Abstract
Objective:
To investigate the protective effects of rhein on IgA nephropathy (IgAN) in the rat model.
Materials and Methods:
Twenty-eight female sprague dawley rats were divided randomly into four groups, namely control, IgAN, rhein-prevented and rhein-treated. The pathologic changes on renal tissue were observed by the H and E, staining and the amount of urinary red blood cells and 24-h urinary protein excretion were measured. The glomerular deposition of immune globulin A (IgA) was measured by immunofluorescence staining. Fibronectin (FN) and α-smooth muscle actin (α-SMA) expression on renal tissue were measured via immunohistochemistry.
Results:
The model of IgAN was established according to Bovine serum albumin-Lipopolysaccharide-Carbon tetrachloride protocol, which was evidenced by histological structural lesions of glomeruli, IgA deposition and urinary measurement. Histological examination of kidney sections from both rhein-prevented group and rhein-treated group showed that glomerular hypertrophy, mesangial expansion, excessive extracellular matrix, and renal capsule dilation were markedly ameliorated compared with IgAN group. Moreover, rhein treatment significantly reduced IgA deposition in glomerulus, the volume of urinary red blood cells and 24-h urinary protein excretion. More importantly, increased FN expression in IgAN was back to normal level in rhein-prevented and rhein-treated group, which was along with the reduction of α-SMA expression in renal tissues.
Conclusions:
These findings indicate that rhein prevents the development of glomerulosclerosis and halts the progression of IgAN via inhibition of FN and α-SMA expression.
KEY WORDS: IgA nephropathy, rat, rhein
Introduction
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide and characterized by deposition of the IgA in the glomerulus.[1] Long-term follow-up studies have shown that up to 25-30% of IgAN patients progress to end-stage kidney disease within 20-25 years.[2] However, the etiology and pathogenesis of IgAN are still uncertain and no disease-specific therapy for IgAN exists. IgAN, as a mesangial proliferative glomerulonephritis, is characterized by extracellular matrix accumulation and deposition. So we selected fibronectin (FN) as a representative of the extracellular matrix in this study. The α-smooth muscle actin (α-SMA)-positive myofibroblasts are the effector cells that are primarily responsible for the overproduction of the extracellular matrix in pathogenic conditions and there is almost no myofibroblasts in the normal renal tissues.[3] Thus, α-SMA was chosen as the marker of myofibroblasts activation.
Rhein (4, 5-dihydroxyanthraquinone-2-carboxylic acid) is one of anthraquinone monomers isolated from rhubarb, which has been broadly used in the treatment of diabetic nephropathy and other chronic renal diseases in experiment and clinic. Rhein can inhibit metabolism in diabetic nephropathy mice, reduce urinary protein, ameliorate glomerular hypertrophy, narrow the area of the renal corpuscle and mesangium, mitigate and suppress renal fibrosis.[4–6] But the effect of rhein on IgAN remains elusive. This study investigated the protective effect of rhein on renal tissue in IgAN rats.
Materials and Methods
Materials
Rhein (the purity of more than 95%) was extracted and identified by Chengdu Mansite, China. Fluorescein isothiocyanate (FITC)-conjugated antibody against IgA, antibodies against α-SMA and FN were purchased from Abcam, U.K. Bovine serum albumin (BSA) was purchased from Roche, Germany. Lipopolysaccharide (LPS) was purchased from Sigma, USA. Carbon tetrachloride (CCl4) and castor oil was purchased from Shanghai Reagents, China. Horseradish peroxidase-conjugated secondary antibodies were purchased from Beijing Zhongshan, China.
Animal Model
Twenty-eight female Sprague Dawley (SD) rats weighted 180-220 g were obtained from the animal center of Nanchang University. They were housed in the animal facilities of the Nanchang University, with free access to food and water. Animals were treated humanely by use of the protocols that were approved by the Institutional Animal Use and Care Committee of Nanchang University. Rats were divided randomly into control group, IgAN group, rhein-prevented group and rhein-treated group (n = 7). IgAN experimental animal models were established with BSA-LPS-CCL4,[7] and the specific implementations are as follows: BSA (400 mg/kg, oral every other day) × 6 weeks + LPS (0.05 mg, intravenous injection at the 6th and 8th weeks) + CCl4 (0.1 ml dissolved in 0.5 ml castor oil, subcutaneous injection weekly) × 9 weeks. The rhein-treated group was given rhein (100 mg/kg/d)[8] from the 7th week until they were sacrificed. The rhein-prevented group was given rhein (100 mg/kg/d) from the 1st week. The control group and IgAN group were given the same volume of normal saline. All of rats were sacrificed at the 10th week. One part of the kidneys was fixed in 4% paraformaldehyde, followed by paraffin embedding for paraffin sections (3 μm). The remaining kidneys were frozen in liquid nitrogen for frozen sections (10 μm). The 24-h urine was collected using metabolic cages before sacrificed for measuring the volume of urinary red blood cells and 24-h urinary protein excretion. Specimens from fresh urine 1 ml were centrifuged at 1500 r/min for 5 min. The supernatants were removed and urine sediment 0.02 ml were mixed well and dropped to the clean glass slides. The glass slides were randomly observed from 10 fields of vision at high magnification, the red blood cells were counted and the mean calculated. Red blood cells ≥3 per field of vision at high magnification, was considered positive for microscopic hematuria. The 24-h urine protein excretion were measured with the automatic biochemical analyzer.
H and E, and Immunohistochemical Staining
Kidney sections from paraffin-embedded tissues were prepared at 3 μm thickness using a routine procedure.[9] Sections were used to perform H and E, staining for general histology and to identify expression of FN and α-SMA by immunohistochemistry. Briefly, after dewaxing, dehydration, rehydration and antigen repair with microwave, paraffin sections were blocked with 3% H2O2 deionized water and 10% normal sheep serum in PBS (0.01 M) and subsequently incubated with the specific primary antibodies againstα-SMA (1:100, Abcam, U.K) and FN (1:200, Abcam, U.K) at 4°C overnight, followed by staining with horseradish peroxidase-conjugated secondary antibodies and counterstained with hematoxylin to visualize the nuclei. The α-SMA-stained paraffin sections were viewed under a common light microscope equipped with a digital camera, identifying α-SMA by brown color. FN-stained paraffin sections were analyzed by a morphological analysis system for semi- quantitatively determining the expression of FN. Briefly, 5 renal glomeruli (upper left, lower left, upper right, lower right and middle) were observed under high magnification per section, with two sections selected from each specimen; the integrated optical density of the positive material in each glomerulus and the glomerular area were measured by the morphological analysis system, the ratio of which showed the relative content of FN in the renal glomeruli.
Direct Immunofluorescence
Direct immunofluorescence staining was performed using an established procedure.[10] Briefly, frozen sections were fixed with cold acetone for 10 min at 4°C. After extensive washing 3 times (5 min per) with cold phosphate buffered saline (PBS), the frozen sections were blocked with 10% normal sheep serum in PBS and then incubated with the FITC-conjugated IgA antibodies (1:100, Abcam, U.K) at 4°C overnight. Stained frozen sections were viewed under Nikon Eclipse E600 Epi- fluorescence microscope equipped with a digital camera, identifying IgA by light green color (excitation light wave length of 490 nm). According to semi-quantitative standard grading at home and abroad, 5 grades were defined from 0 to 4 (Grade 0: Green fluorescence cannot be seen under low magnification and seems to be seen under high magnification; Grade 1: Green fluorescence seems to be seen under low magnification and can be seen under high magnification; Grade 2: Green fluorescence can be seen under low magnification and visible clearly under high magnification; Grade 3: Green fluorescence is clearly visible under low magnification and bright under high magnification; Grade 4: Green fluorescence is glare under high magnification).
Statistical Analysis
All data were expressed as mean ± SE. Statistical analysis was performed using SPSS 17.0 software. Comparison between groups was made using one-way analysis of variance followed by Student-Newman-Keuls test. Comparison between groups in hierarchical grouping data was made using Kruskal-Wallis test. A P value of less than 0.05 was considered to be statistically significant.
Results
Rhein Ameliorated the Structural Lesions of Glomeruli
Figure 1 presents the representative micrographs of the H and E, staining of renal tissue sections from control group, IgAN group, rhein-prevented group and rhein-treated group. The size of renal corpuscle of the control group was normal, with no significant proliferation in the mesangial area. The renal corpuscle of the IgAN group expanded; the mesangial area was significantly widened, mesangial cells and mesangial matrix increased and renal capsule dilated. The kidney sections from rhein-prevented group and rhein-treated group showed that the above-mentioned structural lesions of glomeruli were markedly ameliorated as compared with IgAN group. The effect of rhein-prevented group was better.
Figure 1.
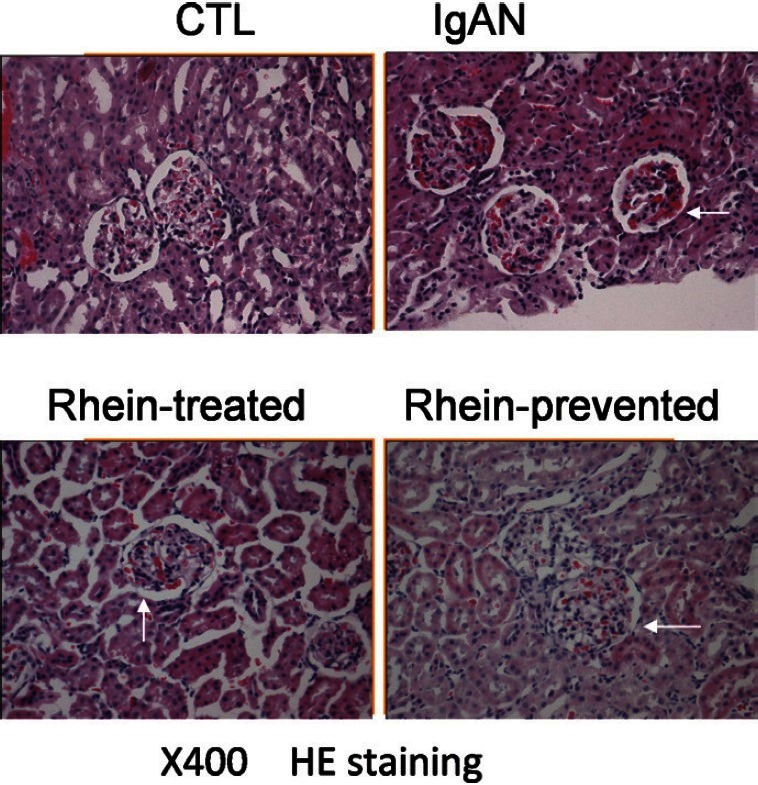
Renal cortex by H and E staining
Rhein Decreased the Glomerular Deposition of IgA
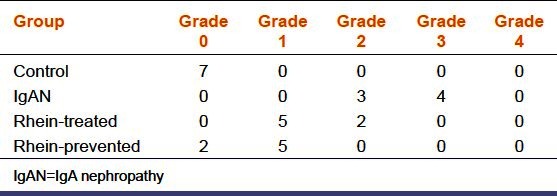
Direct immunefluorescence staining was used to examine the effect of rhein on the glomerular deposition of IgA. As shown in Figure 2, no green fluorescence has been seen in the renal glomerulus of control group, however, bright green fluorescence in a granular pattern in the mesangium has been seen in IgAN group (P < 0.01). Green fluorescence was weaker in rhein-prevented group and rhein-treated group (P < 0.01). Green fluorescence in glomeruli decreased more significantly in the rhein-prevented group compared with the rhein-treated group (P < 0.05) [Table 1].
Figure 2.
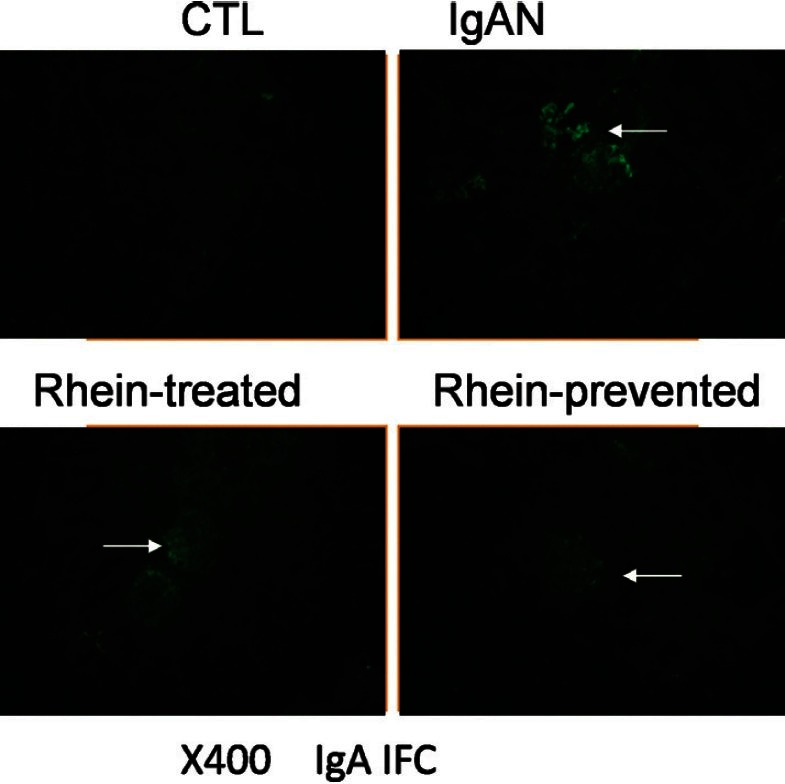
Renal cortex by IgA immunofluorescence staining
Table 1.
The grade of the glomerular deposition of IgA
Rhein Reduced the Volume of Urinary Red Blood Cells and 24-h Urinary Protein Excretion
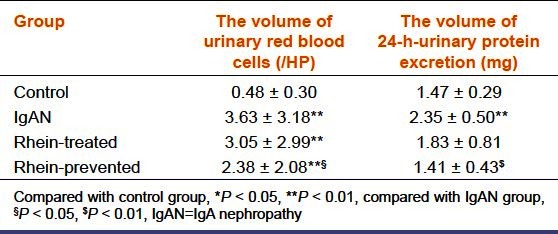
The classic presentation of IgAN is episodic macroscopic hematuria and (or) persistent microscopic hematuria. Compared with the control group, the volume of urinary red blood cells and 24 h-urinary protein excretion increased in the IgAN group (P < 0.01). Those in the rhein-prevented group and rhein-treated group decreased compared with the IgAN group, of which, the decrease in the rhein-prevented group was statistically significant (P < 0.05) [Table 2].
Table 2.
The number of urinary red blood cells and the amount of 24-h urinary protein excretion
Rhein Inhibited Fibronectin Expression in the Renal Glomeruli of IgA Nephropathy
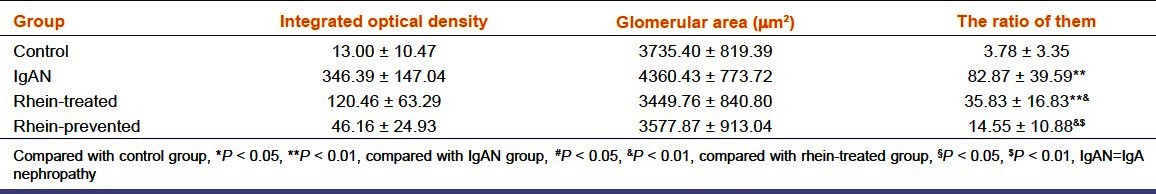
Glomerulosclerosis is characterized by extracellular matrix accumulation and deposition. We selected FN as a representative of the extracellular matrix. Immunohistochemical staining was used to examine the effect of rhein on the FN expression in the renal glomeruli of IgAN. As demonstrated in Figure 3, renal glomeruli of control group express trivial amount of FN. However, compared with the control group, the expression of FN in the renal glomeruli was significantly enhanced in the IgAN group (P < 0.01), indicating extracellular matrix accumulation. Meanwhile, FN expression in glomeruli declined in the rhein-prevented group and rhein-treated group (P < 0.01). FN expression in glomeruli decreased more significantly in the rhein-prevented group compared with the rhein-treated group (P < 0.01). The ratio of the integrated optical density of FN in renal glomerulus and the glomerular area confirmed this result [Table 3]. This result indicated that rhein could inhibit FN expression in the renal glomeruli of IgAN, suppress accumulation of the extracellular matrix and prevent the development of glomerulosclerosis.
Figure 3.
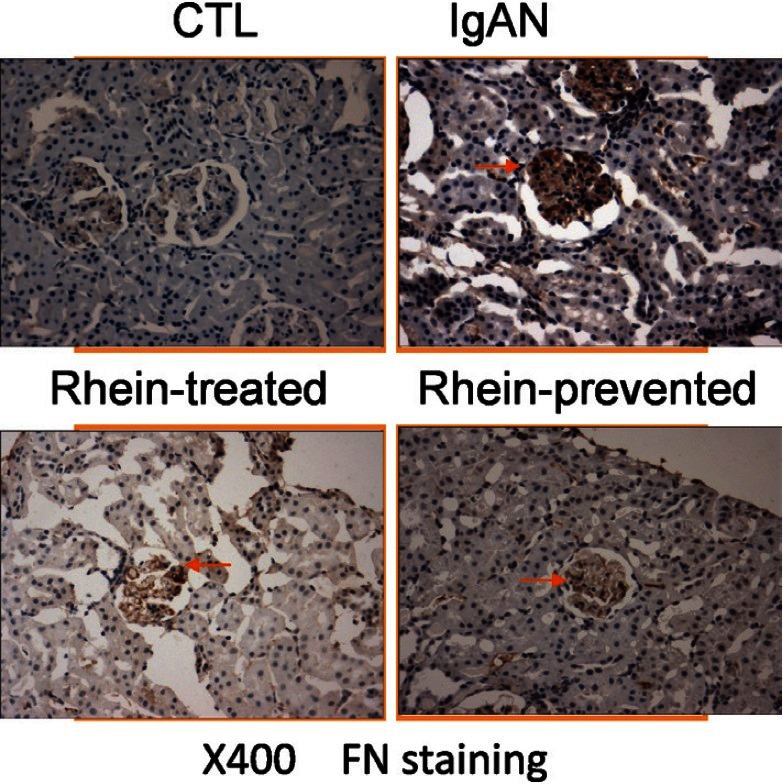
Renal cortex by fibronectin immunohistochemical staining
Table 3.
Relative content of fibronectin in the renal glomeruli
Rhein inhibited α-Smooth Muscle Actin Expression in the Renal Tubulointerstitial of IgA Nephropathy
The α-SMA-positive myofibroblasts are the effector cells that are primarily responsible for the overproduction of the extracellular matrix in pathogenic conditions and there is almost no myofibroblasts in the normal renal tissues. Thus, α-SMA was chosen as the marker of myofibroblasts activation. Figure 4 displays that α-SMA expressed in vascular smooth muscle cells only in the control group, and the renal glomerular, tubular or interstitial expression was not found. However, in the IgAN group, α-SMA expressed not only in vascular smooth muscle cells but also in tubular and interstitial areas, suggesting activation of the renal myofibroblasts. In the rhein-prevented group and rhein-treated group, α-SMA expression of renal tubules and interstitium significantly reduced compared with the IgAN group. The effect of rhein-prevented group was better. This result indicated that rhein could inhibit α-SMA expression in the renal tubulointerstitial of IgAN, suppress the activation of myofibroblasts and prevent the progression of tubulointerstitial fibrosis.
Figure 4.
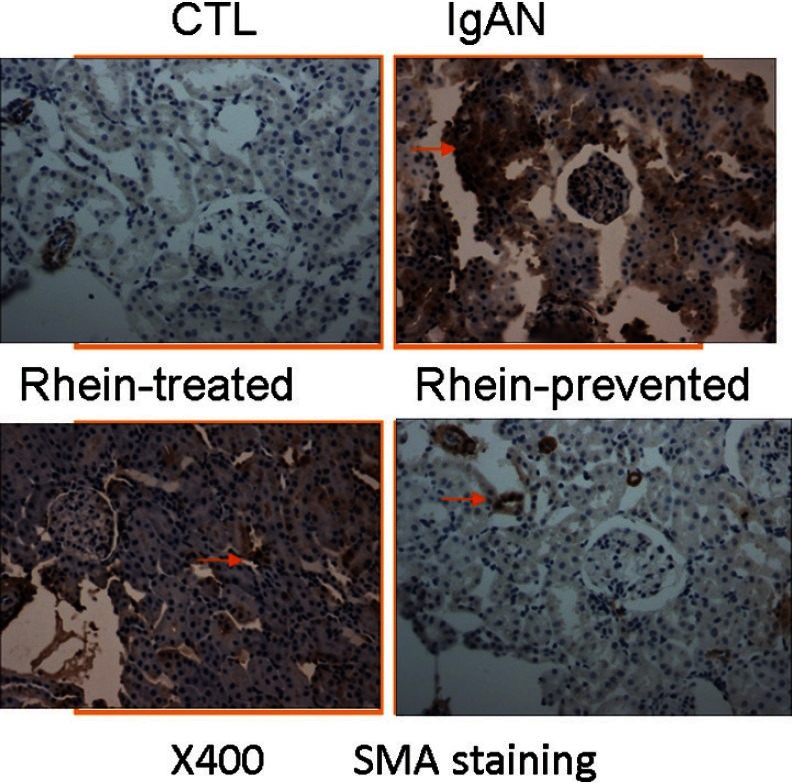
Renal cortex by α-smooth muscle actin immunohistochemical staining
Discussion
A kidney biopsy is necessary to confirm the diagnosis of IgAN. Histologically, IgAN may show widened mesangium, and diffuse mesangial proliferation or crescentic glomerulonephritis may also occur. The mesangium by light microscopy may be hypercellular and show increased deposition of extracellular matrix.[11] End-stage will progress to renal fibrosis, and even nephrosclerosis. Immunoflourescence shows mesangial deposition of IgA often associated with C3 and smaller amounts of other immunoglobulins (IgG or IgM). Hence, it is an effective therapy for IgAN to develop a strategy that blocks the fibrogenic process and reduces the IgA deposition in the glomeruli. The purpose of this study was to determine whether rhein, which has been shown to be preventive in retarding the onset of renal fibrosis in db/db mice with diabetic nephropathy,[12] has therapeutic effects to ameliorate renal fibrosis of IgAN.
Although the pathological mechanism of IgAN is not completely elucidated, the progression of renal fibrosis plays a critical role in permanent loss of the normal structural and functional integrity of the kidney.[13] Our results demonstrate that rhein markedly ameliorates glomerular hypertrophy, mesangial expansion, extracellular matrix accumulation and renal capsule dilation in IgAN rats.
Renal fibrosis is characterized by destruction of normal tissue and excess production and deposition of extracellular matrix. FN is a protein with strong adhesion activity and is the main component of the extracellular matrix. As an adhesion molecule of matrix, it appears,firstly, in the extracellular matrix of the damaged tissue and constitutes the reticular stent for the deposition of the collagen, playing an important role in the process of fibrosis.[14] Moreover, the accumulation and strong chemotaxis of FN and collagen are crucial foundations to cause fibroblasts, mesangial and endothelial cell proliferation.[15] FN controls the cell proliferation, differentiation and apoptosis signal by the cell surface receptor-integrin. Therefore, the deposition of FN in the glomeruli may suggest the activity of glomerular cell proliferation and lesion progress. Our results showed that a small amount of FN expression in the glomeruli of the control group has been observed. The FN expression in the IgAN group was significantly higher than in the control group. Compared with the IgAN group, rhein markedly reduced the expression of FN in the glomeruli of the rhein-prevented group and rhein-treated group. The effect of some plants against renal dysfunction was reported recently,[16] rhein also has immunosuppressive properties. Thus, we speculated that rhein might reduce the FN expression by inhibiting the inflammatory cells to secrete interleukin-6, tumor necrosis factor-α, transforming growth factor-β (TGF-β) and other cytokines to suppress the production of FN by the mesangial cells and fibroblasts.[17]
The α-SMA is an actin found in the cytoplasm of vascular smooth muscle cells, which exists only in the cells derived from smooth muscle. Myofibroblasts are a unique group of smooth-muscle-like fibroblasts that express α-SMA and secrete extracellular matrix. They are responsible for production and deposition of extracellular matrix in diseased kidneys.[18] Suppressing renal myofibroblasts activation can attenuate renal extracellular matrix deposition in kidneys. Myofibroblasts are α-SMA-positive and will not present in normal kidneys. The high expression of α-SMA indicates altered phenotype of renal inherent cells, the expression of α-SMA in the kidney can indirectly reflect the number of the myofibroblasts and the degree of fibrosis.[19] Thus, we can evaluate the activation degree of myofibroblasts by observing the α-SMA expression in the kidney. As seen in Fig 4, no expression of α-SMA has been found in renal tissues except for the vascular smooth muscle cells in the control group. The α-SMA expression in the IgAN group was significantly higher than in the control group, mainly in the tubular and interstitial areas. Rhein observably reduced the expression of α-SMA in the kidneys of the rhein-prevented group and rhein-treated group. That demonstrates rhein can inhibit myofibroblasts activation in the kidneys with IgAN.
The origin of myofibroblasts under pathological conditions is unclear. They are often presumed to be derived from local resident interstitial fibroblasts. Recently, several lines of evidence suggests that these cells may also come from tubular epithelial cells via a process known as epithelial-to-mesenchymal transition.[20,21] In response to tissue injury, renal interstitial fibroblasts and tubular epithelial cells undergo an activation process to become α-SMA-positive myofibroblast and produce abundant extracellular matrix.[22] A variety of cytokines involve in this phenotypic activation, in which TGF-β is the prime stimulator, as shown in previous studies.[23] The influence of other cytokines depends on regulating the generation of TGF-β. So rhein may reduce the extent of renal fibrosis of rats with IgAN, at least in part, by decreasing the TGF-β secreted by inflammatory cells to inhibit activation of renal interstitial fibroblasts and tubular epithelial cells.
Proteinuria caused multiple injury on renal tubular and interstitial, an important factor leading to IgAN deterioration.[24] The filtration and reabsorption imbalances result from the formation of hematuria and proteinuria, the increase of glomerular mesangial cells and mesangial matrix, the hoist of glomerular capillary pressure, the increased permeability of the filtration membrane, the damage of renal tubular epithelial cells and the phenotypic change of renal interstitial fibroblasts.[25] In this study, we found that urinary red blood cell count and 24 h-urinary protein were increased in the IgAN group compared with the control group. The number of urinary red blood cells and the volume of 24 h-urinary protein in the rhein-prevented group and rhein-treated group were lower than the IgAN group, suggesting that rhein can effectively reduce hematuria and proteinuria. The mechanism may be related to the inhibition of renal fibrosis and cell phenotypic change.
The pathogenesis of IgAN is not completely clarified, but the production and deposition of IgA play a critical role in the onset. Our observations indicate that rhein evidently reduce the deposition of IgA in the glomeruli of rhein-prevented group and rhein-treated group, suggesting that rhein may be alleviate the renal lesions by inhibiting the production and deposition of IgA. The process involves many other factors and further research is needed.
In summary, this study highlights that rhein acts as an effective negative regulator of renal fibrosis and halts the progression of IgAN in rats. Its therapeutic mechanism is, at least in part, to inhibit the phenotypic transformation of interstitial fibroblasts and tubular epithelial cells to reduce the deposition of the extracellular matrix. In this regard, rhein may be a potential therapeutic agent for inhibiting renal fibrogenesis in IgAN.
Footnotes
Source of Support: Jiangxi province science and technology support program, 20111BBG70015-3
Conflict of Interest: None declared
References
- 1.Floege J. The pathogenesis of IgA nephropathy: What is new and how does it change therapeutic approaches? Am J Kidney Dis. 2011;58:992–1004. doi: 10.1053/j.ajkd.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–97. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 3.Ina K, Kitamura H, Tatsukawa S, Fujikura Y. Significance of α-SMA in myofibroblasts emerging in renal tubulointerstitial fibrosis. Histol Histopathol. 2011;26:855–66. doi: 10.14670/HH-26.855. [DOI] [PubMed] [Google Scholar]
- 4.Jia ZH, Liu ZH, Zheng JM, Zeng CH, Li LS. Combined therapy of rhein and benazepril on the treatment of diabetic nephropathy in db/db mice. Exp Clin Endocrinol Diabetes. 2007;115:571–6. doi: 10.1055/s-2007-981469. [DOI] [PubMed] [Google Scholar]
- 5.Zheng JM, Zhu JM, Li LS, Liu ZH. Rhein reverses the diabetic phenotype of mesangial cells over-expressing the glucose transporter (GLUT1) by inhibiting the hexosamine pathway. Br J Pharmacol. 2008;153:1456–64. doi: 10.1038/bjp.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He D, Lee L, Yang J, Wang X. Preventive effects and mechanisms of rhein on renal interstitial fibrosis in obstructive nephropathy. Biol Pharm Bull. 2011;34:1219–26. doi: 10.1248/bpb.34.1219. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y, Lou TQ, Cheng CL, Peng H, Guan WM. Improvement of experimental IgA nephropathy model. J Sun Yat-sen University (Medical Sciences) 2006;27:184–7. [Google Scholar]
- 8.Guo MZ, Li XS, Xu HR, Mei ZC, Shen W, Ye XF. Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats. Acta Pharmacol Sin. 2002;23:739–44. [PubMed] [Google Scholar]
- 9.Hatakeyama K, Wakabayashi-Nakao K, Aoki Y, Ogura S, Yamaguchi K, Nakajima T, et al. Novel protein extraction approach using micro-sized chamber for evaluation of proteins eluted from formalin-fixed paraffin-embedded tissue sections. Proteome Sci. 2012;10:19. doi: 10.1186/1477-5956-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Ye F, He Y, Kong D, Han C, Zhao Z, et al. Establishment of a mouse IgA nephropathy model with the MBP-20-peptide fusion protein. Anat Rec (Hoboken) 2010;293:1729–37. doi: 10.1002/ar.21225. [DOI] [PubMed] [Google Scholar]
- 11.Manno C, Strippoli GF, D’Altri C, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am J Kidney Dis. 2007;49:763–75. doi: 10.1053/j.ajkd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Gao Q, Qin WS, Jia ZH, Zheng JM, Zeng CH, Li LS, et al. Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med. 2010;76:27–33. doi: 10.1055/s-0029-1185948. [DOI] [PubMed] [Google Scholar]
- 13.Lim BJ, Kim JH, Hong SW, Jeong HJ. Expression of fibrosis-associated molecules in IgA nephropathy treated with cyclosporine. Pediatr Nephrol. 2009;24:513–9. doi: 10.1007/s00467-008-1055-z. [DOI] [PubMed] [Google Scholar]
- 14.Pavan MV, Ghini B, Castro M, Lopes De Faria JB. Prevention of hypertension attenuates albuminuria and renal expression of fibronectin in diabetic spontaneously hypertensive rats. Am J Nephrol. 2003;23:422–8. doi: 10.1159/000074454. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Pang S, Deng B, Qian L, Chen J, Zou J, et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int J Biochem Cell Biol. 2012;44:629–38. doi: 10.1016/j.biocel.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Singh AP, Singh AJ, Singh N. Pharmacological investigations of Punica granatum in glycerol-induced acute renal failure in rats. Indian J Pharmacol. 2011;43:551–6. doi: 10.4103/0253-7613.84971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu ZH, Li YJ, Chen ZH, Liu D, Li LS. Glucose transporter in human glomerular mesangial cells modulated by transforming growth factor-beta and rhein. Acta Pharmacol Sin. 2001;22:169–75. [PubMed] [Google Scholar]
- 18.Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2992–8. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos E, Gionanlis L, Papayianni E, Kokolina E, Leontsini M, Memmos D. Predictors of outcome in idiopathic rapidly progressive glomerulonephritis (IRPGN) BMC Nephrol. 2006;7:16. doi: 10.1186/1471-2369-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol. 2009;175:1371–3. doi: 10.2353/ajpath.2009.090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Järvinen PM, Laiho M. LIM-domain proteins in transforming growth factor ß-induced epithelial-to-mesenchymal transition and myofibroblast differentiation. Cell Signal. 2012;24:819–25. doi: 10.1016/j.cellsig.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Minz R, Bakshi A, Chhabra S, Joshi K, Sakhuja V. Role of myofibroblasts and collagen type IV in patients of IgA nephropathy as markers of renal dysfunction. Indian J Nephrol. 2010;20:34–9. doi: 10.4103/0971-4065.62098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai JJ, Chen YP, Rui HL. Effects of Hirsutella sinensis on TGF-beta1 and Snail expressions and transdifferentiation of tubular epithelial-myofibroblast in renal tissue of rats with chronic aristolochic acid nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:325–9. [PubMed] [Google Scholar]
- 24.Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, et al. Early prediction of IgA nephropathy progression: Proteinuria and AOPP are strong prognostic markers. Kidney Int. 2004;66:1606–12. doi: 10.1111/j.1523-1755.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- 25.Cirillo M. Evaluation of glomerular filtration rate and of albuminuria/proteinuria. J Nephrol. 2010;23:125–32. [PubMed] [Google Scholar]


