stimuli were generated with a duration of 10 s, and the measured sound pressure levels were expressed in dBHL, following the norm NEN-ISO 389–7 (1996). The resulting spectrum is presented in Figure 2.
FIG. 2.
Clinical measurements of the participants’ hearing thresholds with the average value surrounded by the standard error of the mean. In black the older adults, young adults in red and the children in blue as indicated in the figure. In addition, the spectrum of BB noise is presented. Note that the intensity of the 0.5–5 kHz and 0.5–20 kHz noise stimuli are 55 dBA (over the entire frequency range) and plotted as dotted lines, up to 5 and 12 kHz, respectively. The spectrum was measured in 1/3 octaves and converted to dBHL.
For the young and older adults, each different stimulus was presented 24 times, yielding 96 randomly distributed locations which were presented in pseudorandom order in the two-dimensional frontal hemifield. The target range spanned [−75°, +75°] in azimuth and [−55°, +55°] in elevation. For the children the 0.5-5 kHz stimuli were omitted. Therefore children localized a total of 72 stimuli. When desired, the stimuli were divided into two shorter experimental runs of 24 and 48 trials, respectively. The duration of stimuli was 150 ms, which ensured that the reaction (head saccades towards the sound source) always started after stimulus offset, which denied listeners potential acoustic feedback during their responses.
Sound localization
To become acquainted with the setup, the 15 children who elected the localization experiment, all young and older adults, were first presented with a visual calibration run that enabled the off-line calibration of the head-mounted search coil. The calibration runs demonstrated that listeners had no problem (motoric or otherwise) to point to the stimulus location with their head. We then presented a small set of acoustic stimuli to verify that the listener understood the task to point with the head-fixed laser dot to the sound source, instead of just looking with the eyes. Subsequently, the experiment started and the subject was asked to localize the 72 or 96 sounds. A trial was initiated when the subject pressed a button after lining up the red laser dot with the central LED on the wall. We gave no explicit feedback about performance during or between the experiments, only verbal encouragements.
Data analysis
Sound-source locations were described in the double-pole coordinate system of Knudsen and Konishi (1979). The angular coordinates (azimuth, α, and elevation, ε) are defined relative to a horizontal and vertical plane through the center of the upright head. Azimuth is the horizontal angular deviation from the midsagittal vertical plane, while elevation is the vertical angular deviation from the horizontal plane. We used 17 visual fixation points and final raw head-position signals from the calibration experiment to train two three-layer neural networks that served to calibrate all subsequent head-movement data, using an algorithm based on the Bayesian regularization method (Matlab; The MathWorks). The networks corrected for small inhomogeneities in the magnetic fields and could adequately cope with minor cross talk between the channels, resulting from small deviations from orthogonality of the magnetic field coils. The trained networks were subsequently used to map the raw data to calibrated 2D head positions. Although the number of visual calibration points was limited, the absolute errors of calibrated head positions never exceeded 5 % of the real azimuth and elevation angles over the entire measurement range.
We analyzed the azimuth and elevation responses separately for the four stimulus conditions and for each listener. We determined the best linear fit (in the mean-squared error sense) of the target–response relationship on the data for the different stimulus conditions:
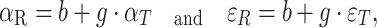 |
2 |
in which α T is the target’s azimuth angle (in degrees) and ε T the target’s elevation angle (in degrees), b is the response bias (in degrees), g the response gain (dimensionless). We also determined the correlation coefficient (r), F and corresponding p value for the full model, and t and p values for the regression coefficients (b and g).
Localization performance was also quantified by determining the mean absolute error (MAE) of the azimuth and elevation responses per stimulus condition according to
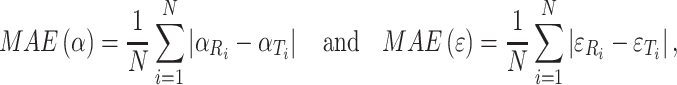 |
3 |
with N the number of trials (Van Wanrooij and Van Opstal 2007).
Results
Hearing loss
Figure 2 shows the averaged audiograms for five children (0.125–8 kHz), all young adults (0.125–12 kHz), and older adults (0.125–12 kHz). Clearly, older adults suffered from a significant hearing loss in the higher frequencies (4, 8, and 12 kHz). Four older adults had an asymmetric hearing loss of 15–20 dB between the left and right ear in the high frequencies. Figure 2 also presents the spectrum of the 0.5–5 kHz and 0.5–20 kHz noise bursts, with an overall intensity of 55 dBA. The sound level for the 0.5–5 kHz noise burst was 7.9 dBHL at 8 kHz.
Ear size
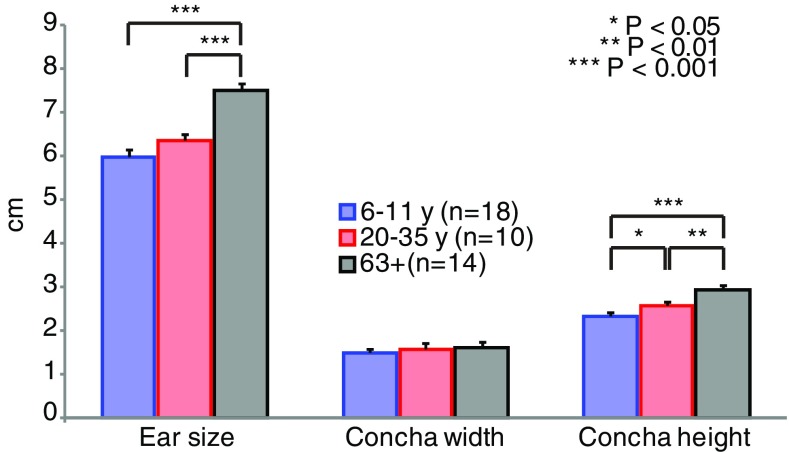
Figure 3 demonstrates an increase in pinna size and concha height with advancing age in our group of listeners. The older adults had an average pinna size of 7.5 cm, which is 1.1 cm longer than the average pinna size in the young adults’ group (unpaired, two-tailed, t test, t 23 = 5.8, P < 10−4) and 1.5 cm larger than the average pinna size in the children’s group (t 28 = 8.7, P < 10−4). The average concha height in the older adults was 2.9 cm, which is 0.4 cm larger than the average in the young adults’ group (t 23 = 3.6, P < 0.01) and 0.6 cm larger than the average in the children’s group (t 28 = 6.8, P < 10−4). The concha width shows no significant difference in size between age groups (between children and older adults: t 28 = 1.4, P = 0.16). These results concur with earlier reports by Niemitz (2007).
FIG. 3.
Average ear size measurements of both ears for the children (white), young adults (black), and older adults (gray). Significant differences after unpaired two-tailed t test displayed with one or more asterisks.
Directional transfer functions
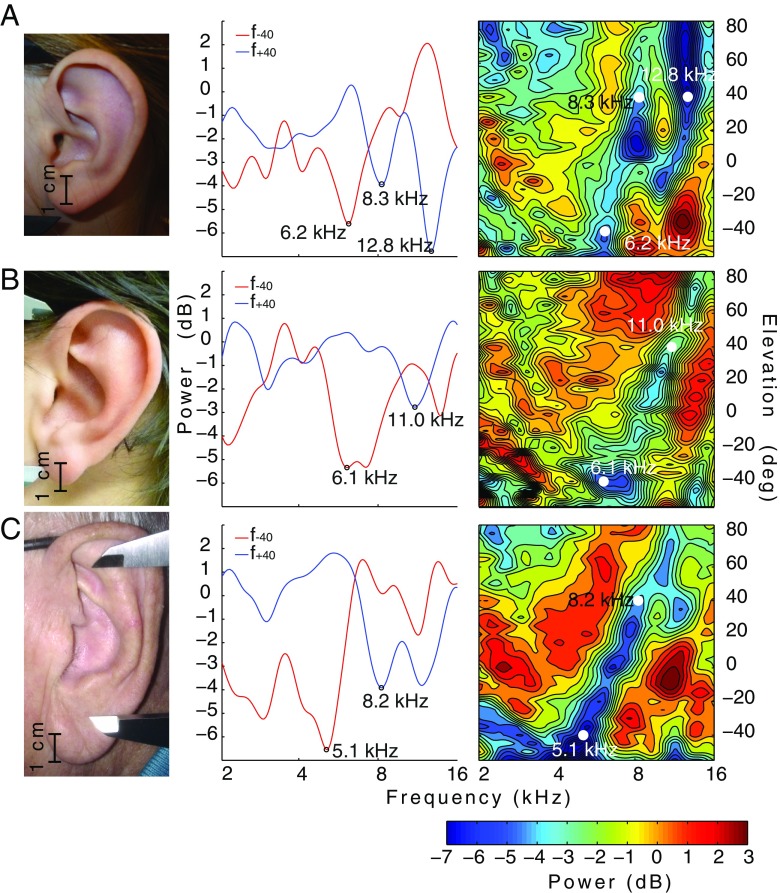
Figure 4 shows a picture of one ear (left column), transfer functions at ±40° elevation (center column), and the DTFs (right column) for a child (Fig. 4A; K7: 9 years old), a young adult (Fig. 4B; YA1: 22 years old), and for an older listener (Fig. 4C; E2: 80 years old). The DTF panels show that different frequencies (x-axis) are attenuated or amplified at different elevation angles (y-axis) due to the differences in size and shape of the pinna (relative intensities indicated by color). The prominent spectral notch (see “Introduction”) of the young adult (Fig. 4B) runs between 6 and 11 kHz for elevation localizations from −40° to +40°, whereas in the older listener (Fig. 4C), the notch is shifted toward lower frequencies, between 5 and 8 kHz for the same elevations. The figure of the child (Fig. 4A) shows the spectral notch between 6.4 and 8.3 kHz but there is an even larger attenuation at 12.7 kHz resulting in a second prominent notch below 16 kHz; this pattern was also found in the other four children DTFs. In the older adults, something similar can be seen but far less prominent. Table 1 provides the data for the spectral notch at ±40° in elevation for five children (both attenuations at +40° in elevation are provided), all young adults, and older adults.
FIG. 4.
A picture of one ear (left column), then the transfer functions (center column) at +40° in elevation (blue line) and −40° in elevation (red line) and finally the DTFs (right column) for a child (Fig. 4A; C7: 9 years old), a young adult (Fig. 4B; YA1: 22 years old) and for an older subject (Fig. 4C; E2: 80 years old). The measured DTF (right) shows the characteristic depression (notch) between 5 and 13 kHz.
TABLE 1.
Age, gender, and hearing levels for both ears averaged for 4, 8, and 11 kHz, offset of the spectral notch in their DTF at ±40° elevation and the azimuth and elevation localization gain (g), correlation coefficient (r), and MAE (in degrees), for the 0.5–20-kHz condition are given for the children (C1–C15), young adults (YA1–YA10), and older adults (OA1–OA14)
| Subject | Age (years) | Gender | HFHL (dBHL) | f −40 (kHz) | f +40 (kHz) | Azimuth 05–20 kHz | Elevation 0.5–20 kHz | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | r | MAE (deg) | g | r | MAE (deg) | ||||||||
| Left | Right | ||||||||||||
| C1 | 11 | M | – | – | – | – | – | 1.04 | 0.97 | 11.7 | 0.31 | 0.75 | 19.0 |
| C2 | 11 | M | – | – | – | – | – | 1.34 | 0.98 | 12.5 | 0.13 | 0.32 | 26.5 |
| C3 | 10 | F | – | – | – | – | – | 0.98 | 0.91 | 15.1 | 0.62 | 0.86 | 15.3 |
| C4 | 9 | F | 5.0 | 7.5 | – | – | – | 1.22 | 0.90 | 17.6 | 0.40 | 0.72 | 21.1 |
| C5 | 9 | F | 5.0 | 5.0 | – | – | – | 1.16 | 0.97 | 17.2 | 0.65 | 0.90 | 14.1 |
| C6 | 9 | F | – | – | 6.2 | 8.3 | 12.8 | 1.21 | 0.98 | 10.3 | 0.71 | 0.94 | 10.6 |
| C7 | 10 | M | – | – | – | – | – | 0.78 | 0.95 | 11.7 | 0.39 | 0.90 | 21.1 |
| C8 | 9 | M | – | – | – | – | – | 1.11 | 0.98 | 10.3 | 0.59 | 0.92 | 14.0 |
| C9 | 9 | M | 5.0 | 7.5 | – | – | – | 1.10 | 0.98 | 11.7 | 0.69 | 0.95 | 10.2 |
| C10 | 10 | M | – | – | 6.0 | 8.2 | 12.6 | 1.24 | 0.95 | 13.7 | 0.21 | 0.26 | 25.7 |
| C11 | 10 | F | 5.0 | 5.0 | – | – | – | 1.06 | 0.96 | 10.4 | 0.83 | 0.96 | 7.6 |
| C12 | 10 | M | 7.5 | 17.5 | – | – | – | 1.13 | 0.98 | 8.1 | 0.56 | 0.80 | 15.1 |
| C13 | 7 | F | – | – | – | – | – | 1.09 | 0.98 | 8.9 | 0.29 | 0.89 | 19.6 |
| C14 | 7 | F | 8.8 | 7.5 | – | – | – | 0.95 | 0.92 | 9.1 | 0.55 | 0.80 | 17.6 |
| C15 | 10 | M | – | – | – | – | – | 1.05 | 0.98 | 10.2 | 0.61 | 0.97 | 11.1 |
| C16 | 11 | M | – | – | 5.9 | 7.7 | 12.8 | – | – | – | – | – | – |
| C17 | 11 | F | – | – | 6.5 | 9.0 | 12.6 | – | – | – | – | – | – |
| C18 | 8 | M | – | – | 6.9 | 9.2 | 13.9 | – | – | – | – | – | – |
| YA1 | 22 | M | 3.3 | 5.0 | 6.1 | 11.0 | 1.03 | 0.96 | 9.2 | 0.65 | 0.88 | 16.7 | |
| YA2 | 21 | F | 13.3 | 10.0 | 6.0 | 8.8 | 1.03 | 0.97 | 7.9 | 0.80 | 0.95 | 9.8 | |
| YA3 | 25 | F | 6.7 | 5.0 | 5.8 | 9.8 | 0.78 | 0.94 | 12.1 | 0.59 | 0.94 | 12.8 | |
| YA4 | 21 | M | 11.7 | 3.3 | 5.2 | 7.6 | 0.93 | 0.98 | 9.3 | 0.61 | 0.93 | 14.2 | |
| YA5 | 21 | F | 5.0 | 0 | 6.5 | 14.3 | 0.94 | 0.98 | 5.6 | 0.49 | 0.91 | 15.4 | |
| YA6 | 22 | M | 16.7 | 6.7 | 6.0 | 8.6 | 1.10 | 0.95 | 9.4 | 0.78 | 0.95 | 9.8 | |
| YA7 | 24 | M | 5.0 | 6.7 | 5.9 | 8.3 | 1.06 | 0.95 | 12.1 | 0.74 | 0.96 | 9.1 | |
| YA8 | 24 | M | 5.0 | 6.7 | 5.6 | 8.3 | 1.14 | 0.97 | 10.6 | 0.75 | 0.97 | 10.6 | |
| YA9 | 34 | M | 1.7 | 5.0 | – | – | 1.13 | 0.97 | 10.4 | 0.69 | 0.96 | 10.4 | |
| YA10 | 33 | M | 6.7 | 0 | – | – | 1.04 | 0.97 | 7.8 | 0.76 | 0.97 | 8.7 | |
| OA1 | 64 | M | 21.7 | 16.7 | – | – | 0.89 | 0.92 | 10.8 | 0.61 | 0.76 | 17.0 | |
| OA2 | 80 | M | 55.0 | 55.0 | 5.1 | 8.2 | 0.83 | 0.95 | 9.0 | 0.20 | 0.71 | 27.7 | |
| OA3 | 63 | M | 18.3 | 23.3 | 5.3 | 7.7 | 0.91 | 0.98 | 6.8 | 0.72 | 0.95 | 8.9 | |
| OA4 | 65 | M | 51.7 | 53.3 | 5.1 | 11.3 | 0.90 | 0.98 | 8.1 | 0.05 | 0.33 | 27.4 | |
| OA5 | 73 | M | 75.0 | 63.3 | 5.6 | 7.4 | 0.64 | 0.96 | 14.4 | 0.05 | 0.21 | 28.4 | |
| OA6 | 67 | M | 53.3 | 55.0 | 4.1 | 9.2 | 0.88 | 0.97 | 14.2 | 0.24 | 0.92 | 22.3 | |
| OA7 | 71 | M | 50.0 | 43.3 | 5.3 | 9.9 | 1.27 | 0.96 | 16.6 | 0.07 | 0.33 | 31.4 | |
| OA8 | 71 | M | 53.3 | 46.7 | – | – | 1.11 | 0.98 | 15.1 | 0.50 | 0.86 | 15.2 | |
| OA9 | 67 | M | 50.0 | 68.3 | 4.7 | 7.0 | 1.04 | 0.97 | 8.9 | 0.33 | 0.53 | 23.5 | |
| OA10 | 66 | M | 8.3 | 10.0 | – | – | 1.03 | 0.97 | 10.2 | 0.53 | 0.91 | 13.7 | |
| OA11 | 65 | M | 25.0 | 25.0 | 5.8 | 9.4 | 0.83 | 0.96 | 11.6 | 0.21 | 0.67 | 24.4 | |
| OA12 | 66 | F | 46.7 | 53.3 | 5.7 | 8.6 | 1.17 | 0.92 | 16.9 | 0.15 | 0.49 | 30.7 | |
| OA13 | 65 | F | 56.7 | 56.7 | 5.9 | 9.8 | 0.77 | 0.98 | 12.6 | 0.08 | 0.44 | 32.8 | |
| OA14 | 74 | F | 28.3 | 30.0 | 5.6 | 11.9 | 0.92 | 0.96 | 10.9 | 0.11 | 0.51 | 25.2 | |
The location of the prominent spectral notch at 0° in elevation has been reported to correlate with concha height (Algazi et al. 2001), where a larger concha results in a spectral notch in the lower frequency range. Linear regression, however, showed no significant correlation between concha height and spectral notch at 0° in elevation in our data (t 17 = −1.0, P = 0.34). However, our data did yield a significant relationship between concha height and the spectral notch at more extreme, especially lower, elevations (for −40° in elevation t 17 = −2.2, P < 0.05). Comparing our data with the CIPIC database (Algazi et al. 2001) also shows a significant relationship between concha height and the spectral notch at the same elevation (for −40° in elevation t54 = −5.0, P < 10−4).
Localization response
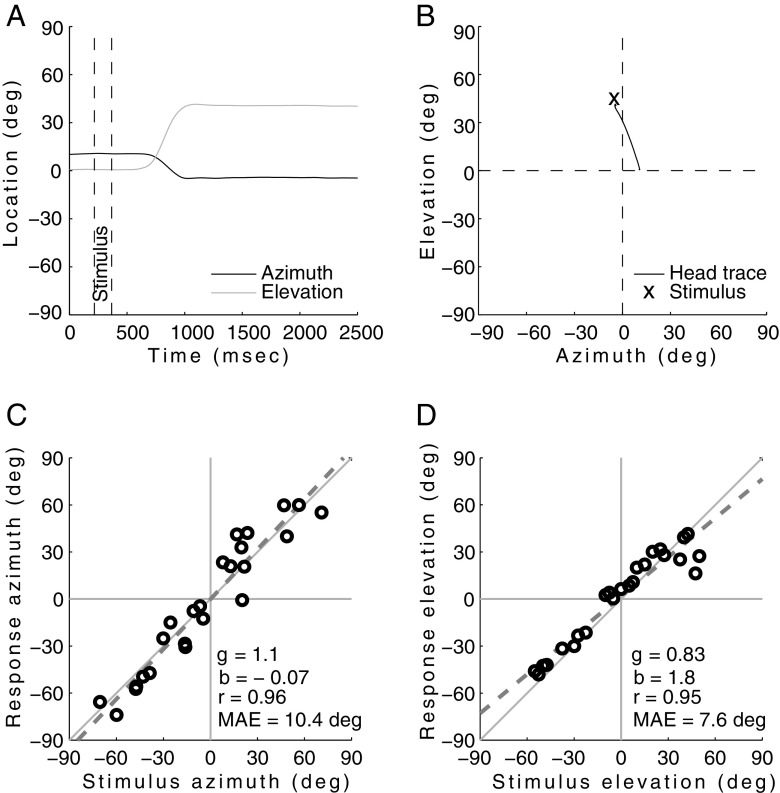
Figure 5A, B show a typical example of a localizing head saccade of child C11 (9 years old) as location versus time (Fig. 5A), as well as its spatial trajectory in azimuth-elevation coordinates (Fig. 5B). The subsequent stimulus–response relations for all localization responses to the 0.5–20-kHz BB noise bursts of this subject are displayed in Figure 5C for azimuth, and in Figure 5D for the elevation direction. The linear regression analysis demonstrates accurate (high gain) and precise (low variability) localization performance in azimuth (g = 1.1, b = −0.07, r = 0.96). Localization in elevation yielded a slightly lower gain than for azimuth (here: g = 0.83, b = 1.8, r = 0.95), but responses were quite consistent. Note that perfect localization would mean that all individual responses would be exactly on the diagonal with slope +1.0.
FIG. 5.
A typical head saccade (A and B) with horizontal and vertical head positions. Subsequently shown is the localization response in azimuth (C) and elevation (D) of all 0.5–20-kHz stimuli for C11 from the children’s group. Also displayed is the mean absolute error (MAE) of the responses.
Azimuth localization
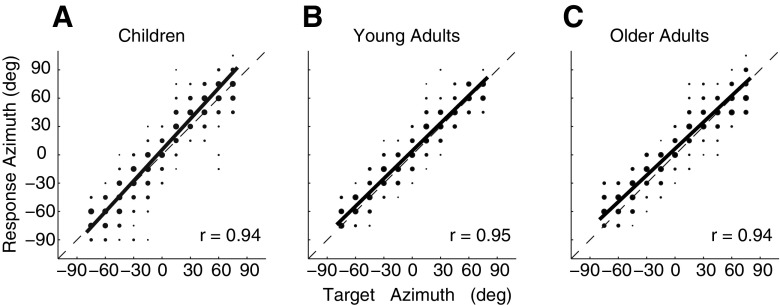
Figure 6 summarizes azimuth localization performance for all stimulus conditions for the children (a), young adults (b), and older adults (c). Localization responses were pooled across stimulus conditions. This figure demonstrates accurate and precise localization in azimuth (high gains, biases close to 0°, and high correlation coefficients) for all three subject groups.
FIG. 6.
Comparison between kids (A), young adults (B), and older adults (C) for the azimuthal localization responses. In this figure, a linear regression is made to the data of all subjects in the corresponding age group.
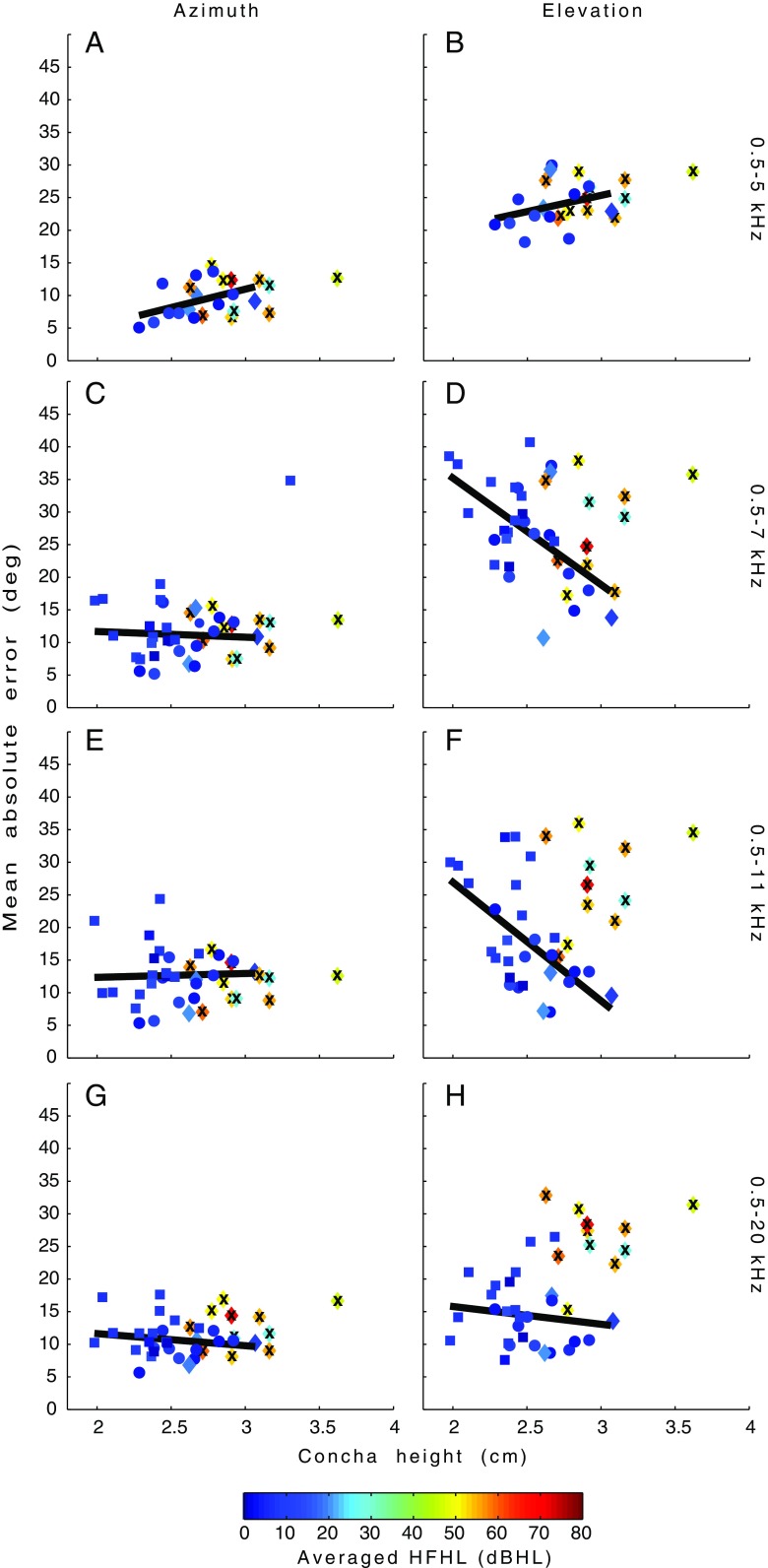
Figure 7 shows the MAE (Eq. 3) in azimuth (left-hand panels) and elevation (right-hand panels) against concha height for each individual listener in the four BB conditions. The amount of HFHL is averaged for both ears for the frequencies 4, 8, and 11 kHz and is indicated by color (in dBHL). The symbol shape indicates whether the MAE was obtained from children (squares), young adults (circles), or older adults (diamonds). Children with unknown audiograms are plotted with the average hearing levels of the measured children. Linear regression on the MAE of all listeners with a hearing loss below 50 dBHL for left or right ear at 11 kHz (indicated by the blue squares, circles, and diamonds; N = 28) shows that azimuth localization performance is independent of concha height. Furthermore, the data indicate that listeners with a high HFHL (indicated by the warm-colored diamonds with cross; N = 11) performed equally well as the other listeners (i.e., low MAEs).
FIG. 7.
Azimuth and elevation MAE for all subjects plotted against their concha height in cm for the 0.5–5-, 0.5–7-, 0.5–11-, and 0.5–20-kHz conditions. The children’s data is plotted in squares and the young adults in circles. The diamonds indicate the older adults. The color represents average HFHL in dBHL as shown in the colorbar. The HFHL is an average of the hearing levels of both ears for 4, 8, and 11 kHz. Data which are not included in the regression due to significant HFHL (>50 dBHL at 11 kHz) are indicated with a “X”.
Elevation localization
Panels b, d, f, and h (Fig. 7) quantify the relation between concha height, age group, and averaged HFHL versus the measured MAE in elevation for the four different stimuli (same format as the azimuth data). Panel b shows the poor elevation performance for BB noises that only contain frequencies up to 5 kHz. When stimuli also contain higher frequencies (Fig. 7D, 7) subjects with limited hearing loss and with larger ears start to improve their localization responses (four older adults, and two young adults in Fig. 7D), whereas subjects with smaller ears (especially found among the children) improve less, or not at all. Panel h shows that the latter group only yields good localization performance when stimuli also contain the highest frequencies. Furthermore, panel h indicates no significant improvement in elevation localization for the older adults with significant HFHL (warm colors and crosses) even for the largest-bandwidth sounds (0.5–20 kHz).
For the 0.5–11- and 0.5–20-kHz BB noises, subjects with HFHL from 30–40 dBHL and higher do not receive sufficient high-frequency information to enable localization in the vertical plane. Their hearing loss is situated in the 4–11-kHz region, which is the most informative region of the DTFs (see Table 1).
Linear regression on the MAEs versus concha height for listeners with hearing loss better than 50 dBHL at 11 kHz (blue circles and diamonds without “X”) shows that elevation performance is independent of the listener’s concha height in the 0.5–5- and 0.5–20-kHz conditions. Interestingly, the significant slope of the regression lines in the 0.5–7-kHz (panel d) and 0.5–11-kHz (panel f) conditions (t 26 = −3.19, P < 0.01 and t 26 = −3.48, P < 0.01, respectively) indicate that listeners with larger conchas and limited HFHL were able to localize the band-limited stimuli more accurately than (younger) listeners with smaller conchas.
For the 0.5–20-kHz condition, the MAEs in elevation were the lowest of all four sound bandwidths, although they were still slightly larger than the MAEs in azimuth (cf. Fig. 7 G, H). This is further corroborated when comparing the gains and correlation coefficients for this condition in azimuth and elevation (see Table 1).
Discussion
Azimuth localization
Our results demonstrate that despite significant HFHL, older adults were able to accurately localize sounds in the azimuth direction. Our data therefore suggest that the use of binaural sound-localization mechanisms in our group of older adults has not deteriorated. These results contrast with Dobreva et al. (2011), who reported reduced localization performance in the horizontal plane with advancing age. Furthermore, this is the first study to demonstrate accurate sound-localization performance under open-loop conditions in a completely dark environment by children from 7 to 11 years of age. Our results thus extend and better quantify observations from earlier studies (Van Deun et al. 2009; Lovett et al. 2012).
Elevation localization
Interestingly, older adults with large ears and a limited HFHL demonstrated a statistically significant improvement in their elevation localization abilities for the limited-bandwidth noises (0.5–7 kHz and 0.5–11 kH; panels D and F, Fig. 7), although none of them could accurately localize the 0.5–5 kHz stimuli in elevation (panel B, Fig. 7). Subjects with smaller ears (especially children) demonstrated less accurate elevation performance in the 0.5–7- and 0.5–11-kHz conditions, when compared to the listeners with larger ears (especially young and older adults) and limited hearing loss. The children’s localization performance for the 0.5–20 kHz stimuli was as well as for the young and older adults with the smallest HFHL. We conclude that larger ears significantly, but not completely, compensate for the hearing loss at higher frequencies.
Although the stimulus–response correlations were typically high for stimuli with the highest frequency content (the 0.5–20-kHz condition), the elevation gains were generally somewhat lower than the azimuth gains (Table 1). Different factors could underlie these lower elevation gains. First, subjects could have adopted a strategy to undershoot in elevation as a general feature of head-motor pointing behavior. Indeed, eye-head gaze-motor studies have demonstrated that in the vertical direction, the contribution of the eye movement to the total gaze shift is typically larger than in the horizontal direction. Thus, in normal gaze-motor control to auditory and visual stimuli, the head tends to move more in the horizontal direction, and less in the vertical direction, possibly to minimize effort against gravity (Goossens and Van Opstal 1997). However, because similar undershoots in elevation were reported for head-fixed saccadic eye movements to sounds (Hofman et al. 1998), for auditory target localization with a joystick (Dobreva et al. 2012), and because we used a head-fixed visual pointer to direct the head to the perceived sound location, a simple head-motor strategy is an unlikely explanation for the lower elevation gains in the present experiments.
An alternative explanation for lower elevation gains might be provided by an optimal statistical response strategy, as dictated by Bayesian inference. According to this idea, the sensorimotor response is optimized to reduce overall endpoint variability (i.e., maximize precision) and mean localization error (maximizing accuracy), by accounting for the inherent noise in the neural processing pathways, uncertainty about the stimulus environment, and by using prior (learned, expected) information about potential target locations (Kording and Wolpert 2006; Fischer and Pena 2011). Since the spectral elevation cues are more complex than the robust binaural difference cues, and hence their processing more vulnerable to perturbation by noise, sensory uncertainty about elevation may be considered larger than for azimuth. This is corroborated by the larger variance in response elevation than in azimuth (Hofman and Van Opstal 1998). In case that the prior estimate assumes a Gaussian distribution of potential targets around straight ahead (elevation zero), the Bayesian theory then predicts that the optimal strategy to deal with such uncertainty is to let the straight-ahead prior estimate dominate the responses. As a result, the elevation response gain will be reduced more than the azimuth gain.
Ear morphology
In contrast to an earlier study by Algazi et al. (2001), our data showed no significant relation between concha height and the spectral notch at 0° in elevation (f 0). However, our data did show a significant relation at more extreme elevations (data not shown). In a simplified acoustic model of the pinna, one could assume that the location of the spectral notch at a given elevation is primarily caused by interference between sound waves that enter the ear canal directly, and the first reflection within the pinna. In such a model, the location of f 0 would be primarily attributed to the concha width (which showed no significant increase with age). In contrast, the location of the spectral notch, e.g., −40° in elevation, would be mainly affected by the height of the concha (which does increase with age, leading to a shift of f −40 toward lower frequencies). According to this simple model, when a reflective surface at distance x from a point P lengthens with ∆x, the frequency notch at point P will shift:
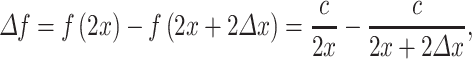 |
4 |
with c the speed of sound. This leads to:
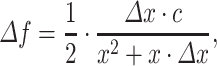 |
5 |
Increasing the reflection distance by a small amount (e.g., ∆x=1 mm) leads to a change in the frequency notch of:
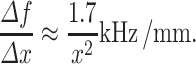 |
6 |
This gives an approximation of how much the frequency notch shifts per millimeter of concha growth. Applying this simple model to the shape of the concha leads to the intuitive idea that the growing concha might pivot the elevation-dependent spectral notch (at more extreme elevations) around the relatively constant f 0. A more sophisticated quantitative model about the specific frequencies of multiple notches and about how the concha’s size and shape determine the DTFs requires further research that includes exact (3D) measurements of the concha and ear canal in relation to the DTFs.
Summary
We investigated whether sound-localization abilities of subjects of three different age groups to brief BB sound bursts could be explained by the age-related factors of high-frequency hearing loss and ear size. Previously considered, but qualitative (potentially negative) effects such as age-related immaturity (in children) or degeneration (in the older adults) of the neural processing of binaural acoustic cues do not explain the sound-localization performance of our listeners.
Younger listeners
The present study demonstrates that children from 7 to 11 years already localize BB noises in azimuth and elevation (0.5–20 kHz) as well as adults, even in a completely dark environment under open-loop conditions. However, because of their smaller ears, children require frequencies exceeding 11 kHz to localize accurately in elevation. Listeners with larger ears (especially young and older adults) require less high-frequency information (Fig. 7D, F), provided the high-frequency cut-off in the BB noise exceeded 5 kHz (Fig. 7B).
Older listeners
Older adults demonstrated poor localization performance in elevation due to significant high-frequency hearing loss, which was found in the majority of older adults (Fig. 2). In contrast, sound-localization performance in azimuth was unaffected (Figs. 6 and 7A, C, E, G), which contradicts earlier results by Dobreva et al. (2011). Possibly, this difference in results might originate from methodological differences in target pointing (a natural head-orienting response vs. instructed, more indirect, joystick pointing). Although not specifically dissociated in our experiments, our results suggest that the subcortical processing of binaural ITD and ILD acoustic cues for sound localization is not deteriorated in older adults. To what extent ITDs contribute to the sound-localization abilities of older adults requires further study that particularly targets the ITD pathway for azimuth, e.g., by presenting low-pass noise stimuli (frequencies < 1.5 kHz).
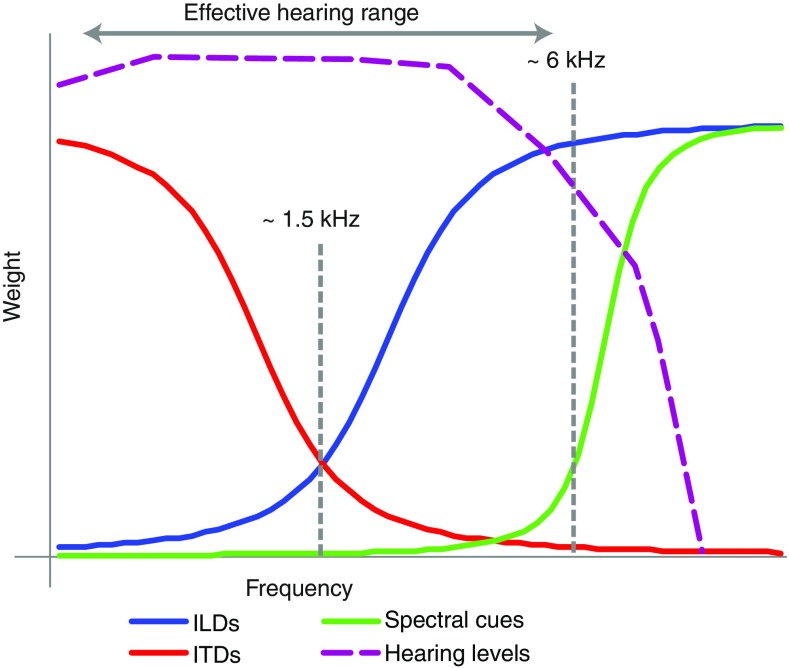
Figure 8 summarizes the available localization cues to an average older listener with HFHL (purple dashed line). The effective hearing range (defined as the stimulus intensity minus the hearing thresholds) of the older adults limits the useable cues to the ITDs (red line) and lower-range ILDs (blue line), while the high-frequency spectral cues (green line) are largely lost. This scheme explains the poor elevation performance, while azimuth localization remains unaffected. Ideally, larger ears could compensate for the HFHL through a considerable shift of the green line (Fig. 8) into the effective hearing range, which indeed seemed to be the case for some listeners (Fig. 7D, F). Nonetheless, our results show that the modest shift of the spectral cues due to ear growth cannot keep up with the considerable hearing loss found in most older adults. Hence, the apparent compensation of HFHL by ear growth found in some listeners is not a general feature of the auditory system of older adults. Rather, some older adults benefit from a relatively limited HFHL, combined with considerable ear growth and their neural plasticity to cope with these changes (panel f, Fig. 7).
FIG. 8.
Relative weight of the ILDs (blue line), ITDs (red line), DTF (green line), and hearing levels (purple dotted line) plotted against frequency. The effective hearing range is the stimulus intensity minus the hearing levels.
Acknowledgments
We thank H. Kleijnen, P. Bens, T. Arts and M. Verbruggen for their technical support. We thank teachers and children from the primary school Het Talent (Lent, The Netherlands) for their enthusiast cooperation. This research was funded by the William Demants og Hustru Ida Emilies Fond and the Dutch Organization for Scientific Research, through a VICI grant within Earth and Life Sciences of NWO (project grant ALW/VICI 865.05.003; AJVO, MMVW), the Radboud University Nijmegen (AJVO), the Donders Centre for Neuroscience (MJHA), and the Department of Otorhinolaryngology at the Radboud University Medical Centre (AFMS).
References
- Abel SM, Hay VH. Sound localization. The interaction of aging, hearing loss and hearing protection. Scand Audiol. 1996;25:3–12. doi: 10.3109/01050399609047549. [DOI] [PubMed] [Google Scholar]
- Abel SM, Giguère C, Consoli A, Papsin BC. The effect of aging on horizontal plane sound localization. J Acoust Soc Am. 2000;108:743–752. doi: 10.1121/1.429607. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Snik AF, Hol MK, van Esch TE, Cremers CW, Van Wanrooij MM, Van Opstal AJ. Improved horizontal directional hearing in bone conduction device users with acquired unilateral conductive hearing loss. J Assoc Res Otolaryngol. 2011;12:1–11. doi: 10.1007/s10162-010-0235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algazi VR, Duda RO, Thompson DM, Avendano C. The CIPIC HRTF database. In: IEEE Workshop on Applications of Signal Processing to Audio and Acoustics. New York: New Paltz; 2001. pp. 99–102. [Google Scholar]
- Ashmead DH, Clifton RK, Perris EE. Precision of auditory localization in human infants. Dev Psychol. 1987;23:641–647. doi: 10.1037/0012-1649.23.5.641. [DOI] [Google Scholar]
- Batteau DW. The role of the pinna in human sound localization. Proc R Soc Lond B Biol Sci. 1967;168:158–180. doi: 10.1098/rspb.1967.0058. [DOI] [PubMed] [Google Scholar]
- Blauert J. Spatial hearing. The psychophysics of human sound localization. Cambridge: MIT; 1997. [Google Scholar]
- Brant LJ, Fozard JL. Age-changes in pure-tone hearing thresholds in a longitudinal-study of normal human aging. J Acoust Soc Am. 1990;88:813–820. doi: 10.1121/1.399731. [DOI] [PubMed] [Google Scholar]
- Bremen P, van Wanrooij MM, van Opstal AJ. Pinna Cues determine orienting response modes to synchronous sounds in elevation. J Neurosci. 2010;30:194–204. doi: 10.1523/JNEUROSCI.2982-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge M, Burger W. Ear biometrics. Springer Int Ser Eng Comput Sci. 1997;479:273–285. [Google Scholar]
- Cheng CI, Wakefield GH. Introduction to head-related transfer functions (HRTFs): representations of HRTFs in time, frequency, and space. J Audio Eng Soc. 2001;49:231–249. [Google Scholar]
- Dobreva MS, O’Neill WE, Paige GD. The influence of aging on human sound localization. J Neurophysiol. 2011;105:2471–2486. doi: 10.1152/jn.00951.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva MS, O’Neill WE, Paige GD. Influence of age, spatial memory, and ocular fixation on localization of auditory, visual, and bimodal targets by human subjects. Exp Brain Res. 2012;223:441–455. doi: 10.1007/s00221-012-3270-x. [DOI] [PubMed] [Google Scholar]
- Fischer BJ, Pena JL. Owl’s behavior and neural representation predicted by Bayesian inference. Nat Neurosci. 2011;14:1061–U1163. doi: 10.1038/nn.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens HH, Van Opstal AJ. Human eye-head coordination in two dimensions under different sensorimotor conditions. Exp Brain Res. 1997;114:542–560. doi: 10.1007/PL00005663. [DOI] [PubMed] [Google Scholar]
- Heathcote JA (1995) Why do old men have big ears? BMJ:23–30 [DOI] [PMC free article] [PubMed]
- Hofman PM, Van Opstal AJ. Spectro-temporal factors in two-dimensional human sound localization. J Acoust Soc Am. 1998;103:2634–2648. doi: 10.1121/1.422784. [DOI] [PubMed] [Google Scholar]
- Hofman PM, Van Riswick JGA, Van Opstal AJ. Relearning sound localization with new ears. Nat Neurosci. 1998;1:417–421. doi: 10.1038/1633. [DOI] [PubMed] [Google Scholar]
- Kording KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci. 2006;10:319–326. doi: 10.1016/j.tics.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Konishi M (1979) Mechanisms of sound localization in the barn owl (Tyto alba). J Comp Physiol A 133:13–21
- Lopez-Poveda EA, Meddis R. A physical model of sound diffraction and reflections in the human concha. J Acoust Soc Am. 1996;100:32–48. doi: 10.1121/1.417208. [DOI] [PubMed] [Google Scholar]
- Lovett RE, Kitterick PT, Huang S, Summerfield AQ. The developmental trajectory of spatial listening skills in normal-hearing children. J Speech Lang Hear Res. 2012;55:865–878. doi: 10.1044/1092-4388(2011/11-0096). [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Narrow-band sound localization related to external ear acoustics. J Acoust Soc Am. 1992;92:2607–2624. doi: 10.1121/1.404400. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annu Rev Psychol. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Morrongiello BA. Infants localization of sounds along the horizontal axis—estimates of minimum audible angle. Dev Psychol. 1988;24:8–13. doi: 10.1037/0012-1649.24.1.8. [DOI] [Google Scholar]
- Niemitz C. Human ears grow throughout the entire lifetime according to complicated and sexually dimorphic patterns—conclusions from a cross-sectional analysis. Anthropol Anz. 2007;65:391–413. [PubMed] [Google Scholar]
- Noble W, Byrne D, Lepage B. Effects on sound localization of configuration and type of hearing impairment. J Acoust Soc Am. 1994;95:992–1005. doi: 10.1121/1.408404. [DOI] [PubMed] [Google Scholar]
- Rakerd B, Van der Velde TJ, Hartmann WM. Sound localization in the median sagittal plane by listeners with presbyacusis. J Am Acad Audiol. 1998;9:466–479. [PubMed] [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci. 2007;27:11172–11178. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Age-related changes in temporal processing: implications for speech perception. Semin Hear. 2001;22:227–239. doi: 10.1055/s-2001-15628. [DOI] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104:2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114:1332–1343. doi: 10.1016/S1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Van Deun L, Van Wieringen A, Wouters J. Sound localization, Sound lateralization, and binaural masking level differences in young children with normal hearing. Ear Hear. 2009;30:178–190. doi: 10.1097/AUD.0b013e318194256b. [DOI] [PubMed] [Google Scholar]
- Van Grootel TJ, Van Opstal AJ. Human sound-localization behaviour after multiple changes in eye position. Eur J Neurosci. 2009;29:2233–2246. doi: 10.1111/j.1460-9568.2009.06761.x. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Relearning sound localization with a new ear. J Neurosci. 2005;25:5413–5424. doi: 10.1523/JNEUROSCI.0850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Sound localization under perturbed binaural hearing. J Neurophysiol. 2007;97:715–726. doi: 10.1152/jn.00260.2006. [DOI] [PubMed] [Google Scholar]
- Yin TC. Neural mechanisms of encoding binaural localization cues in the auditory brainstem. In: Oertel DFR, Popper AN, editors. Integrative functions in the mammalian auditory pathway. Heidelberg: Springer; 2002. pp. 99–159. [Google Scholar]
- Young ED, Davis KA. Circuitry and function of the dorsal cochlear nucleus. In: Oertel DFR, Popper AN, editors. Integrative functions in the mammalian auditory pathway. Heidelberg: Springer; 2002. pp. 160–206. [Google Scholar]
- Zwiers MP, Versnel H, Van Opstal AJ. Involvement of monkey inferior colliculus in spatial hearing. J Neurosci. 2004;24:4145–4156. doi: 10.1523/JNEUROSCI.0199-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]