Dear Editor,
Calreticulin (CRT) is an endoplasmic reticulum (ER)-localized chaperone-like lectin that plays a crucial role in promoting the folding and maturation of newly synthesized glycoproteins and retaining incompletely/mis-folded proteins in the ER through its specific binding to monoglucosylated asparigine-linked glycans (GlcMan9GlcNAc2 with Glc, Man, and GlcNAc indicating glucose, mannose, and N-acetylglucosamine, respectively) (Michalak et al., 2009). CRT contains a cleavable signal peptide at its N-terminus and a tetrapeptide HDEL (His-Asp-Glu-Leu) ER retention/retrieval signal at its C-terminus. CRT3 and its ER membrane-anchored homolog calnexin (CNX) share a three-domain structure composed of a sugar-binding β-sandwich globular N-domain, an extended central proline-rich P-domain, and an acidic C-domain (Michalak et al., 2009). In vitro biochemical studies coupled with crystallographic analysis of recombinant CRT proteins have identified amino acid residues in the globular N-domain critical for its lectin function (Thomson and Williams, 2005; Gopalakrishnapai et al., 2006; Kozlov et al., 2010). However, no study verifying these in vitro findings in a living organism has been reported so far.
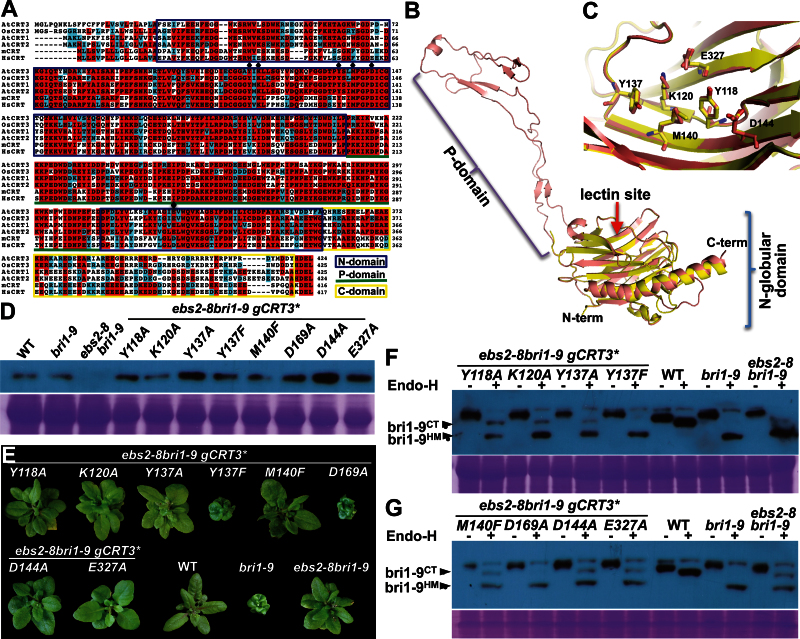
In this study, we used an Arabidopsis ems-mutagenized bri1 suppressor 2 (ebs2) mutant as a convenient genetic system to investigate the physiological importance of several amino acid residues of a predicted sugar-binding site of an Arabidopsis CRT in a plant protein quality-control process. Our previous studies showed that loss-of-function mutations in a plant-specific CRT, CRT3, suppress a unique Arabidopsis dwarf mutation, brassinosteroid-insensitive 1–9 (bri1-9) of the plant brassinosteroid (BR) receptor BRI1, which keeps a structurally imperfect but biochemically competent BR receptor in the ER via a CRT3–bri1-9 binding (Jin et al., 2007, 2009). Sequence alignment of the three Arabidopsis CRTs, a rice CRT3 homolog, and two mammalian CRTs coupled with molecular modeling of a 3-D structure of CRT3 identified conserved amino acids directly involved in binding monoglucosylated N-glycans, including Tyr118 (Y118), Lys120 (K120), Tyr137 (Y137), Met140 (M140), Asp144 (D144), and Glu327 (E327) (Figure 1A–1C). To verify the physiological importance of these residues in the predicted lectin function of CRT3, we performed site-directed mutagenesis experiments to mutate each of these residues to alanine (A) or phenylalanine (F) in a genomic CRT3 transgene (gCRT3) that could complement a null ebs2 mutation (Jin et al., 2009). We also mutated Asp169 (D169) to alanine because its corresponding residue in a mammalian CNX was previously shown to be involved in glucose binding (Schrag et al., 2001). We reasoned that, if these conserved residues are involved in binding monoglucosylated N-glycans of the ER-retained bri1-9, their mutations should destroy the ‘bri1-9-retention’ activity of CRT3 and their corresponding CRT3 transgenes should fail to complement an ebs2 mutation in the bri1-9 background. To test our hypothesis, we individually transformed each of the mutant gCRT3 transgenes into the Arabidopsis ebs2-8 bri1-9 double mutant that carries a T-DNA insertional allele of CRT3 with the DNA being inserted in the second intron (Jin et al., 2009).
Figure 1.
Bioinformatic and Mutational Analyses of the Predicted Sugar-Binding Site of the Arabidopsis CRT3 Protein.
(A) Sequence alignment of CRTs from Arabidopsis thaliana (At), Oryza sativa (Os), Mus musculus (m), and Homo sapiens (Hs). Multiple sequence alignments of amino acid residues of AtCRT3 (NP_563816), OsCRT3 (BAC06263), AtCRT1 (NP_176030), AtCRT2 (NP_172392), mCRT (AAH03453), and HsCRT (AAB51176) were performed using the ClustalW program (http://mobyle.pasteur.fr/cgi-bin/portal.py). Aligned sequences were color-shaded at a BoxShade 3.31 web server (http://mobyle.pasteur.fr/cgi-bin/portal.py). Residues identical in at least four proteins are shaded in red while similar amino acid residues are shaded in cyan. Diamonds indicate residues predicted to be involved in N-glycan binding. The N-globular domain, the central P-domain, and the C-terminal domain are indicated by blue box, green underline, and yellow box, respectively.
(B) Superimposition of the N-globular domain of the mCRT structure (pdb code 30oW; yellow) and the predicted 3-D structural model of AtCRT3 (salmon). The N- and P-domains, the predicted lectin site, and two termini are indicated.
(C) A close-up view of the sugar-binding site revealing similar positioning of key amino acid residues of the predicted lectin sites of mCRT and AtCRT. The numbers shown are amino acid residue positions of AtCRT.
(D) Immunoblot analysis of CRT3 in the wild-type (WT), bri1-9, ebs2-8 bri1-9, and 8 representative ebs2-8 bri1-9 gCRT3* transgenic lines (* substitutes for each of the eight italicized amino acid mutations listed beneath the line), each carrying a mutant gCRT3 transgene containing one of the following amino acid changes: Y118A, K120A, Y137A, Y137F, M140F, D169A, D144A, and E327A. Total protein extracts from 5-week-old soil-grown plants were separated by 10% SDS–PAGE and analyzed by immunoblotting using anti-CRT3 antibody. Coomassie-blue-stained SDS–PAGE gel is shown to serve as a loading control.
(E) Images of 5-week-old soil-grown plants of WT, bri1-9, ebs2-8 bri19, and 8 representative gCRT3* ebs2-8 bri1-9 transgenic lines shown in (D).
(F, G) The Endo-H sensitivity of BRI1 in WT, bri1-9, ebs2-8 bri1-9, and 8 representative ebs2-8 bri1-9 gCRT3* lines shown in (D). Equal amounts of total proteins extracted from rosette leaves of 5-week-old plants were treated with 1 μl Endo Hf for 1.5 h at 37°C, separated by 10% SDS–PAGE, and analyzed by either Coomassie-blue-staining (to serve as a loading control) or by immunoblotting with anti-BRI1 antibody.
Because a missense mutation of a key residue in CRT3 could lead to protein misfolding and subsequent ER-associated degradation (ERAD) (Jin et al., 2009), we performed an immunoblotting experiment to ensure that each of the mutant gCRT3 transgenes did lead to accumulation of detectable amounts of CRT3 in the transgenic ebs2-8 bri1-9 plants. As shown in Figure 1D, each representative transgenic line did accumulate CRT3 at a level equal to or higher than that of the wild-type or the bri1-9 mutant, indicating that the introduced mutations did not lead to significant degradation of the mutant CRT3 proteins.
We first analyzed the overall plant morphology to determine whether a mutant gCRT3 transgene was able to complement the ebs2-8 mutation in the ebs2-8 bri1-9 double mutant. As shown in Figure 1E, all introduced mutant gCRT3 transgenes except gCRT3(Y137F) and gCRT3(D169A) failed to rescue the ebs2-8 mutation, as the morphology of the corresponding representative transgenic plants were indistinguishable from that of ebs2-8 bri1-9. Consistently with earlier in vitro studies and a recent structural analysis (Thomson and Williams, 2005; Kozlov et al., 2010), the D169A mutation had no detectable effect on the CRT3 function, indicating that the mutant CRT3(D169A) largely maintains the ‘bri1-9-retaining’ activity of the wild-type CRT3. Interestingly, while the Y137A mutation completely destroys the biological activity of CRT3, the Y137F change that removes the hydroxyl group from Y137 retained the activity of the gCRT3 transgene to rescue the ebs2-8 mutation, suggesting that the aromatic ring is crucial for binding monoglucosylated N-glycans. While our result is similar to an earlier in vitro study that revealed reduced but not abolished sugar-binding activity by the Y–F mutation (Thomson and Williams, 2005), it contradicts the recent structural analysis suggesting an essential role of the hydroxyl group in forming a key hydrogen bond with the glucose moiety of a monoglucosylated N-glycan (Kozlov et al., 2010). A structural analysis of a sugar-bound CRT3 is needed to determine the exact contributions of the two functional groups of the polar aromatic residue for glucose binding.
In addition to the morphological analysis, we also performed a convenient biochemical assay, widely known as endoglycosidase (Endo-H) sensitivity assay, which can directly assess the ‘bri1-9-retention’ activity of various mutant CRT3 proteins (Jin et al., 2009). Endo-H only removes the high-mannose-type (HM) N-glycans on the ER-localized glycoprotein but can not cut Golgi-processed complex-type (CT) N-glycans. Consistently with our previous study (Jin et al., 2007, 2009), the wild-type BRI1 is largely Endo H-resistant, the mutant bri1-9 protein is extremely sensitive to the Endo-H treatment, and the ebs2-8 mutation compromises the ER retention of bri1-9, allowing its ER export and plasma membrane targeting. As expected from their plant morphological phenotypes, the Endo-H-resistant band of bri1-9 was still detectable in all ebs2-8 bri1-9-like gCRT3 transgenic plants but became almost undetectable in the phenotypically rescued ebs2-8 bri1-9 gCRT3(D169A) and ebs2-8 bri1-9 gCRT3(Y137F) transgenic plants. These results further confirmed that the D169A/Y137F-mutated CRT3 could still retain bri1-9 in the ER.
Taken together, our experiments provided an in vivo genetic confirmation for the critical role of the five conserved amino acid residues of the predicted lectin site of a plant CRT for retaining structurally imperfect but biochemically competent glycoprotein in the ER. Our study also suggested that the aromatic ring of the Y137 residue is more important than its hydroxyl group for the biological function of CRT3. Given the fact that the sugar-binding N-domain and the central P-domain are interchangeable between CRT3 and CRT1/CRT2 in Arabidopsis (Jin et al., 2009), our conclusion on the roles of the five conserved residues also applies to the other two Arabidopsis CRTs and other plant CRTs that have diverse functions in plant growth/development and stress responses (Jia et al., 2009).
FUNDING
This work was supported in part by grants from the National Institutes of Health (grant number GM060519) and the National Science Foundation (grant number IOS1121496). No conflict of interest declared.
REFERENCES
- Gopalakrishnapai J., Gupta G., Karthikeyan T., Sinha S., Kandiah E., Gemma E., Osccarson S., Surolia A. (2006). Isothermal titration calorimetric study defines the substrate binding residues of calreticulin. Biochem. Biophys. Res. Commun. 351, 14–20 [DOI] [PubMed] [Google Scholar]
- Jia X.Y., He L.H., Jiang R.L., Li R.Z. (2009). Calreticulin: conserved protein and diverse functions in plants. Physiol. Plant. 136, 127–138 [DOI] [PubMed] [Google Scholar]
- Jin H., Hong Z., Su W., Li J. (2009). A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc. Natl Acad. Sci. U S A. 106, 13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Yan Z., Nam K.H., Li J. (2007). Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol. Cell. 26, 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov G., Pocanschi C.L., Rosenauer A., Bastos-Aristizabal S., Gorelik A., Williams D.B., Gehring K. (2010). Structural basis of carbohydrate recognition by calreticulin. J. Biol. Chem. 285, 38612–38620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M., Groenendyk J., Szabo E., Gold L.I., Opas M. (2009). Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 417, 651–666 [DOI] [PubMed] [Google Scholar]
- Schrag J.D., Bergeron J.J., Borisova S., Hahn M., Thomas D.Y., Cygler M. (2001). The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell. 8, 633–644 [DOI] [PubMed] [Google Scholar]
- Thomson S.P., Williams D.B. (2005). Delineation of the lectin site of the molecular chaperone calreticulin. Cell Stress Chaperones. 10, 242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]