Abstract
Objectives:
The present study was designed to evaluate the effect of aqueous extract of Trigonella foenum-graecum(AqE-TFG) seeds on monosodium glutamate (MSG)-induced dyslipidemia and oxidative stress in Wistar rats.
Materials and Methods:
Neonatal Wistar rats were treated subcutaneously with MSG (4 g/kg b.w.) from day 2 to 14 after birth, on alternate days. After attaining six-weeks of age, MSG-treated rats were administered with AqE-TFG (0.5 and 1 g/kg b.w., orally) or orlistat (10 mg/kg b.w., orally) for 28 days, respectively. Serum chemistry and relevant enzymes in hepato-cardiac tissues were assessed on day 29.
Results:
AqE-TFG produced significant reduction in serum total cholesterol (TC), triglycerides (TGs), lactate dehydrogenase (LDH), aspartate amino transferase (AST), alanine amino transferase (ALT), hepatic and cardiac lipid peroxides (MDA) levels and elevation in serum high density lipoprotein cholesterol (HDL-C), hepatic and cardiac antioxidant enzymes [glutathione (GSH), and superoxide dismutase (SOD) and catalase (CAT)] levels.
Conclusion:
Results were comparable with orlistat, a standard anti-obesity drug, and provide clear evidence that the AqE-TFG treatment offered significant protection against MSG-induced dyslipidemia and oxidative stress, and may play an important role in amelioration of the free radical generated consequences like dyslipidemia and atherosclerosis.
KEY WORDS: Antihyperlipidemic, monosodium glutamate, neonatal, oxidative stress, Trigonella foenum-graecum
Introduction
Monosodium glutamate, one of the most abundant naturally occurring amino acids, is frequently added as a flavor enhancer. MSG is known to have some adverse effects in humans and experimental animals. These include the Chinese restaurant syndrome,[1] neuroexcitotoxicity,[2,3] and obesity.[4] Chronic administration of MSG (4 g/kg b.w.) induces oxidative stress in hepatic and cardiac tissues in experimental animals due to metabolic shifting.[5–7] Increased oxidative stress brings change in the membrane lipids and proteins, which could be responsible for the initiation of metabolic disorders. Oxidative stress is abiochemical disequilibrium occurring due to excessive production of free radicals and reactive oxygen species, which aggravates oxidative damage to biomolecules that cannot becounteracted by antioxidative defense systems.[8,9]
Although some information is available on MSG-induced dyslipidemia and oxidative stress, the studies on the effect of antioxidants, especially those consumed in food, in MSG-induced dyslipidemia and oxidative stress are lacking. Trigonella foenum-graecum Linn. (Fenugreek, family - Fabaceae) has been shown to possess antioxidant activity in different experimental animal models.[10] The purpose of the present study was to investigate the protective effect of aqueous extract of Trigonella foenum-graecum on MSG-induced dyslipidemia and oxidative stress in rats. The dose of aqueous extract of Trigonella foenum-graecum was based on previous inquiries that were held to establish and assess the effect of aqueous extract of Trigonella foenum-graecum(AqE-TFG) and soluble dietary fibre of TFG (Tf-sdf) in different metabolic disorders (i.e. diabetes and dyslipidemia), respectively.[11,12]
Materials and Methods
Trigonella foenum-graecum seeds were purchased from Indian Council for Agricultural Research, New Delhi. The seeds were botanically identified and authenticated by Head, Raw Materials Herbarium and Museum, NISCAIR, New Delhi and a voucher specimen (ref. NISCAIR/RHMD/Consult/-2011-12/1743/43) has been deposited in the herbarium of the Institute.
Preparation of Aqueous Extract of Trigonella foenum-graecum (AqE-TFG) Seeds
Trigonella foenum-graecum seeds were dried at 40°C and finely powdered. Twenty five grams of powdered seeds were extracted with 500 ml of boiling distilled water for 45 min. The heated decoction was left overnight for complete socking, at room temperature. The decoction was filtered; the filtrate was lyophilized and stored in the refrigerator. On lyophilization, the resulting material weighed 1.133 g (4.53% yield). The extract was subjected to the preliminary phytochemical screening as per standard procedure.[13]
Drugs and Chemicals
Monosodium glutamate (MSG) (Sigma, St. Louis, USA), orlistat (Biocon Lab., Bangalore, India), LDH kit (Reckon diagnostics Pvt. Ltd., Vadodara, Gujarat, India), total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), aspartate amino transferase (AST), and alanine amino transferase (ALT) kits (Span Diagnostics Ltd., Vadodara, Gujarat, India) were used in the study. All chemicals were of analytical grade and those required for sensitive tissue assays were purchased from Sigma Chemical Co., St. Louis, USA, HiMedia, and SD Fine Chemicals.
Experimental Design
The experimental protocol was approved by the Institutional Animal Ethics Committee of Hamdard University, New Delhi, which is registered with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (registration no. 173/CPCSEA, dated January 28, 2000).
Newborn Wistar rat pups were injected subcutaneously with MSG at a dose of 4 g/kg body weight dissolved in normal saline on alternate days seven times, i.e. on postnatal day 2, 4, 6, 8, 10, 12, and 14, respectively.[14] Normal control animals received only normal saline on these days. After weaning on postnatal day 21, the female rat pups were excluded from the study and the male rat pups were housed in polypropylene cages under controlled conditions (room temperature 25 ± 2°C, air humidity 50 ± 15%, and photoperiod of 12 h Light:Dark cycle)[15] and had free access to commercial pellet diet (Amrut Rat Feed, Nav Maharashtra Chakan Oil Mills Ltd., Delhi, India) and water ad libitum. After attaining six-weeks of age, normal control rats (n=10) were treated with normal saline (2 ml/kg b.w., orally), while MSG-treated rats (n=10 pups in each group) were administered with AqE-TFG (0.5 and 1 g/kg b.w., orally) or orlistat (10 mg/kg b.w., orally) dissolved in normal saline for 28 days, respectively.
Determination of Biochemical Parameters
On the 29th day, blood was collected from the retro-orbital plexus of overnight fasted rats under light ether anesthesia and serum was separated by centrifugation at 3000 rpm for 15 min and frozen at –20°C for estimation of TC and HDL-C,[16] TGs,[17] LDH,[18] AST, and ALT[19] with commercially available kits using ultra violet (UV)-visible spectrophotometer (Shimadzu, UV-1601, Japan). The liver and heart were isolated and washed in ice-cold physiological saline and stored at –20°C for biochemical estimations of hepatic and cardiac lipid peroxides (MDA), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT).
MDA, a product of membrane lipid peroxidation, was estimated by the method of Okhawa et al.,[20] and expressed as nmoles/mg protein. Protein was estimated by the method of Lowry et al.[21] GSH content was estimated by the method of Sedlack and Lindsay.[22] The absorbance of reaction mixture was read within five min of addition of DTNB at 412 nm using UV-spectrophotometer, against a reagent blank. GSH activity was expressed as μmol of P liberated/min/mg protein. SOD content was estimated by the method of Marklund and Marklund.[23] The absorbance of reaction mixture was read at 420 nm at one-minute intervals for three minutes after addition of pyrogallol, which was inhibited by the presence of SOD. The results were expressed as U/mg protein. CAT content was estimated by the method of Clairborne.[24] The disappearance of H2O2was monitored at 240-nm wavelength at one-minute interval for three minutes. Catalase activity was expressed as nmol H2O2/min/mg protein.
All statistical analyses were performed using GraphPad Prism 3.0 (GraphPad, San Diego, CA). All results were expressed as mean ± S.E.M. The data were analyzed with one-way ANOVA followed by Dunnett's test. A statistical difference of P< 0.05 was considered significant in all cases.
Results
The preliminary phytochemical screening of AqE-TFG, revealed the presence of saponin glycoside, carbohydrates, and proteins.
Effect of AqE-TFG on Serum Biochemical Parameters
The serum TC, TGs, and HDL-C levelsare depicted in Table 1. MSG control group showed a significant (P< 0.01) increase in serum TC and TGs levels and a significant (P< 0.01) decrease in HDL-C level when compared with the normal control group. Administration of AqE-TFG (0.5 and 1 g/kg b.w., orally) or orlistat (10 mg/kg b.w., orally) for a period of 28 days caused a significant (P< 0.01) decrease in serum TC and TGs and a significant (P< 0.01) increase in serum HDL-C as compared to the MSG control group. Also, a significant (P < 0.01) decrease in serum TC and TGs levels was observed in the orlistat (10 mg/kg b.w., orally) treated groupas compared to the AqE-TFG (0.5 g/kg b.w., orally) treated group, while there was no significant (P > 0.05) difference between orlistat (10 mg/kg b.w., orally)and the AqE-TFG (1 g/kg b.w., orally) treated groups for serum TC and TGs levels in MSG-treated rats. Furthermore, there was no significant (P > 0.05) difference between serum HDL levels in the orlistat (10 mg/kg b.w., orally) and the AqE-TFG (0.5 and 1 g/kg b.w., orally) treated groups.
Table 1.
Effect of aqueous extract of Trigonella foenum-graecum (AqE-TFG) on serum lipid levels in monosodium glutamate (MSG) treated rats

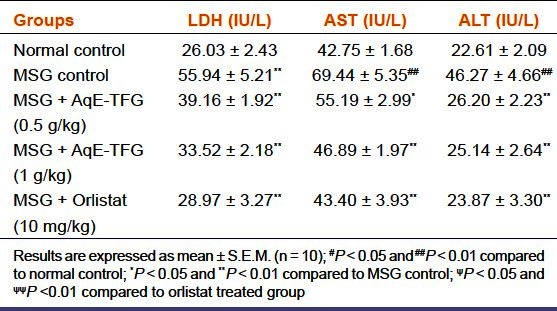
The serum LDH, AST, and ALT levels are depicted in Table 2. MSG control group showed a significant (P < 0.01) increase in serum LDH, AST, and ALT levels as compared to the normal control group. AqE-TFG (0.5 and 1 g/kg b.w., orally) or orlistat (10 mg/kg b.w., orally) administration caused a significant (P < 0.05 and P< 0.01) decrease in these levels as compared to the MSG control group. Also, there was no significant (P > 0.05) difference between orlistat(10 mg/kg b.w., orally)and AqE-TFG (0.5 and 1 g/kg b.w., orally) treated groups for serum LDH, AST, and ALT levels in MSG-treated rats.
Table 2.
Effect of aqueous extract of Trigonella foenum-graecum(AqE-TFG) on serum lactate dehydrogenase (LDH), aspartate transaminase (AST), and alanine transaminase (ALT) levels in monosodium glutamate (MSG) treated rats
Effect of AqE-TFG on lipid peroxides (MDA), GSH, SOD and CAT levels
MSG control rats showed a significant (P< 0.01) increase in hepatic and cardiac MDA levels as compared to the normal control group. Administration of AqE-TFG (0.5 and 1 g/kg b.w., orally) or orlistat (10 mg/kg b.w., orally) caused a significant (P < 0.05 and P< 0.01) decrease in both hepatic and cardiac MDA levels as compared to the MSG control group [Figure 1]. Also, there was no significant (P > 0.05) difference between the orlistat (10 mg/kg b.w., orally) and AqE-TFG (0.5 and 1 g/kg b.w., orally) treated groups for hepatic and cardiac MDA levels in MSG-treated rats.
Figure 1.
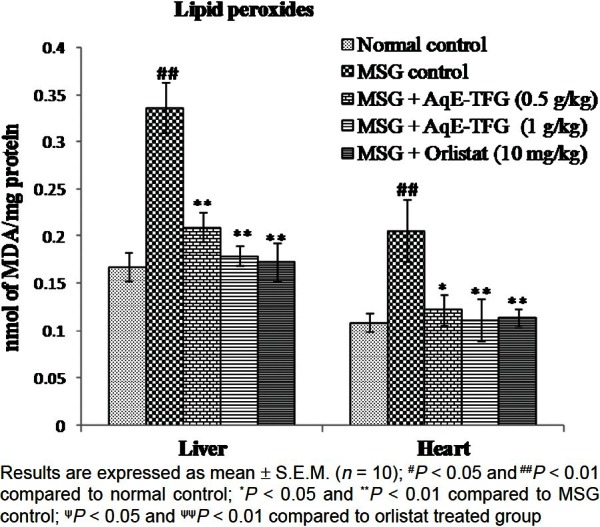
Effect of aqueous extract of Trigonella foenum-graecum (AqE-TFG) on hepatic and cardiac lipid peroxides (MDA) levels in monosodium glutamate (MSG) treated rats
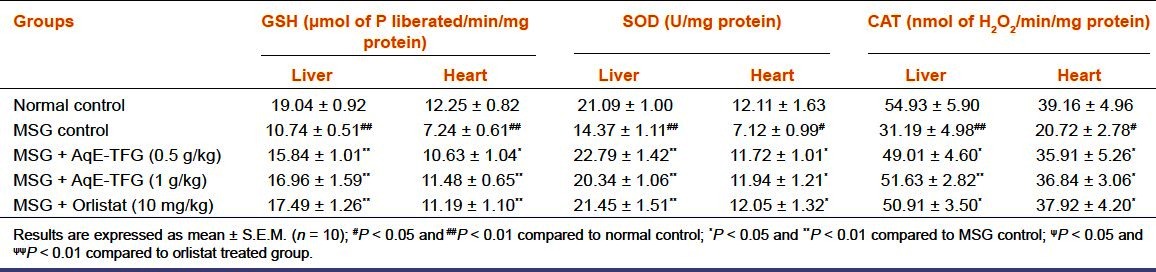
The MSG control group showed a significant (P < 0.01) depletion in hepatic and cardiac GSH, SOD, and CAT levels as compared to normal control group. Administration of AqE-TFG (0.5 and 1 g/kg b.w., orally) or orlistat (10 mg/kg b.w., orally) caused a significant (P < 0.05 and P < 0.01) increase in these levels as compared to MSG control group [Table 3]. Also, there was no significant (P > 0.05) difference between orlistat (10 mg/kg b.w., orally) and AqE-TFG (0.5 and 1 g/kg b.w., orally) treated groups for hepatic and cardiac GSH, SOD and CAT levels in MSG-treated rats.
Table 3.
Effect of aqueous extract of Trigonella foenum-graecum (AqE-TFG) on hepatic and cardiac reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) levels in monosodium glutamate (MSG) treated rats
Discussion
In the present study, the MSG control rats showed dyslipidemia and oxidative stress perhaps due to the destruction of hypothalamic arcuate nucleus,which is in agreement with previous reports.[25–28] The increased total cholesterol levels and hypertriglyceridemia in MSG-treated animals is a high risk dysmetabolic situation. Metabolic disturbance is the main cause of dyslipidemia, which is a major risk factor for cardiovascular diseases.[29] Administration of AqE-TFG or orlistat resulted in significant reduction in levels of TC, TGs and elevation in HDL-C which is similar to other findings.[30] It is well known that hyperlipidemia decreases the strength of the antioxidative defense system.[31] Thus, the present study hypothesizes that the possible explanation for improvement in dyslipidemia following administration of AqE-TFG or orlistat may be due to reduction in oxidative stress in MSG-treated rats.
As per earlier reports,[32] the ALT enzyme is a sensitive marker of liver damage and AST levels are predictive of damage to the liver and other organs with high metabolic activity (brain, heart, and lungs). Moreover, LDH is a sensitive biomarker for cardiac diseases. Hence any necrosis or membrane damage to the liver and heart leads to leakage of these enzymes into the blood circulation.[28,33–35] The results of our study showed that MSG-treated rats were more prone to hepatotoxicity and cardiotoxicity as evidenced by increased levels of serum LDH, AST, and ALT. Administration of AqE-TFG significantly reduced the elevated LDH, AST, and ALT levels, which could be attributed to the protective effect on hepatic and cardiac tissues.
The serum malondialdehyde (MDA) concentration, a marker of lipid peroxidation, increased in MSG-treated rats. GSH play an important role in catalysis, metabolism, and transport. It protects the cells against free radical peroxides and other toxic compounds.[36,37] The reduction in GSH levels in our study are in agreement with Onyema et al.,[28] who suggested that MSG-induced lipid peroxidation contributed to the depletion of tissue levels of GSH. SOD catalyzes the breakdown of superoxide anion into oxygen and hydrogen peroxide,[38] and CAT is a key component of the antioxidant defense system, which catalyzes the conversion of hydrogen peroxide to water and oxygen, using an iron or manganese cofactor.[39] In our study, MSG control rats showed decreased activities of SOD and CAT enzymes, which is again in agreement with earlier reports.[5,6] Therefore, it appears that MSG causes the induction of oxidative stress in the liver and heart, which as a consequence, may lead to liver disorders (like dyslipidemia) and cardiac complications (like atherosclerosis).
Treatment with AqE-TFG caused significant reduction in lipid peroxides levels and elevation in antioxidant enzymes GSH, SOD, and CAT indicating the protection of the hepatic and cardiac tissues from the damaging effect of MSG. The results of our study show that the indices of hepatic and cardiac risk predictor (TC, TGs, HDL-C), hepatic and cardiac risk biomarkers (LDH, AST, and ALT), indices of lipid peroxidation (MDA) and the hepatic and cardiac tissue antioxidant enzymes (GSH, SOD and CAT) were significantly restored to normal levels with AqE-TFG treatment. Results were also comparable with orlistat, a standard antiobesity drug.
The major constituents of AqE-TFG are saponin glycoside, carbohydrates, and proteins. It is well-established that saponins are known to be hypocholesterolemic and antioxidant in animal species.[40,41] Therefore, it is possible that the presence of saponins in AqE-TFG is responsible for the observed lipid lowering and antioxidant activity. In conclusion, Trigonella foenum-graecum seems to have an important role in preventing the development of MSG-induced dyslipidemia and oxidative stress. The protective effect of Trigonella foenum-graecum may be related to its free radical scavenging and membrane stabilizing property, and may be helpful in protection from the metabolic disorders like dyslipidemia and atherosclerosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Schaumburg HH, Byck R, Gerstl R, Mashman JH. Monosodium L-glutamate: Its pharmacology and role in the Chineserestaurant syndrome. Science. 1969;163:826–8. doi: 10.1126/science.163.3869.826. [DOI] [PubMed] [Google Scholar]
- 2.Park CH, Choi SH, Piao Y, Kim S, Lee YJ, Kim HS, et al. Glutamate and aspartate impair memory retention and damage hypothalamic neurons in adult mice. Toxicol Lett. 2000;115:117–25. doi: 10.1016/s0378-4274(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 3.Singh P, Mann KA, Mangat HK, Kaur G. Prolonged glutamate excitotoxicity: Effects on mitochondrial antioxidants and antioxidant enzymes. Mol Cell Biochem. 2003;243:139–45. doi: 10.1023/a:1021668314070. [DOI] [PubMed] [Google Scholar]
- 4.Gobatto CA, Mello MA, Souza CT, Ribeiro IA. The monosodium glutamate (MSG) obese rat as a model for the study of exercise in obesity. Res Commun Mol Pathol Pharmacol. 2002;111:89–101. [PubMed] [Google Scholar]
- 5.Diniz YS, Fernandes AA, Campos KE, Mani F, Ribas BO, Novelli EL. Toxicity of hyper caloricdiet and monoso diumglutamate: Oxidative stress and metabolic shifting in hepatictissue. Food Chem Toxicol. 2004;42:313–9. doi: 10.1016/j.fct.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Abdel Baky NA, Mohamed AM, Faddah LM. Protective effect of N-acetyl cysteine and/or pro vitamin A against monosodium glutamate-induced cardiopathy in rats. J Pharmacol Toxicol. 2009;4:178–93. [Google Scholar]
- 7.Soliman AM. Extract of Coelaturaaegyptiaca, a freshwater clam, ameliorates hepatic oxidative stress induced by monosodium glutamate in rats. Afr J Pharm Pharmacol. 2011;5:398–408. [Google Scholar]
- 8.Foyer CH, Pellny TK, Locato V, De Gara L. Analysis of redox relationships in the plant cell cycle: Determinations of ascorbate, glutathione and poly (ADPribose) polymerase (PARP) in plant cell cultures. Methods Mol Biol. 2009;476:193–209. doi: 10.1007/978-1-59745-129-1_14. [DOI] [PubMed] [Google Scholar]
- 9.Minibayeva F, Kolesnikov O, Chasov A, Beckett RP, Lüthje S, Vylegzhanina N, et al. Wound-induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant Cell Environ. 2009;32:497–508. doi: 10.1111/j.1365-3040.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaviarasan S, Ramamurty N, Gunasekaran P, Varalakshmi E, Anuradha CV. Fenugreek (Trigonellafoenumgraecum) seed prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006;41:267–73. doi: 10.1093/alcalc/agl020. [DOI] [PubMed] [Google Scholar]
- 11.Zia T, Hasnain SN, Hasan SK. Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L. (methi) in normal mice. J Ethnopharmacol. 2001;75:191–5. doi: 10.1016/s0378-8741(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 12.Hannan JM, Ali L, Rokeya B, Khaleque J, Akhter M, Flatt PR, et al. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr. 2007;97:514–21. doi: 10.1017/S0007114507657869. [DOI] [PubMed] [Google Scholar]
- 13.Kokate CK. 4th ed. New Delhi: Vallabh Prakashan; 1994. Practical pharmacognosy; pp. 107–11. [Google Scholar]
- 14.Li PP, Shan S, Chen YT, Ning ZQ, Sun SJ, Liu Q, et al. The PPAR alpha/gamma dual agonist chiglitazar improves insulin resistance and dyslipidemia in MSG obese rats. Br J Pharmacol. 2006;148:610–8. doi: 10.1038/sj.bjp.0706745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oluba O, Onyeneke E, Idonije B, Eidangbe G. Effect of soy protein on monosodium glutamate (MSG)-induced obesity in rats. Asian J Phar Biol Res. 2011;1:8–14. [Google Scholar]
- 16.Wybenga DR, Pileggi VJ, Dirstine PH, Di Giorgio J. Direct manual determination of serum total cholesterol with a single stable reagent. Clin Chem. 1970;16:980–4. [PubMed] [Google Scholar]
- 17.Stein EA, Myers GL. National Cholesterol Education Program recommendations for triglyceride measurement: Executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995;41:1421–6. [PubMed] [Google Scholar]
- 18.Lum G, Gambino SR. A comparison of serum versus heparinized plasma for routine chemistry tests. Am J Clin Pathol. 1974;61:108–13. doi: 10.1093/ajcp/61.1.108. [DOI] [PubMed] [Google Scholar]
- 19.Rietman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Bio Chem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Sedlak J, Lindsay RH. Estimation of total, protein bound and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 23.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Bio Chem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Clairborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook of methods for oxygen radical research. Boca Raton, Florida, USA: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 25.Malik VB, Ahluwalia P. Studies on effect of monosodium glutamate (MSG) on various fractions of lipids and certain carbohydrate metabolic enzymes in liver and blood of adult male mice. Toxicol Lett. 1994;74:69–77. doi: 10.1016/0378-4274(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 26.Ahluwalia P, Tewari K, Choudhary P. Studies on the effects of monosodium glutamate (MSG) on oxidative stress in erythrocytes of adult male mice. Toxicol Lett. 1996;84:161–5. doi: 10.1016/0378-4274(95)03612-1. [DOI] [PubMed] [Google Scholar]
- 27.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;(10 Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 28.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–4. [PubMed] [Google Scholar]
- 29.Rizvi F, Iftikhar M, George JP. Beneficial effects of fish liver preparations of sea bass (Lates calcarifer) versus gemfibrozil in high fat diet-induced lipid-intolerant rats. J Med Food. 2003;6:123–8. doi: 10.1089/109662003322233521. [DOI] [PubMed] [Google Scholar]
- 30.Vijayakumar RS, Nalini N. Efficacy of piperine, an alkaloidal constituent from Piper nigrum on erythrocyte antioxidant status in high fat diet and antithyroid drug induced hyperlipidemic rats. Cell Bio Chem Funct. 2006;24:491–8. doi: 10.1002/cbf.1331. [DOI] [PubMed] [Google Scholar]
- 31.Liou W, Chang LY, Geuze HJ, Strous GJ, Crapo JD, Slot JW. Distribution of CuZn superoxide dismutase in rat liver. Free Radic Biol Med. 1993;14:201–7. doi: 10.1016/0891-5849(93)90011-i. [DOI] [PubMed] [Google Scholar]
- 32.Al-Mamary M, Al-Habori M, Al-Aghbari AM, Baker MM. Investigation into the toxicological effects of Catha edulis leaves: A short term study in animals. Phytother Res. 2002;16:127–32. doi: 10.1002/ptr.835. [DOI] [PubMed] [Google Scholar]
- 33.Bain PJ. Liver. In: Latimer KS, Mahaffey EA, Prasse KW, editors. Duncan and Prasse's Veterinary Laboratory Medicine Clinical Pathology. 4th ed. Ames, Iowa: Iowa State Press; 2003. pp. 193–214. [Google Scholar]
- 34.Pari L, Murugan P. Protective role of tetrahydrocurcumin against erythromycin estolate- induced hepatotoxicity. Pharmacol Res. 2004;49:481–6. doi: 10.1016/j.phrs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Farombi EO, Onyema OO. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: Modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol. 2006;25:251–9. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 36.Harlan JM, Levine JD, Callahan KS, Schwartz BR, Harker LA. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984;73:706–13. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiraishi H, Terano A, Ota S, Mutoh H, Sugimoto T, Harada T, et al. Protection of cultured rat gastric cells against oxidant-induced damage by exogenous glutathione. Gastroenterology. 1994;106:1199–207. doi: 10.1016/0016-5085(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 38.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 39.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oakenfull DG, Fenwick DE, Hood RL, Topping DL, Illman RL, Storer GB. Effects of saponins on bile acids and plasma lipids in the rats. Br J Nutr. 1979;42:209–16. doi: 10.1079/bjn19790108. [DOI] [PubMed] [Google Scholar]
- 41.Hamden K, Jaouadi B, Salami T, Carreau S, Bejar S, Elfeki A. Modulatory effect of fenugreek saponins on the activities of intestinal and hepatic disaccharidase and glycogen and liver function of diabetic rats. Biotechnol Bioprocess Eng. 2010;15:745–53. [Google Scholar]


