Abstract
Purpose:
The purpose of the present study was to investigate the effect of nimodipine (NMD), a calcium channel blocker, on N-methyl-N-nitrosourea (MNU)-induced retinal degeneration.
Materials and Methods:
60 mg/kg MNU was given intraperitoneally to 6-week-old female Sprague-Dawley rats, and NMD was injected intraperitoneally for up to 5 days after MNU. The effect of NMD was evaluated by electron microscopy and electroretinography (ERG). Proteins of Bax, Bcl-2, Caspase-3, and mitochondrial membrane potential (MMP) were analyzed with flow cytometry. The expressions of phosphodiesterase (PDE) and Caspase-3 were detected by reverse transcriptase polymerase chain reaction (RT-PCR).
Results:
The apparent preservation of NMD to the photoreceptor cell was demonstrated by electron microscopy. After NMD treatment, both a- and b-waves of ERG were significantly higher compared with the control group, and had a protective effect on MNU-damaged retinal ERG. Flow cytometric assays showed that NMD decreased the level of Bax and Caspase-3 and increased the activity of Bcl-2 in retina. NMD significantly restored the mitochondrial membrane potential (MMP). RT-PCR analysis demonstrated that NMD treatment significantly decreased mRNA level of Caspase-3, and mRNA level of PDE was clearly upregulated.
Conclusions:
These data suggest that NMD may regulate the expressions of Bax, Bcl-2, Caspases-3, and PDE, and protection on the functions of retinal cell mitochondria inhibit MNU-induced photoreceptor cell apoptosis and protect retinal function in rats.
KEY WORDS: Nimodipine, N-methyl-N-nitrosourea, retinal degeneration
Introduction
Retinitis pigmentosa (RP) refers to a heterologous group of inherited human disorders which caused primary retinal degeneration and are characterized by loss of photoreceptor cells via apoptosis.[1] The incidence of RP is about 1/4,000, and almost 1.5 million people worldwide are affected. The effective medicines are in various stages of development, no drugs are actually available to treat people with RP.
MNU-induced photoreceptor cell apoptosis in rats provides a good model for human RP, both in understanding the disease and in providing a mechanism for developing and evaluating therapeutic interventions.[2] In previous study, a dose of 60 mg/kg MNU was considered optimal to induce maximum damage in retina and cause photoreceptor cell apoptosis in rats.[3]
In recent years, there has been an upsurge of interest in unraveling the roles of Ca2+ antagonists in the pathophysiology of different human diseases including neurodegenerative and retinal degenerative diseases. For example, systemic administration of nilvadipine delays photoreceptor degeneration of heterozygous retinal degeneration slow (RDS) mouse.[4]
Nimodipine (NMD) is a dihydropyridine calcium channel antagonist, which has been shown to dilate cerebral arterioles and increase cerebral blood flow in animals and humans. In the clinic practice, it is used to treat cerebral vasospasm and migraine headaches, and had the neuroprotective potential in the recent study. In addition, NMD may be considered as a protective agent against severe head trauma related neuronal injuries.[5] Studies have demonstrated NMD can protect retinal neurons from ischemic injury and it was significantly effective vasodilators in human and pig retinal arterioles.[6,7] Several researchers reported that oral administration of NMD had beneficial effect in visual field tests color vision tests and contrast sensitivity.[8] NMD has been shown to improve ocular haemodynamics and to increase visual field in patients with normal-pressure glaucoma and primary open angle glaucoma, and it might also be effective in ameliorating cerebral haemodynamics in glaucoma. In vitro, NMD has a direct neuroprotective effect against retinal ganglion cell (RGC) damage related to hypoxia.[9–11]
All these show that NMD as a calcium channel blocker is potentially useful for ophthalmic treatment. However, the effect of NMD on the nervous system has not been explored and even less is known about its effects on retinal degenerative diseases. In the present study, we adopted the animal model of MNU-induced retinal degeneration to assess the protective effect of NMD. The retinal changes were measured after NMD treatment to MNU-induced damage rats on morphology with light and electron microscopy, electroretinography (ERG) and mitochondrial membrane potential, the possible mechanisms of apoptotic cell death of photoreceptor cells are performed by flow cytometry and reverse transcriptase polymerase chain reaction (RT-PCR) in rats.
Materials and Methods
Animals and Procedures
MNU (Sigma) was kept at −20°C in the dark. Before use, MNU was dissolved in phosphate-buffered saline (PBS) just before use. Rats received a single intraperitoneal (i.p.) injection of MNU at a dose of 60 mg/kg or an injection of vehicle. Anti-caspase-3 (rabbit polyclonal antibody), rabbit anti-Bcl-2, rabbit anti-Bax, Cy3 conjugated goat anti-mouse IgG and all other chemicals were purchased from commercial sources.
6-week-old female Sprague-Dawley rats were purchased from the animal facility at the China Medical University, P.R. China. Animal experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the study was approved by the local Medical Ethics Committee. Rats were reared under standard laboratory conditions (22 ± 2°C, 60% ± 10% relative humidity, and a 12 h light-dark cycle) and had free access to food and water throughout the experiment.
To investigate the effect of NMD on MNU-induced retinal damage, rats were randomly divided into three groups, including control group (NC group), MNU-treated group (MNU group) and NMD-treated group (NMD group). In the NMD treatment group, rats received a single i.p. injection of 60 mg/kg body weight MNU, followed immediately by once daily injection of 2 mg/kg NMD for 5 days. In the MNU group, after a single i.p. of MNU injection, PBS (10 ml/kg, once a day) was administrated instead of NMD for 5 days. NC group animals were injected with a corresponding bolus of PBS. Then, the animals were prepared for ERG analysis, and killed at 5 days after MNU treatment.
The eyes were enucleated, and the retinas were dissected away from the pigmented epithelium and resolved in PBS on ice in 1 ml of PBS. After centrifugation at 1,000 rpm for 5 min., retinal cells were examined the expression of the protein of Bcl2, Bax, and Caspase-3. Total RNA was extracted with Trizol reagent for RT-PCR.
Electromicroscopy Assay
The eyes reserved for electromicroscopy assay was kept in 2.5% glutaraldehyde overnight at 4°C. Then a portion of cornea and lens were removed and the remaining eye cups containing the retinas were placed in 2.5% glutaraldehyde overnight at 4°C. The samples were postfixed in 2% osmium solution for 20 min, then dehydrated and embedded in epon, and polymerased at 60°C. Thin sections (50 nm thick) stained with uranyl acetate and lead citrate were examined using a electron microscope (JEM-100CXII, Japan).
Electroretinogram (ERG)
Rats were dark adapted overnight and anesthetized with an i.p. injection of 50 mg/kg chloral hydrate and prepared for the recordings under dim red light. Pupils were dilated with topical 1% tropicamide. A gold wire loop record electrode was placed on the surface of the cornea anesthetized with 1% tetracaine. A drop of 1% methylcellulose was used to assure good contact between the cornea and the electrode. A reference electrode was placed under the lip and the ground electrode on the ear. A photic stimulator (VISION TECH CO., LTD CLASS 1 TYPE B) provided white flashes of 3.4 cd-s/m2 against a dark background. The inter-stimulus interval was set at 10 s to control the adaptive effect. The bandwidths of signal extraction were set from 0.3 to 300 Hz.
ERGs were monitored by electrophysiological recorder at 5 days after MNU injection in each group. ERG wavelets were recorded for three or four times, and the average amplitudes of a- and b-waves were calculated. The amplitude of the a-wave was measured as the distance from the baseline to the bottom of the a-wave, while the amplitude of the b-wave was measured as the distance from the bottom of the a-wave to the peak of the b-wave.
Flow Cytometric Analysis of Bcl-2, Bax, and Caspase -3
Retinal cells were used for Bcl-2, Bax, and Caspase-3 detection. The first appropriate antibody (1:100) was added for 60 min at room temperature. The cells were then washed twice with PBS, treated with the anti-mouse or anti-rabbit Cy3 conjugated antibody (1:200) and incubated in darkness. Samples were allowed to incubate in the dark for 30 min at room temperature, then, washed and filtered through a 200 mesh filter before being resuspended in 500 ul of PBS, ready to be analyzed by flow cytometry.
Flow Cytometric Analysis of Mitochondrial Membrane Potential
The mitochondrial transmembrane potential (ΔΨm) value was determined using rhodamine 123, a membrane-permeable cationic fluorescent dye.[12] Retinal cells were stained with 5 uM rhodamine 123 in PBS at 37°C for 1 h. After washing with PBS, cells were analyzed by flow cytometry.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
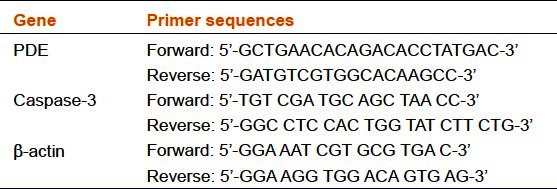
Rat eyes were enucleated and the retinas dissected. Total RNA was extracted from the neural retinas using reagent Trizol according to manufacturer's instructions.[13] RNA was quantitated by measuring the Optical Density (OD) 260 and reverse transcribed into a single stranded cDNA with the RevertAid H Minus First Strand cDNA Synthesis Kit, K1632 (Fermentas AB, Vilnius, Lithuania). cDNA was amplified for 25 cycles so that the PCR product remained in the linear range. PCR was performed with gene-specific primers for β-actin, PDE, and Caspase-3 gene [Table 1]. β-actin was used as an internal control to confirm mRNA integrity. The identity of all PCR products was confirmed by comparison with the correct size based on the known length of the DNA sequence on 1% agarose gel stained by ethidium bromide. The optical density of the bands was analyzed on the GeneSnap system.
Table 1.
Primer sequences for mRNAs amplified by RT-PCR
Statistical Analyses
Values are expressed as mean ± standard error. Normal distribution of all data was examined using the Shapiro–Wilk normality test. One-way ANOVA was used followed by Fisher's least-significant difference (LSD) for the homogeneity testing of variance (Levene's test), and the data were analyzed by Dunnett's T3 for the heteroschedasticity of variance test. P < 0.05 was considered statistically significant. All statistical procedures were performed using Statistical Product and Service Solutions (SPSS) 13.0 software for Windows (SPSS Inc., USA).
Results
Morphological Observation of NMD On MNU-Treated Rats
At the ultrastructural level [Figure 1], electron microscopy showed that morphology of photoreceptor outer segments was normal in the NC group [Figure 1a], whereas vacuoles and irregularity of disc membranes are more numerously seen in the MNU group [Figure 1b]. Electron microscopic analysis disclosed that discs of photoreceptor outer segments were apparently less deteriorated in the NMD group [Figure 1c] than in the MNU group. Five days after MNU injection, 2 mg/kg NMD partially protected against MNU-induced photoreceptor cell damage at the ultrastructural level. These observations suggest that NMD has a beneficial effect on MNU-induced retinal degeneration.
Figure 1.
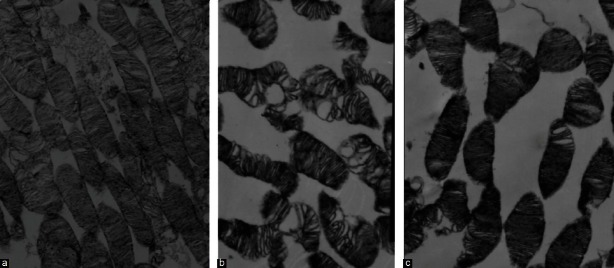
Effects of NMD on photoreceptor outer segments after MNU treatment at the ultrastructural level in rats. (a) NC group (× 5000), the normal photoreceptor outer segment. (b) MNU group (× 6000), the apparent vacuoles and irregularity of disc membrane (c) NMD group (× 6000), the less deterioration of photoreceptor outer segment
The Effect of NMD on Retinal Function Detected By ERG
ERG recordings provided a- and b-wave amplitudes representing the effect of NMD on the retinal damage induced by MNU. In the MNU group, both a- and b-wave amplitudes were significantly decreased compared with the NC group. In NMD -treated rats, both a- and b-wave amplitudes were significantly elevated compared with the MNU group [Figure 2a–c], but wave amplitudes were still lower than the NC group. In brief, NMD-treated eyes exhibited more moderate loss in both a- and b-wave amplitudes compared with MNU group. In a word, NMD showed the protective effect on retinal function detected by ERG.
Figure 2.
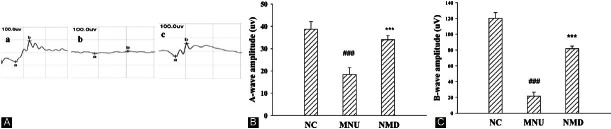
Effects of NMD on MNU-induced retinal ERG. (A) Representative ERG recording from an eye of (a) NC group, (b) MNU group and (c) NMD group. (B) a-wave amplitudes of NC group, MNU group and NMD groups. (C) b-wave amplitudes of NC group, MNU group and NMD groups. In rats received NMD treatment, both amplitudes significantly increased more than those of rats in the MNU group. Data are expressed as mean ± standard error (n = 6), ***P< 0.001 versus NC group, ***P< 0.001 versus MNU group
The Effect of NMD on Protein Expressions of Bax, Caspase-3, and Bcl-2 in Rat Retina Induced by MNU
It was measured that NMD affected on the expression of Bax, Caspase-3, and Bcl-2 in rat retina induced by MNU with flow cytometry. Representative results of Bax, Caspase-3, and Bcl-2 in retina at 5 d in every group are presented in Figure 3. The expression of Bax and Caspase-3 were up-regulated, while Bcl-2 down-regulated in MNU group compared to NC group. Treatment with NMD decreased the expression of Bax and Caspase-3, and increased the expression of Bcl-2 in MNU-induced retinal cells.
Figure 3.
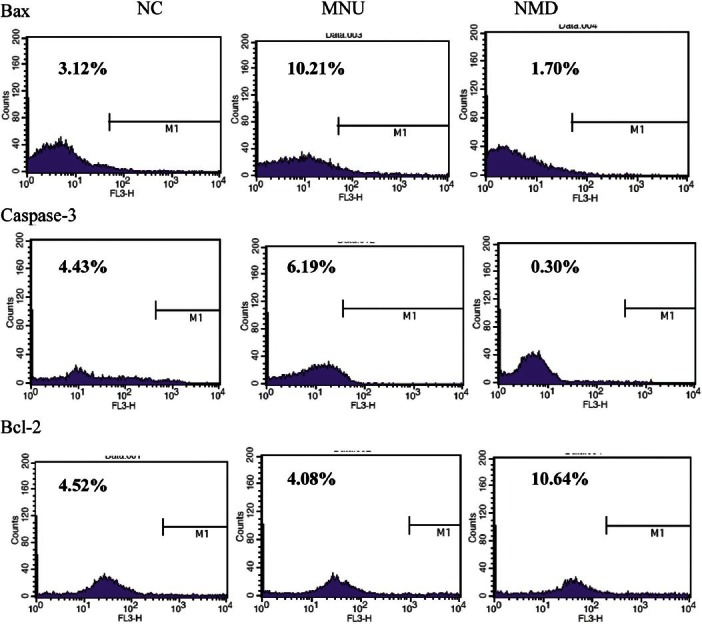
Flow cytometric analysis of NMD on the expression of Bax, Caspase-3, and Bcl-2 in rat retinal cells. Representative cytometric profile of Bax, Caspase-3, and Bcl-2 in retinal cells are presented in NC group, MNU group and NMD group the percentage of bax was successively 3.12%, 10.21%, and 1.70%, the percentage of Caspase-3 was successively 4.43%, 6.19%, and 0.30%, the percentage of bcl-2 was successively 4.52%, 4.08%, and 10.64% in NC group, MNU group and NMD group
The Effect of NMD on ΔΨm of Retinal Mitochondria Induced by MNU
To elucidate the protective effect of NMD to the mitochondria, we monitored the changes in MMP in retina cells by measuring the fluorescence intensity of Rhodamine 123 with flow cytometry. As shown in Figure 4, control (NC) retinal cells incorporated and retained the dye, exhibiting higher fluorescence intensity, and MNU exposure resulted in a marked decrease in fluorescence intensity as compared with control group. Treatment with NMD (2 mg/kg) restored the fluorescence intensity.
Figure 4.
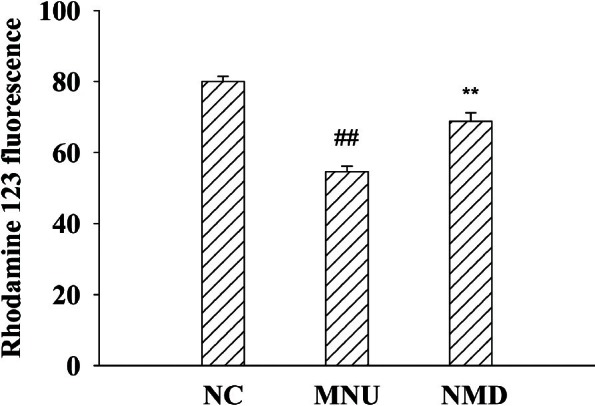
Effects of NMD on MNU-induced ΔΨm of retinal mitochondria. ΔΨm of retinal mitochondria was calculated according to the measured values of flow cytometry. Data are expressed as mean ± standard error (n = 6), ##P< 0.01 versus NC, **P< 0.01 versus MNU
The Effect of NMD on the Retinal Cells of Caspase-3 and PDE Induced by MNU
We investigated whether NMD could modulate retinal cell survival after MNU injection by analyzing the transcription changes of related genes Caspase-3 and PDE with NMD treatment. Representative RT-PCR results for Caspase-3 and PDE mRNA expression are shown in Figure 5a, and quantitative data are summarized in Figure 5b–c. Caspase-3 significantly upregulated at 5 d after MNU injection compared with NC group. In the NMD group, Caspase-3 mRNA expression was significantly lower than in the MNU group at 5 d (P < 0.01). In the MNU group, PDE mRNA levels decreased at 5 d after MNU injection, the values were significantly lower than in the NC group (P < 0.01). Likewise, the expression of PDE mRNA was found, and the values were significantly higher in the NMD group than in the MNU group (P < 0.01).
Figure 5.
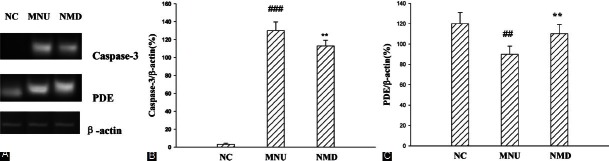
Effects of NMD on MNU-induced mRNA expression of Caspase-3 and PDE in rat retinal measured by RT-PCR. (A) DNA strips show expression levels of Caspase-3 and PDE on agarose gel electrophoresis. (B) Effects of NMD on MNU-induced mRNA expression of Caspase-3 in rat retinal. (C) Effects of NMD on MNU-induced mRNA expression of PDE in rat retinal. Data are expressed as mean ± standard error (n= 6), ##P< 0.01, ###P< 0.001 versus NC, **P< 0.01 versus MNU
Discussion
NMD, an L-type calcium channel antagonist, has been mainly used in treatment of cardiovascular related diseases, including angina pectoris, cardiac arrhythmia, and hypertension. Recently, NMD has been found to be beneficial in many central nervous system (CNS) disorders, The clinical studies demonstrated a favorable effect of NMD on the severity of neurological deficits caused by cerebral vasospasm following subarachnoid haemorrhage.[14,15] NMD-was chosen after a range of Ca2+ entry blockers (NMD, nifedipine, verapamil, and ditiazem) were screened for vasodilatory activity in the pig retinal arteriole, the result showed that NMD was the most potent.[16] Takayuki conformed that total calcium increased in retinas after MNU treatment.[17] In the present study, systemic administration of NMD morphologically and functionally demonstrated a beneficial effect on retinal degeneration caused by MNU rat. For the above reasons, we speculated that regulation of intracellular Ca2+ concentration by NMD and vasodilator effect in retinal arterioles might be involved in the preservation of retinal function in different ways.
Similar to the human RP, MNU-induced retinopathy can be diagnosed by the changes in the ERG.[18] In our study, the quantified visual ability of the animal detected by ERG represents the summed responses of various retinal neurons.[19] In the dark-adapted flash ERG, the a-wave primarily represents the mixed function of cones and rods, while b-wave mainly reflects light-induced depolarization of ON-bipolar cells but may be shaped by the activity of other cells, including the responses of Müller cells.[20,21] Our studies found that daily administration of 2.0 mg/kg NMD had significantly the protective effect compared with untreated controls. These results are in agreement with the previous report that NMD exert a slight beneficial effect on the recovery of the amplitude of the b-wave during reperfusion.[22]
MNU-induced retinal damage is due to selective 7-methyldeoxyguanosine DNA adduct formation in photoreceptor cell nuclei followed by photoreceptor cell death via an apoptotic mechanism 6, the apoptosis cascade involves up-regulation of Bax protein, down-modulation of Bcl-2 protein, and activation of Caspase-3, -6, and -8.[23] Proteins of the Bcl-2 family, as an anti-apoptotic protein, induce and integrate signals of survival and death, correlated with apoptosis in photoreceptor cells. In contrast, the increased expression of the pro-apoptotic Bax mediates enhancement of apoptosis. Likewise, inhibition of Caspase-3 can suppress MNU-induced retinal apoptosis.[3] Our present study provides the evidences that NMD down-regulated the expression of Bax and Caspase-3 and up-regulated the expression of Bcl-2 in MNU-induced retinal degeneration rats. These facts lead us to postulate that NMD may block MNU-induced photoreceptor cell apoptosis by regulating the expression of Bax, Bcl-2, and Caspase-3.
In the present study, MNU was used to induce a selective and progressive photoreceptor degeneration, the degeneration is different from genetic animal models and human disease in the etiopathogenesis, but they all lead to apoptosis-mediated cell death. It has been reported that there are significant changes of gene expression in the early stage of MNU-induced retinal degeneration.[24] Some studies found that retinal degeneration in rd mouse is caused by a defect in the β-subunit of rod cGMP-phosphodiesterase. Following this, the identical mutation is also recognized in patients with autosomal recessive RP. In our present studies, RT-PCR analyses revealed that Caspase-3 mRNA expression was significantly lower in the NMD group than in the MNU group, while PDE mRNA levels increased at 5 d after NMD injection, these results suggest that NMD treatment may modulate protein expression of pro-apoptotic gene Caspase-3 and PDE to inhibit MNU-induced apoptosis.
In conclusion, our studies indicate the possibly protective effect of NMD on MNU-induced retinal cell damage. Although further studies are required to clarify the direct protective mechanism of NMD such as up-regulation of bcl-2 and suppression of Bax and Caspase-3, the administration of NMD appear to be importantly involved in the NMD-dependent protection against MNU-induced retinal photoreceptor apoptosis in vivo.
Footnotes
Source of Support: This work was supported by China Medical University
Conflict of Interest: None declared
References
- 1.Van Soest S, Westerveld A, de Jong PT, Bleeker-Wagemakers EM, Bergen AA. Retinitis pigmentosa: Defined from a molecular point of view. Surv Ophthalmol. 1999;43:321–34. doi: 10.1016/s0039-6257(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizawa K, Tsubura A. Characteristics of N-methyl-N-nitrosourea-induced retinal degeneration in animals and application for the therapy of human retinitis pigmentosa. Nihon Ganka Gakkai Zasshi. 2005;109:327–37. [PubMed] [Google Scholar]
- 3.Miki K, Uehara N, Shikata N, Matsumura M, Tsubura A. Poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide rescues N-methyl-N-nitrosoureainduced photoreceptor cell apoptosis in Sprague-Dawley rats through preservation of nuclear factor-kappa B activity. Exp Eye Res. 2007;84:285–92. doi: 10.1016/j.exer.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi K, Nakazawa M, Mizukoshi S. Systemic administration of nilvadipine delays photoreceptor degeneration of heterozygous retinal degeneration slow (rds) mouse. Exp Eye Res. 2008;86:60–9. doi: 10.1016/j.exer.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Aslan A, Gurelik M, Cemek M, Goksel HM, Buyukokuroglu ME. Nimodipine can improve cerebral metabolism and outcome in patients with severe head trauma. Pharmacol Res. 2009;59:120–4. doi: 10.1016/j.phrs.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Crosson CE, Willis JA, Potter DE. Effect of the calcium antagonist, nifedipine, on ischemic retinal dysfunction. J Ocul Pharmacol. 1990;6:293–9. doi: 10.1089/jop.1990.6.293. [DOI] [PubMed] [Google Scholar]
- 7.Yu DY, Su EN, Cringle SJ, Alder VA, Yu PK, Desantis L. Effect of betaxolol, timolol and nimodipine on human and pig retinal arterioles. Exp Eye Res. 1998;67:73–81. doi: 10.1006/exer.1998.0495. [DOI] [PubMed] [Google Scholar]
- 8.Boehm AG, Breidenbach KA, Pillunat LE, Bernd AS, Mueller MF, Koeller AU. Visual function and perfusion of the optic nerve head after application of centrally acting calcium-channel blockers. Graefes Arch Clin Exp Ophthalmol. 2003;241:34–8. doi: 10.1007/s00417-002-0592-6. [DOI] [PubMed] [Google Scholar]
- 9.Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 10.Flammer J. Therapeutic aspects of normal-tension glaucoma. Curr Opin Ophthalmol. 1993;4:58–64. [Google Scholar]
- 11.Niwa Y, Yamamoto T, Harris A, Kagemann L, Kawakami H, Kitazawa Y. Relationship between the effect of carbon dioxide inhalation or nilvadipine on orbital blood flow in normal-tension glaucoma. J Glaucoma. 2000;9:262–7. doi: 10.1097/00061198-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, Lin CF, Chiang CW, Jan MS, Lin YS. Lithium inhibits ceramide- and etoposide-induced protein phosphatase 2A methylation, Bcl-2 dephosphorylation, caspase-2 activation, and apoptosis. Mol Pharmacol. 2006;70:510–7. doi: 10.1124/mol.106.024059. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Deng XG, Sun QN, Zhong ZQ. Ganoderma spore lipid inhibits N-methyl-N-nitrosourea-induced retinal photoreceptor apoptosis in vivo. Exp Eye Res. 2010;90:397–404. doi: 10.1016/j.exer.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Murray GD, Teasdale GM, Schmitz H. Nimodipine in traumatic subarachnoid haemorrhage: A re-analysis of the HIT I and HIT II trials. Acta Neurochir (Wien) 1996;138:1163–7. doi: 10.1007/BF01809745. [DOI] [PubMed] [Google Scholar]
- 15.Vergouwen MD, Vermeulen M, Roos YB. Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: A systematic review. Lancet Neurol. 2006;2:1029–32. doi: 10.1016/S1474-4422(06)70582-8. [DOI] [PubMed] [Google Scholar]
- 16.Yu DY, Su EN, Cringle SJ. Effect of beta blockers and Ca2+ entry blockers on ocular vessels. In: Drance SM, editor. Vascular risk factors and neuroprotection in glaucoma. Amsterdam: Kugler Publications; 1996. pp. 123–245. [Google Scholar]
- 17.Oka T, Nakajima T, Tamada Y, Shearer TR, Azuma M. Contribution of calpains to photoreceptor cell death in N-methyl-N-nitrosourea-treated rats. Exp Neurol. 2007;204:39–48. doi: 10.1016/j.expneurol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Petrin D, Baker A, Coupland SG, Liston P, Narang M, Damji K, et al. Structural and functional protection of photoreceptors from MNU-induced retinal degeneration by the X-linked inhibitor of apoptosis. Invest Ophthalmol Vis Sci. 2003;44:2757–63. doi: 10.1167/iovs.02-0729. [DOI] [PubMed] [Google Scholar]
- 19.Homma K, Osakada F, Hirami Y, Jin ZB, Mandai M, Takahashi M. Detection of localized retinal malfunction in retinal degeneration model using a multielectrode array system. J Neurosci Res. 2009;87:2175–82. doi: 10.1002/jnr.22024. [DOI] [PubMed] [Google Scholar]
- 20.Yang PZ, Chen JQ, Ge J. 1st ed. Beijing: People's Medical Publishing House; 2006. Clinical and Basic Research in Ophthalmology; pp. 233–40. [Google Scholar]
- 21.Mojumder DK, Sherry DM, Frishman LJ. Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol. 2008;586:2551–80. doi: 10.1113/jphysiol.2008.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block F, Schwarz M. The b-wave of the electroretinogram as an index of retinal ischemia. Gen Pharmacol. 1998;30:281–7. doi: 10.1016/s0306-3623(97)00359-5. [DOI] [PubMed] [Google Scholar]
- 23.Podestà F, Romeo G, Liu WH, Krajewski S, Reed JC, Gerhardinger C, et al. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000;156:1025–32. doi: 10.1016/S0002-9440(10)64970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Li D, Chen J, Yang J, Xue L, Hu S, et al. Microarray expression analysis of the early N-methy-N-nitrosourea-induced retinal degeneration in rat. Neurosci Lett. 2007;418:38–43. doi: 10.1016/j.neulet.2007.02.084. [DOI] [PubMed] [Google Scholar]


