Abstract
Objective:
The main objective of this study was to evaluate antidepressant activity of vanillin in mice models of depression.
Materials and Methods:
Animals were divided into five groups, consisting six mice in each group. Out of these, three groups served as control (distilled water, imipramine,and fluoxetine) and the remaining two groups received test drug in two different doses (10mg/kg and 100mg/kg). All the drugs were administered orally one hour before the test procedure for acute study and daily for ten days for chronic study. Mice were subjected to forced swim (FST) and tail suspension tests (TST).
Results:
Both the doses of vanillin reduced the immobility duration in TST as well as in FST. In TST, there was a statistically significant decrease in the immobility in all the groups when compared to the control (distilled water) group. But the reduction of immobility in FST did not show statistically significant reduction in immobility in the groups treated with vanillin when compared with control. In the chronic study group that received vanillin at a dose of 100mg/kg, the immobility reduction was significantly lower when compared to the group receiving fluoxetine.
Conclusion:
Vanillin at the dosage of 100mg/kg has demonstrated antidepressant activity in mice, which is comparable with fluoxetine.
KEY WORDS: Antidepressant, depression, fluoxetine, imipramine, vanillin
Introduction
Major depression represents a significant public health problem worldwide. The high prevalence of suicide in depressed patients (up to 15%), coupled with complications arising from stress and its effects on the cardiovascular system, have suggested that it will be the second leading cause of death by the year 2020 and studies show depression as a contributory factor to fatal coronary disease.[1] Depression is a heterogeneous disorder that affects a person's mood, physical health, and behaviour. Patients with major depression have symptoms that reflect changes in brain monoamine transmitters, specifically norepinephrine, serotonin, and dopamine.[2] Despite the advent of new molecules in the pharmacotherapy of depression, many cases of depression are undiagnosed and untreated. Although the currently prescribed molecules provide some improvement in the clinical condition of the patient, it is at the cost of bearing the burden of their adverse effects.[3] The use of alternate medicines is increasing worldwide day by day especially in non-communicable diseases like endogenous depression, diabetes mellitus, etc.
Vanilla (Vanilla planifolia), a monocotyledonous orchid native of Central America, is grown for the attractive aroma produced by its fruit.[4] Because vanilla is so much in demand and expensive, synthetic vanilla are often used instead of natural vanilla. Synthetic vanilla contains only one organic component - vanillin - the flavor and fragrance that we most associate with vanilla. Vanillin is one of the primary chemical components of the extract of the vanilla bean. It is a pleasant aromatic compound that occurs naturally in vanilla beans (Vanilla planifolia); it is a fine, white to slightly yellow crystal, usually needle-like, having an odor and taste suggestive of vanilla. Synthetic vanillin is used as a flavoring agent in foods, beverages, and pharmaceuticals.[5] Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin. The largest single use of vanillin is as flavoring, usually in sweet foods. Several studies demonstrated that it has antimutagenic,[6] antinvasive[7], and antimetastatic potential by suppressing enzymatic activity of matrix metaloproteinase-9. It has also shown to have antinocioceptive property in acetic acid-induced visceral inflammation pain model(analgesic vanillin dose); this property has been attributed to α-adrenergic and opioid receptors-mediated action.
There is always a need for the development of new drugs for the treatment of depression as the existing classes of antidepressants are associated with several limitations like adverse effects, delay in the onset of antidepressant action, poor response in certain individuals, etc. The development of newer and safer therapeutic agents would benefit the treatment modalities of depression. The natural compound vanillin having multiple properties may also have antidepressant like effects. Vanilla has been commonly used as a home remedy from the 17th century for treating anxiety, depression etc. However, studies regarding its antidepressant action were lacking in the literature. Hence, this study was undertaken with the objective of evaluating antidepressant like activity of vanillin in mice.
Materials and Methods
Adult albino mice (Swiss Strain) of either sex, weighing 20-30grams, inbred in our own central animal house were used for the study. Mice were housed in clean polypropylene cages, six mice were accommodated in each cage in a controlled environment (26°-28°C) with a 12 hour light and dark cycle along with standard chow containing fat 4.15%, protein 22.15%, carbohydrates 4%, and water ad libitum. The mice were allowed to acclimatize for these conditions for one week. All procedures were performed during the light phase of the cycle (10:00-17:00 hrs). The study has been approved by the institutional animal ethics committee.
Vanillin [IUPAC name: 4-hydroxy-3-methoxybenzaldehyde, chemical formula: (CH3O)(OH)C6H3CHO, and molecular weight: 152.15] was obtained from HiMedia laboratories, Mumbai. Fluoxetine (CadilaPharma) and imipramine (Sarabhai Piramal) were purchased from the medical stores.
Imipramine was given at a dose of 15 mg/kg, orally (0.1ml/10g) for ten days.[7] Fluoxetine was given at a dose of 20 mg/kg orally (0.1 ml/10 g) for ten days.[8] Drugs were separately dissolved in double distilled water to make up the above concentrations.
Mice were randomly assigned to five groups of six mice each. The feeding and treatment schedule is as follows.
Group 1: Distilled water (Normal control).
Group 2: Imipramine at the dose of 15 mg/kg, orally (0.1 ml/10g) for ten days.[7]
Group 3: Fluoxetine at the dose of 20 mg/kg orally (0.1 ml/10 g) for ten days.[8]
Group 4: Vanillin is dissolved in distilled water given orally at a dose of 10 mg/kg[9](V1).
Group 5: Vanillin is dissolved in distilled water given orally at a dose of 100 mg / kg[9](V2).
All the drugs were administered one hour before the test procedure for acute study and daily for ten days for chronic study.
The Forced Swim test (FST) was performed according to the method described by Porsolt et al.[10,11] Animals were pre-screened on the previous day by placing the animals individually in the five liter glass beakers, filled to a height of 15 cm with water (room temperature). After 5-6 min., immobility reaches a plateau where the rats remained immobile for approximately 80% of the time. After 15minutes in the water, the mice were removed and allowed to dry in a heated enclosure (32°C) before being returned to their home cages.
On the day of experiment, mice were again placed individually in the five liter glass beakers, filled to a height of 15 cm with water and the duration of immobility is recorded during the last four minutes of a six minutes test. A mouse is considered immobile when floating motionless or making only those movements necessary to keep its head above water surface. The water is changed after each test. Antidepressants decrease the immobility time. The test has been validated by most current types of antidepressants.
Tail suspension test (TST) was performed according to Steru et al.[10,12] The tail suspension test is a facile means of evaluating potential antidepressants. The immobility displayed by rodents,when subjected to an unavoidable and inescapable stress, has been hypothesized to reflect behavioral despair,which in turn may reflect depressive disorders in humans. Clinically effective antidepressants reduce the immobility that mice display after active and unsuccessful attempts to escape when suspended by the tail.
Animals were transported from the housing room to the testing area in their own cages and allowed to adapt to the new environment for one hour before testing. Mice were suspended on the metal rod stand 50cm above the table top by the adhesive tape placed approximately one cm from the tip of the tail. Immobility time was recorded during eight minute period. The immobility during the first two minute due to vigorous activity is not taken into account and in the remaining six minutes immobility was recorded.
Results
Forced Swim Test
In the acute study, there was a significant reduction of immobility period of imipramine group (15 ± 6.73 seconds) when compared to the control (66.83 ± 12.92 seconds) (P < 0.05). Surprisingly, there was a slight increase in the immobility period in fluoxetine group (107 ± 20.39 seconds) and group 5 (75.17 ± 10.41 seconds), which was not statistically significant [Figure 1]. A similar picture was seen in the chronic study with immobility reduction being maximum in imipramine group (6.67 ± 4.22 seconds) [Figure 2].
Figure 1.
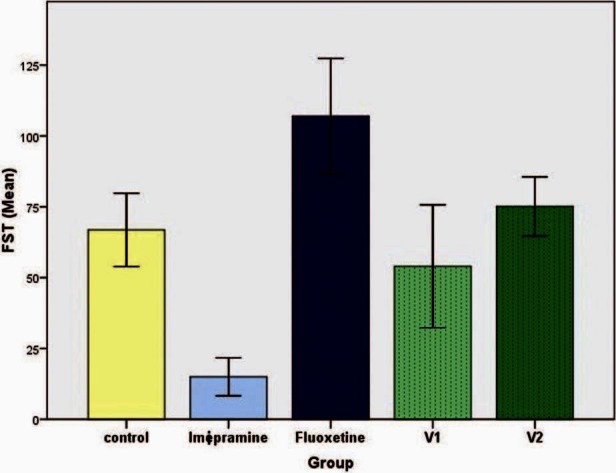
Effect of vanillin on duration of immobility in forced swim test (acute study). Each histogram represents mean duration of immobility in seconds (n = 6). Vertical line on top represents SD. Values are significant for imipramine when compared with control (P< 0.05)
Figure 2.
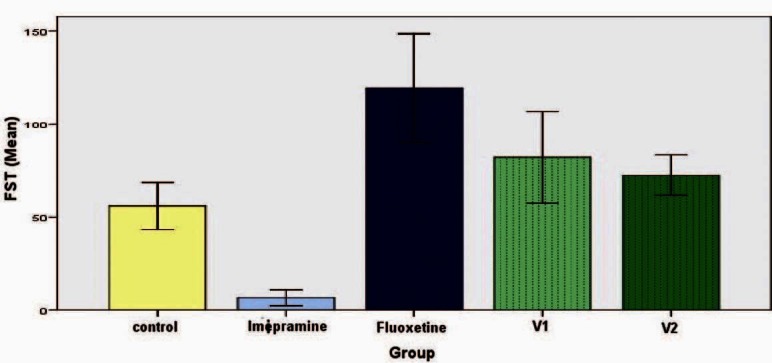
Effect of vanillin on duration of immobility in forced swim test (chronic study). Each histogram represents mean duration of immobility in seconds (n = 6). Vertical line on top represents SD. Values are significant for imipramine when compared with control (P< 0.05)
Tail Suspension Test
There was a statistically significant decrease in the immobility in all the groups except imipramine group(172.33 ± 30.84 seconds) when compared to the control group (225 ± 17.90seconds) (P < 0.05). Even though there was reduction in immobility period seen in vanillin 10 mg/kg group (115.00 ± 22.77 seconds), vanillin 100mg/kg group (122.17 ± 17.94 seconds) as well as fluoxetine group (58.33 ± 19.43 seconds), when compared to imipramine group, it was not statistically significant [Figure 3]. In the chronic study, a similar result was seen with reduction of immobility among fluoxetine group (90 ± 24.36 seconds) and both the doses of vanillin. But in group five, which received vanillin at a dose of 100 mg/kg, the immobility reduction (49.67 ± 6.16 seconds) was significantly lower when compared to the group receiving fluoxetine (P < 0.05) [Figure 4].
Figure 3.
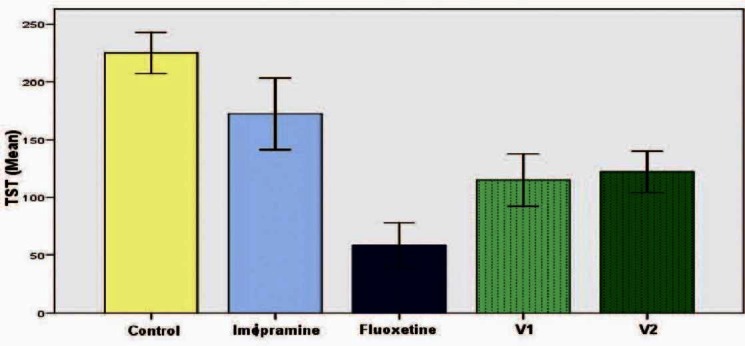
Effect of vanillin on duration of immobility in tail suspension test (acute study). Each histogram represents mean duration of immobility in seconds (n = 6). Vertical line on top represents SD. Values are significant for fluoxetine, vanillin 10 mg/kg (V1), vanillin 100 mg/kg (V2) when compared with control (P< 0.05)
Figure 4.
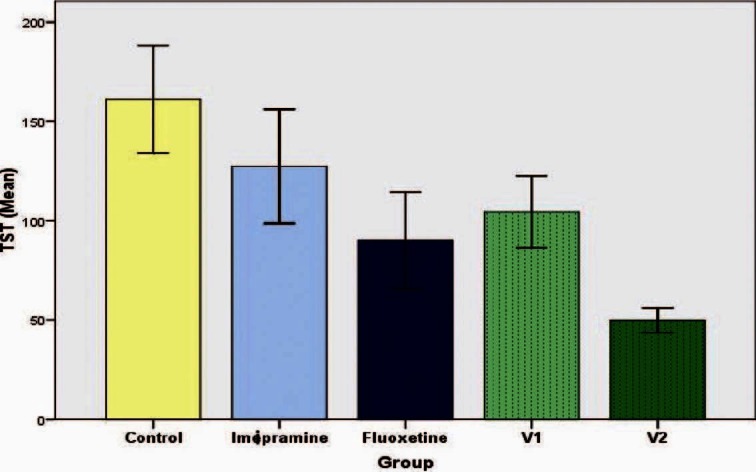
Effect of vanillin on duration of immobility in tail suspension test(chronic study). Each histogram represents mean duration of immobility in seconds (n = 6). Vertical line on top represents SD. Values are significant for fluoxetine, vanillin 10 mg/kg (V1), vanillin 100mg/kg (V2) when compared with control (P< 0.05). Values were also significant for vanillin 100mg/kg (V2) when compared with fluoxetine (P< 0.05)
Discussion
In our study, both the standard drugs imipramine and fluoxetine reduced the immobility duration. The reduction in immobility by imipramine, which is a monoamine reuptake inhibitor, was better in FST model than TST and vice versa for fluoxetine, a selective serotonin reuptake inhibitor. Both the doses of vanillin reduced the immobility duration in TST as well as in FST. This was evident on acute as well as on chronic administration of vanillin. In the acute study, there was not much of differentiation in the reduction of immobility by both the doses. However, in the chronic study there was a dose dependent decrease in the same with vanillin 100 mg/kg displaying a better immobility reduction.
Fluoxetine and also vanillin at 100 mg/kg dose has shown increase in duration of immobility in FST in both acute and chronic study. This paradoxical response could not be explained easily, though can be attributed as false negative. FST model is purely behavioral without presuppositions concerning the mechanism of action of potential antidepressants. It is sensitive to a large number of atypical antidepressants, otherwise inactive in the more classical tests. The rat version seems to be more selective (fewer false positives) and the mouse version more sensitive (fewer false negatives). It has been reported that some strains of mice may be resistant to behavioral despair test especially to TST.
Vanillin, a compound widely used in foods, beverages, cosmetics, and drugs, has been reported to exhibit multifunctional effects such as antioxidant, antimutagenic, antiangiogenetic, and analgesic effects.[13,14] The antinocioceptive activity has been proposed to be due to vanillin's action on α2 adrenergic receptors or opioid receptors,and not due to activity on the serotonergic system.[9] As vanillin is known to have an adrenergic agonistic activity, this effect could be leading to its antidepressant effect. However, its activity on other receptors including opioid receptor, which might contribute to the antidepressant effect, cannot be ruled out.
Oxidative stress represents a loss of balance in oxidation-reduction reactions. It is characterized by the reduced ability of the antioxidant defense system to efficiently eliminate the excess of the oxygen-derived species production, eliciting the toxicity of oxygen and its detrimental effects. Increased oxidative stress is seen in patients suffering from depression.[15,16] Antioxidants such as N-acetylcysteine has been tried as a newer modality for the treatment for depression with encouraging results.[17] Antioxidant property of vanillin[5] could also contribute to its antidepressant activity.
To conclude, vanillin at a dose of 100 mg/kg has demonstrated antidepressant activity in mice which is comparable with fluoxetine. Vanillin's antidepressant activity could be due to the agonistic action at α2 adrenergic or opioid receptors or due to the antioxidant property. The results of the present study give us future scope to explore the mechanism of antidepressant action of vanillin that may involve antioxidant or α2 agonistic activities. Hence, further studies are required in this direction to establish the mechanism of action of vanillin in depression.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.WHO report on mental illness released October 4, 2001. [Last accessed on 2012 Aug 7]. Available from: http://www.who.int/whr/2001/media_centre/press_release/en/index.html .
- 2.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology stress (1) N Engl J Med. 1988;319:348–53. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 3.Shalam M, Shantakumar SM, Narasu ML. Pharmacological and biochemical evidence for the antidepressant effect of the herbal prepration Trans-01. Indian J Pharmacol. 2007;39:231–4. [Google Scholar]
- 4.Odoux E, Escoute J, Verdeil JL, Brillouet JM. Localization of beta-D-glucosidase activity and glucovanillin in vanilla bean (Vanilla planifolia Andrews) Ann Bot. 2003;92:437–44. doi: 10.1093/aob/mcg150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta. 2011;1810:170–7. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 6.King AA, Shaughnessy DT, Mure K, Leszczynska J, Ward WO, Umbach DM, et al. Antimutagenicity of cinnamaldehyde and vanillin in human cells: Global gene expression and possible role of DNA damage andrepair. Mutat Res. 2007;616:60–9. doi: 10.1016/j.mrfmmm.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhingra D, Sharma A. Evaluation of antidepressant-like activity of glycyrrhizin in mice. Indian J Pharmacol. 2005;37:390–4. [Google Scholar]
- 8.Kalra BS, Tayal V, Chawla S. Antidepressant-like activity of tramadol in mice. Indian J Psychiatry. 2008;50:51–3. doi: 10.4103/0019-5545.39760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SH, Sim YB, Choi SM, Seo YJ, Kwon MS, Lee JK, et al. Antinociceptive profiles and mechanisms of orally administered vanillin in the mice. Arch Pharm Res. 2009;32:1643–9. doi: 10.1007/s12272-009-2119-8. [DOI] [PubMed] [Google Scholar]
- 10.Vogel GH. 3rd ed. Germany: Spinger Publication; 2007. Drug discovery and evalution-pharmacological assays; pp. 788–93. [Google Scholar]
- 11.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 12.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 13.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, et al. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.D’Aquila PS, Canu S, Sardella M, Spanu C, Serra G, Franconi F. Dopamine is involved in the antidepressant-like effect of allopregnanolone in the forced swimming test in female rats. Behav Pharmacol. 2010;21:21–8. doi: 10.1097/FBP.0b013e32833470a7. [DOI] [PubMed] [Google Scholar]
- 15.Kumar SS, Priyadarsini KI, Sainis KB. Inhibition of peroxynitrite-mediated reactions by vanillin. J Agric Food Chem. 2004;52:139–45. doi: 10.1021/jf030319d. [DOI] [PubMed] [Google Scholar]
- 16.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 17.Machado-Vieira R, Salvadore G, DiazGranados N, Ibrahim L, Latov D, Wheeler-Castillo C, et al. New therapeutic targets for mood disorders. Sci World J. 2010;10:713–26. doi: 10.1100/tsw.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]


