Abstract
Objective:
To compare the pattern, efficacy, and tolerability of self-medicated drugs and to assess the adequacy of their dose in primary dysmenorrhea (PD).
Materials and Methods:
A survey using a self-developed, validated, objective, and structured questionnaire as a tool was conducted among subjects with PD. Statistical analysis was carried out using Chi-square test and ANOVA with post-hoc Tuckey's test.
Results:
Out of 641 respondents, 42% were self-medicated. The pattern of drugs used was: Dicyclomine, an unknown drug, mefenamic acid, mefenamic acid + dicyclomine, and metamizole by 35%, 29%, 26%, 9%, and 1% of respondents, respectively. Mefenamic acid + dicyclomine, the combination was the most efficacious in comparison to other drugs in moderate to severe dysmenorrhea. There was better tolerability with mefenamic acid + dicyclomine group compared to other drugs. Sub-therapeutic doses were used by 86% of self-medicating respondents.
Conclusions:
The prevailing self-medication practices were inappropriate in a substantial proportion of women with inadequate knowledge regarding appropriate drug choice, therapeutic doses, and their associated side effects.
KEY WORDS: Efficacy, primary dysmenorrhea, self-medication, survey, tolerability
Introduction
Primary dysmenorrhea (PD), a common gynaecological disorder affecting nearly 50% of menstruating women, is characterized by painful menstruation in the absence of any underlying pelvic pathology.[1,2] It is mostly confined to the adolescent age group, appearing within 6-12 months after menarche and coinciding with the onset of regular ovulatory cycles. The prevalence is particularly high among adolescents (50-70%) disrupting educational and social life, leading to school absenteeism and loss of labor.[3]
Dysmenorrhea results from increased prostaglandins (PGs), which induce intense uterine contractions, decrease uterine blood flow, and increase peripheral nerve hypersensitivity, resulting in pain. Clinical presentation of PD consists of colicky suprapubic pain and may be accompanied by lumbosacral backache, pain radiating down the anterior thigh, nausea, vomiting, diarrhea, and rarely syncopal attack.[1]
The mainstay of treatment includes various classes of non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, mefenamic acid, naproxen, ketoprofen, and celecoxib.[4] Hormonal therapy in the form of oral contraceptive pills (OCPs) to inhibit ovulation and to decrease endometrial proliferation and PG synthesis is particularly useful in patients with severe PD. Other hormonal agents used are medroxyprogesterone acetate, levonorgestrel-releasing intrauterine devices (IUDs), and GnRH analogues like leuprolide acetate.[5]
Self-medication is defined as the use of medication by a patient on his own initiative or on the advice of a pharmacist or a layperson instead of consulting a medical practitioner.[6] Self-medication for PD is common with an incidence of 38-80%.[7] With extensive accessibility to over-the-counter drugs for PD, there exists lack of awareness regarding appropriate choice of drugs and adequate therapeutic dose.[8,9]
However, to the best of our knowledge the published data regarding self-medication for PD in India is found lacking and yet to be established and hence the present study was undertaken to evaluate the existing self-medication pattern, to compare the efficacy, adequacy, and tolerability of self- medicated drugs in PD.
Materials and Methods
We conducted a survey in various educational institutions in and around Bangalore from January 2011 to June 2011 with prior approval from Institutional Ethics Committee.
The inclusion criteria were females aged between 18- 30 years, with regular menstrual cycle, with at least four painful menstrual cycles during the preceding six months, prior normal abdomino-pelvic scan, if available, and willing to give written informed consent. Exclusion criteria included women <18 years or > 30 years of age, history of irregular menstrual cycle, with <4 painful menstrual cycles during the preceding six months, not willing to give written informed consent, subjects receiving concomitant medications including antipsychotics, antidepressants, sedative hypnotics, antispasmodics, corticosteroids, taking physician prescribed medications, resorting to only non-pharmacological measures, incomplete questionnaires, and with no available normal abdomino-pelvic scan. The verbal multidimensional scoring system was used for assessment of dysmenorrhea severity.[7] The survey questionnaire was pre-validated for reliability coefficient by initially administering it to a sample of 40 women across different professions and strata of society.
Following data was collected: Demographic characteristics, menstrual history including age at menarche, severity and duration of dysmenorrhea, number of days missed at work/class due to dysmenorrhea and associated symptoms, and details of self-medication including pattern, adequacy of dose, efficacy (based on verbal rating scale), and tolerability.
Statistical analysis was done using SPSS version 19. The characteristics of demographic and menstrual pain and self-medication patterns were described using descriptive statistics. The association of self-medication pattern and severity of pain was analysed using ANOVA and post-hoc Tuckey's test. The efficacy and tolerability assessment was done using Chi-square test; P values were calculated, with P < 0.05 considered statistically significant.
Results
The mean age and mean age at menarche of 641 respondents was 23.5 ± 2.5 years and 12.6 ± 1.6 years, respectively. The survey included respondents studying in various professions like nursing 28%, medical 26%, agricultural sciences 21%, engineering 7%, pharmacy 6%, dental 5%, and physiotherapy 7%. The mean duration of dysmenorrhea was 2.2 ± 0.5 days/month. Sixty three percent reported missing of school/work for 2 - 7 days/month. The most commonly associated symptoms which were overlapping included nausea/vomiting (76%) followed by headache (63%), sweating (39%), diarrhea (38%), dizziness (36%), loss of appetite (35%), and fainting (28%).
Among 641 respondents, 42% (n = 269) resorted to self-medication, 53% followed non pharmacological methods, and only 5% took physician-prescribed medication. The data obtained from self-medicating respondents was considered for the study.
Self-medication was practiced by 27% nursing, 56% medical, 36% agricultural, 47% engineering, 66% pharmacy, 47% dental, and 40% of physiotherapy students. Pharmacy and medical students significantly resorted to self-medication in comparison to respondents of other professions (P = 0.0001).
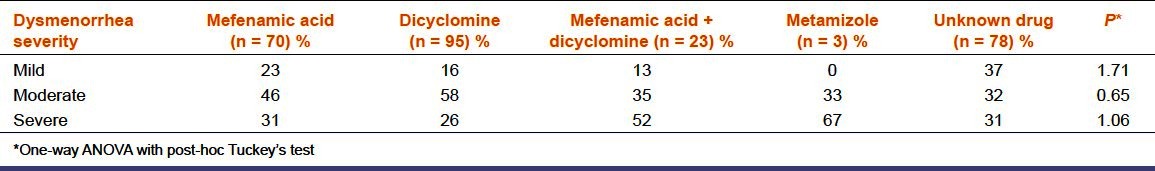
Self-medication was initiated by physician/nurse, pharmacist and relatives/self in 41%, 8%, and 51%, respectively. The existing pattern of self-medication was as follows: Dicyclomine, an unknown drug, mefenamic acid, mefenamic acid + dicyclomine, and metamizole in 35%, 29%, 26%, 9%, and 1% of respondents, respectively. Severity of dysmenorrhea among respondents was as follows: 23% mild, 45% moderate, and 32% severe. The pattern of self-medication in relation to severity of dysmenorrhea was assessed [Table 1]. The prevalence of self-medication was more in respondents with moderate and severe dysmenorrhea (86%, P = 0.0001). In respondents with mild dysmenorrhea, the least commonly used was mefenamic acid + dicyclomine. Dicyclomine, mefenamic acid were used more frequently, while 37% consumed an unknown drug. In respondents with moderate dysmenorrhea, most commonly used was dicyclomine followed by mefenamic acid, mefenamic acid + dicyclomine, metamizole, while 32% took an unknown drug. In respondents with severe dysmenorrhea, metamizole was most commonly used followed in descending order by mefenamic acid + dicyclomine, mefenamic acid, unknown drug, and dicyclomine. The severity of dysmenorrhea did not influence the pattern of self-medication [Table 1, P > 0.05].
Table 1.
Comparison of severity of dysmenorrhea & pattern of self medication (n = 269)
The efficacy of the self-medicated drugs was assessed with respect to dysmenorrhea severity based on the verbal rating scale [Table 2]. In mild dysmenorrhea – mefenamic acid, dicyclomine, mefenamic acid + dicyclomine, and unknown drug were equally efficacious (P = 0.7960). In moderate and severe dysmenorrhea, mefenamic acid + dicyclomine was perceived to be more efficacious compared to other drugs [Table 2, P = 0.0006, P = 0.0403]. The daily dose adequacy of self-medicated drugs was calculated and compared with standard recommended daily dose for that medication.[10,11] Eighty six percent, 5%, and 9% respondents consumed <99%, 100%, and >101% of maximum daily doses of self-medicated drugs, respectively [Figure 1]. The most commonly associated side effects were nausea (72%), followed by abdominal pain (27%), headache (27%), and dizziness (16%). Mefenamic acid + dicyclomine was better tolerated than other drugs (P = 0.0001).
Table 2.
Pain relief assessment based on verbal rating scale (n = 269)
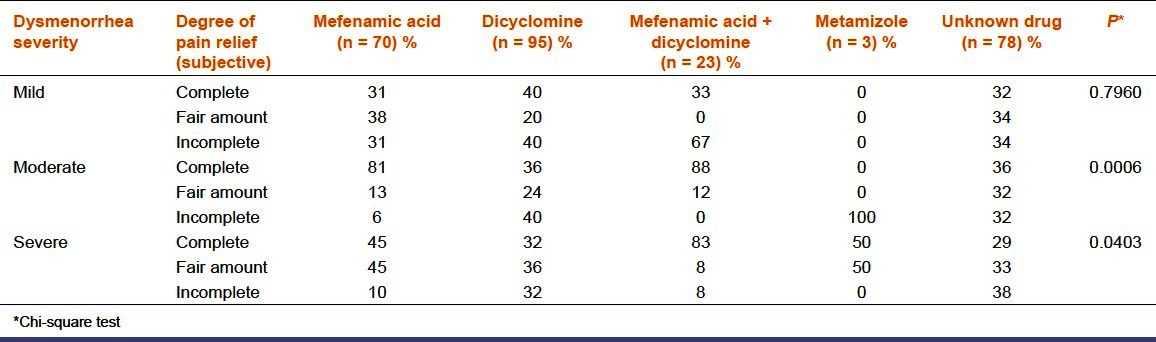
Figure 1.
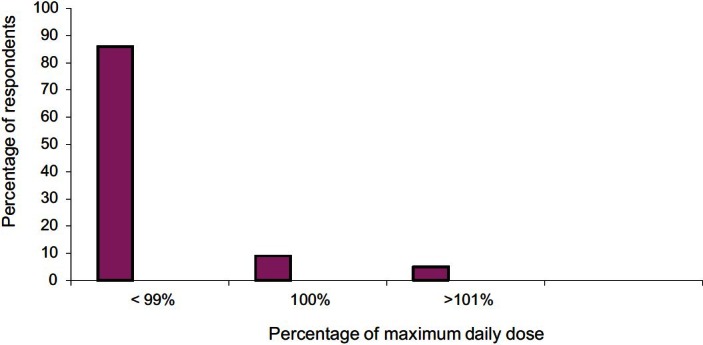
Comparison of dose adequacy of self medicated drugs with their standard recommended daily dose* (n = 191). *Not assessed for respondents taking unknown drugs.
Discussion
It is inferred from our study that the prevalence of self-medication is significant among the pharmacy and medical students with similar results from previous studies, probably due to better awareness of self-medication and greater access to drug information during their curriculum.[12] Similar to various other studies, majority reported missing school/work, implicating a negative impact on quality of life with substantial social, economical, and educational consequences.[13] Fifty three percent respondents used non-pharmacological measures such as acupressure, massage, topical heat/cooling therapy, exercises which are found to be generally less effective and often harmful.[9,14,15] In majority of women (51%), self-medication was initiated by self/relatives, akin to previous studies.[15,16] Reasons like lack of initiative to seek medical help, inaccessibility to medical care, dysmenorrhea considered as insignificant physiological menstrual pain, lack of time to approach physician as majority of the respondents were students, confidence in self/relatives regarding drug choice based on their prior experience, economical and convenient access to non-pharmacological measures, and readily available OTC drugs may be attributed to the existing above self-medication practices followed by majority of women.
Our study showed that majority of women with moderate to severe dysmenorrhea resorted to self-medication probably due to increased burden of morbidity associated with it necessitating the need for self-medication.[17] These results were similar to previous studies.[9,17] Though dicyclomine was the most commonly used, there existed no statistically significant association between self-medication pattern and severity of dysmenorrhea which is similar to the results of a pre-existing study [Table 1].[9] In our study, mefenamic acid+ dicyclomine was found to be more effective in reducing moderate and severe dysmenorrhea when compared to other drugs [Table 2].[11] Mefenamic acid, a NSAID, relieves PD primarily by suppressing endometrial prostaglandin (PG) production, thus alleviating cramps and restoring normal uterine activity.[13] It is also found to decrease the volume of menstrual flow and relieve PG-induced symptoms like headache, bloating, diarrhea, and breast tenderness.[18,19] In addition, it is also reported to have direct analgesic action on Central Nervous System (CNS) mediated by interactions with descending serotonergic pathways, together with modulation of neurotransmission at glycine or N-methyl-D-aspartate receptors independent of cyclo-oxygenase inhibition.[20] In PD, which is spasmodic in nature, the combination of mefenamic acid with dicyclomine is likely to be synergistic [Table 2].[21] Majority of the women consumed dicyclomine alone which was less efficacious in moderate and severe dysmenorrhea [Table 2], as a non PG synthesis inhibitor was chosen for its antispasmodic effect. It probably reflects the lack of awareness regarding appropriate drug choice. Similar results in the past have shown that women rely on medications whose efficacy in reducing dysmenorrhea is not known.[22] In mild dysmenorrhea, all drugs were equally efficacious probably due to self-limiting physiological nature of dysmenorrhea. One percent respondents used metamizole which is associated with agranulocytosis. Seventy eight percent respondents were not aware of the name of the drug. This ignorant unsafe act carries serious implications like harm to the patient and a constant risk of medication error. Majority of the respondents (86%) used the medications at considerably less than the recommended therapeutic dose with comparable observations from past studies; probably due to lack of awareness of appropriate dose [Figure 1].[8,9] Nine percent of respondents used more than the recommended daily doses of the medications which may implicate serious consequences. Their observations indicated the negative consequences of self-medication. Seventy one percent of the women were not aware of the side effects of the self medicated drugs (P = 0.0001). The combination of mefenamic acid + dicyclomine is better tolerated (P = 0.034). This is probably because dicyclomine, being an antimuscarinic and spasmolytic, relaxes the gut reducing diarrhea associated with mefanamic acid. These results matched with another study where fixed dose combination of mefenamic acid with dicyclomine was highly effective and well-tolerated in spasmodic dysmenorrhea.[11]
The limitations of this study are the small sample size, with only women of from urban population included. Being a questionnaire-based study the data was retrospective from recollection of memory, and pain relief was based on subjective assessment of the respondents.
This, however, does not undermine our observation that though prevalence of self-medication is high among women with PD, there is no adequate knowledge of appropriate selection of effective medication, correct dosing, and awareness of associated side effects. The findings suggest a need to create appropriate pharmacological management of primary dysmenorrhea (PD).
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Ropkin AJ, Howe NC. Pelvic pain and dysmenorrhea. In: Berek JS, editor. Novak's Gynecology. 14th ed. Philadelphia: Lippincot Williams & Wilkins; 2007. pp. 505–40. [Google Scholar]
- 2.Umland EM, Weinstein LC, Buchanan E. Menstruation related disorders. In: Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LW, editors. Pharmacotherapy a pathophysiologic approach. 7th ed. New York: McGraw Hill; 2008. pp. 1329–44. [Google Scholar]
- 3.Agarwal AK, Agarwal A. A study of dysmenorrhea during menstruation in adolescent girls. Indian J Community Med. 2010;35:159–64. doi: 10.4103/0970-0218.62586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKay HT. Dysmenorrhea. In: McPhee ST, Papadakis MA, Gonzales R, Zeiger R, editors. Current Medical Diagnosis and Treatment. 49th ed. New York: McGraw Hill Lange; 2010. pp. 655–86. [Google Scholar]
- 5.Sanfilippo J, Erb T. Evaluation and management of dysmenorrhea in adolescents. Clin Obstet Gynecol. 2008;51:257–67. doi: 10.1097/GRF.0b013e31816d2307. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organisation: Guidelines for the regulatory assessment of medicinal products for use in self-medication. [Last accessed on 2011 Dec 2]. Available from: http://apps.who.int/medicinedocs/pdf/s2218e/s2218e.pdf .
- 7.Andersch B, Milsom I. An epidemiological study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144:655–60. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- 8.Campbell MA, McGrath PJ. Use of medication by adolescents for the management of menstrual discomfort. Arch Pediatr Adolesc Med. 1997;151:905–13. doi: 10.1001/archpedi.1997.02170460043007. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell K, Davis AR, Westhoff C. Self-treatment patterns among adolescent girls with dysmenorrhea. J Pediatr Adolesc Gynecol. 2006;19:285–9. doi: 10.1016/j.jpag.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kastrup EK, editor. USA: Wolters Kluwer Health; 2012. Drug facts and comparisons. [Google Scholar]
- 11.Dabholkar KM. Mefenamic acid with dicyclomine is a highly effective and well tolerated treatment for spasmodic dysmenorrhea. Indian Pract. 1999;52:767. [Google Scholar]
- 12.Abay SM, Amelo W. Assessment of self medication practices among medical, pharmacy and health science students in Gondar University, Ethiopia. J Young Pharm. 2010;2:306–10. doi: 10.4103/0975-1483.66798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolhe S, Deb S. Dysmenorrhea. Obstet Gynaecol Reprod Med. 2011;21:311–6. [Google Scholar]
- 14.Eryilmaz G, Ozdemir F. Evaluation of menstrual pain management approaches by Northeastern Anatolian adolescents. Pain Manag Nurs. 2009;10:40–7. doi: 10.1016/j.pmn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. 2000;154:1226–9. doi: 10.1001/archpedi.154.12.1226. [DOI] [PubMed] [Google Scholar]
- 16.El-Gilany AH, Badawi K, El-Fedawy S. Epidemiology of dysmenorrhea among adolescent students in Mansoura, Egypt. East Mediterr Health J. 2005;11:155–63. [PubMed] [Google Scholar]
- 17.Tzafettas J. Painful menstruation. Pediatr Endocrinol Rev. 2006;3(Suppl 1):160–3. [PubMed] [Google Scholar]
- 18.French L. Dysmenorrhea in adolescents: Diagnosis and treatment. Pediatr Drugs. 2008;10:1–7. doi: 10.2165/00148581-200810010-00001. [DOI] [PubMed] [Google Scholar]
- 19.Patruno JE. Dysmenorrhea. In: Ehrenthal D, Hoffman MK, Hillard PJ, editors. Menstrual disorders. USA: Versa Press; 2006. pp. 97–125. [Google Scholar]
- 20.Bovill JG. Mechanisms of actions of opioids and non-steroidal anti-inflammatory drugs. Eur J Anaesthesiol Suppl. 1997;15:9–15. doi: 10.1097/00003643-199705001-00003. [DOI] [PubMed] [Google Scholar]
- 21.Porwal A, Mahajan AD, Oswal DS, Erram SS, Sheth DN, Balamurugan S, et al. Efficacy and tolerability of fixed dose combination of dexketoprofen and dicyclomine injection in acute renal colic. Pain Res Treat. 2012;2012:295926. doi: 10.1155/2012/295926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J. Level of knowledge among adolescent girls regarding effective treatment for dysmenorrhea. J Adolesc Health Care. 1988;9:398–402. doi: 10.1016/0197-0070(88)90036-8. [DOI] [PubMed] [Google Scholar]


