Abstract
Purpose
The narrow therapeutic index and large interpatient variability in sirolimus pharmacokinetics (PK) make therapeutic drug monitoring necessary. Factors responsible for PK variability are not well understood, and published PK studies do not include pediatric patients with neurofibromatosis type 1 (NF1). The objectives of this study were to estimate sirolimus clearance in a cohort of children with NF1 using data collected in a concentration-guided trial, to evaluate the effect of treatment duration on clearance and dose requirements, and to evaluate the association of sirolimus clearance with patient-specific factors including age, weight, body surface area, race, and sex.
Methods
Sirolimus concentration-time data were collected from an ongoing prospective trial in children with NF1. An iterative two-stage Bayesian method was used for the pharmacokinetic parameter analyses.
Results
Data from 44 patients with NF1 were included in the analyses. Mean age was 8.4 years (SD 4.5, range 3-18), and mean weight was 29.8 kg (SD 16.7, range 12-85.8) Mean sirolimus clearance was 11.8 L/hr (SD 4.6, range 2.2-24.1), and the mean dose to obtain a target trough concentration of 10-15 ng/mL was 2.0 mg/m2 BID (SD 0.72, range 0.77-3.85). A nonlinear relationship between age and clearance was observed. Total body weight and body surface area were strong predictors of sirolimus clearance (r2=0.67 and 0.65, respectively).
Conclusions
Sirolimus clearance in children with NF1 is comparable to that in pediatric transplant patients. Clearance was most associated with body size parameters (body surface area and total body weight) in children with NF1. When normalized for size, an age effect on clearance was observed in the youngest patients, most likely due to maturational changes in drug absorption and metabolism. A mean dose of 2.0 mg/m2 twice a day was required for attainment of target trough concentrations of 10-15 ng/mL in children greater than 3 years of age who have NF1. The updated model will allow PK guided individualized dosing of sirolimus in patients with NF1.
Keywords: sirolimus, neurofibromatosis, pharmacokinetics, pediatric
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder that affects approximately 1/3000 individuals worldwide1. The pathogenesis of NF1 is thought to be related to mutations in the NF1 gene located on chromosome 17q11.2, leading to decreased production of the tumor suppressor protein, neurofibromin2. NF1 is a progressive disorder characterized by cutaneous, neurologic, skeletal, and neoplastic manifestations. Patients with NF1 are at higher risk for developing tumors of the central and peripheral nervous systems, including plexiform neurofibromas (PNs)3,4. PNs are benign nerve sheath tumors that often grow rapidly in young children, and may be highly debilitating. There is currently no proven first line treatment for NF1-related tumors other than surgery. Surgery may be difficult due to the extensive growth and highly invasive nature of these tumors, and up to 44% of tumors progress following surgery5.
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase regulated by phosphoinositol 3 kinase (PI3K). mTOR plays a key role in the regulation of cellular catabolism and anabolism, protein translation, angiogenesis, cell motility, and proliferation6-12. The NF1 tumor suppressor, neurofibromin, regulates the mTOR pathway activity, with increased mTOR activation reported in NF1-deficient cells and tumors from NF-1 patients13,14. Sirolimus and its analogues have been reported to inhibit mTOR activity, preventing phosphorylation (and activation) of p70S6K, 4E-BP1, and other proteins involved in cell motility, angiogenesis, and control of cell growth6-8,12,15-17. Inhibitors of mTOR, including sirolimus, have shown promise in preclinical NF studies, displaying the ability to decrease mTOR activation and cell proliferation; therefore, sirolimus may have a role in treating NF-1 related tumors13,14.
Sirolimus has a narrow therapeutic index and its pharmacokinetics exhibit high interpatient variability; thus, some patients treated with sirolimus are at greater risk for therapeutic failure or adverse reactions, and it is not clear who these patients are a priori. Under- and over-exposure to sirolimus remain great concerns, and rapid attainment of target sirolimus concentrations is challenging, yet essential for preventing therapy related complications. In patients with NF1, underexposure may lead to a lack of therapeutic effect and tumor progression, whereas overexposure may increase the risk for toxicities such as renal dysfunction, hypertension, pneumonitis, and infection. These may lead to increased morbidity and mortality, decreased quality of life, and increased health care costs. Identifying and measuring factors that affect the variability in sirolimus pharmacokinetics will contribute to more rapid target attainment thereby optimizing therapeutic outcomes at reduced cost.
There is a paucity of data on sirolimus pharmacokinetics in children, particularly those with NF1. In adults and children, sirolimus clearance has been reported to be associated with patient age18-20. Dansirikul and colleagues reported sirolimus clearance was inversely related to age in 25 adult renal transplant patients18. Similarly, Schachter and colleagues reported the sirolimus half-life to be shorter in children compared to adult data (10-24 hours vs 49-70 hours, respectively) based on their analysis of 24 sirolimus pharmacokinetic profiles in 13 pediatric renal transplant patients19. This study also reported that terminal half-life was significantly shorter in children ≤ 6 years of age (median 8.2 hours, range 4.4-10.6) compared to those > 6 years of age (median 12.6 hours, range 4.7-95.2) (p < 0.05). The authors attributed these findings to increased rates of drug metabolism in children. Another study of sirolimus pharmacokinetic profiles in 49 pediatric renal transplant patients also reported that sirolimus clearance was inversely related to age20. The investigators also suggested that the increased sirolimus clearance observed in pediatric patients may be attributable to higher metabolic rates reported in children.
The present study aimed to estimate sirolimus clearance in a cohort of children with NF1 and plexiform neurofibromas with at least potential significant morbidity using data collected in a concentration-controlled trial, and to evaluate the effect of treatment duration on clearance and dose requirements. Sirolimus clearance has been associated with patient-specific factors including age, weight, body surface area (BSA), race, sex, and drug metabolizing enzyme gene polymorphisms in other studies21-25. Therefore, a secondary objective of this study was to evaluate the association of available patient-specific factors with sirolimus clearance in pediatric patients with NF1. Ultimately, identification of patient-specific factors that influence sirolimus pharmacokinetics may facilitate development of an algorithm to minimize under- and over- exposure to sirolimus in the treatment of NF1.
MATERIALS AND METHODS
Study data were retrospectively collected from an ongoing prospective multi-center clinical concentration-controlled trial26. Inclusion criteria for the population pharmacokinetics study were diagnosis of NF1, enrollment in the prospective study, and age < 19 years. Exclusion criteria for the trial included the use of cytochrome P450 3A4 (CYP3A4) inhibitors or inducers at study entry, chemotherapy within 4 weeks of study entry, and renal or hepatic dysfunction. Additional exclusion criteria for the clinical trial are described elsewhere26. Data collected included age, sex, race (patients stratified as White, Black, Asian, undefined), ethnicity (patients stratified as Hispanic, non-Hispanic, undefined), height, weight, sirolimus concentrations, dosing regimens, and concomitant medications.
The sirolimus starting dose in the trial was 0.8 mg/m2 by mouth twice a day. The starting dose was based on extrapolation of the recommended sirolimus dose in older children and adult transplant recipients25. Subsequent dosing was pharmacokinetically guided to achieve a target trough concentration of 10 to 15 ng/mL. This target range was based on a case series of five sirolimus-treated patients (ages 3-21 years) with astrocytoma who exhibited lesion regression and tolerated treatment well with trough levels of 10-15 ng/mL27. The first sirolimus concentration measurement was at steady-state after 7-10 days of treatment. Subsequent pre-dose concentrations were obtained every two weeks until stable, defined as two consecutive trough concentrations between 10 and 15 ng/mL. Once the patient was stable, pre-dose concentrations were measured every 4 weeks. Patients were given a diary in which they recorded all outpatient sirolimus administration dates and times for a five-day period prior to the visit. This diary was reviewed prior to the drawing of blood for sirolimus concentration measurements to document adherence and provided the actual dosing time information for the PK assessment.
Sirolimus whole blood concentrations were centrally determined by a validated tandem mass spectrometry assay performed using electrospray on a Waters Quattro Micro API triple quadrupole mass spectrometer (Milford, MA) interfaced with an Acquity Ultra Performance Liquid Chromatography (UPLC) instrument. The assay range was 0.5-100.0 ng/mL. The LLOQ of the assay was 1.0 ng/mL and within and between-batch variability (CV) was 12.8% and 14.0%, respectively. Lab results were reported via a web-based protocol management tool and included real time dosing recommendations generated with a Bayesian estimator (MW/Pharm version 3.6, Mediware, Groningen, Netherlands)28. The system automatically generated email notifications to the clinical teams and included the recording of acceptance of dosing adjustments.
The pediatric population parameter estimates for PK-guided dosing were derived from published pharmacokinetic data in stable renal transplant patients treated with sirolimus23, 25. Population parameters and their distributions were defined as means (± SD) and were as follows: 17.3 (7.9) L/hr/1.85m2 for clearance, 12.0 (5.0) L/kg for volume of distribution, and 2.77 (1.33) h-1 for the absorption rate constant. For this study, therapeutic dose was defined as the sirolimus dose at the time the patient reached the target trough concentration.
The patient demographics, dosing and concentration-time data sets entered into the MW/Pharm program were used for the iterative two-stage Bayesian (IT2B) estimation.28 As concentration data were mostly pre-dose trough concentrations, only clearance was estimated. To analyze changes in sirolimus clearance over time, patient data were divided into four time periods: initial 3 months of therapy, 6-9 months into therapy, 9-12 months into therapy, and 12-15 months into therapy. Patients were excluded from a time period analysis if data were lacking for a given time interval.
Statistical analyses were performed using Prism (version 4.03, Graphpad, San Diego, California) and MYSTAT (version 12.02, SYSTAT, Chicago, Illinois). Normality of data was analyzed using the D’Agostino & Pearson omnibus test, Shapiro-Wilk test, and the Kolmogorov-Smirnov (KS) normality test. The relationships of continuous variables age, height, weight, BSA, and lean body mass (LBM) on sirolimus clearance were evaluated by linear and non-linear regression as recently described29. Covariate analysis was performed using the initial three months of the patients’ sirolimus concentration-time data. The relationships of clearance with sex, and initial clearance vs. clearance at 6-9 months, 9-12 months, and 12-15 months were analyzed by Student’s t-tests; clearance and ethnicity were analyzed by a t-test, including Welsh correction for inequality of variance. The relationship between clearance and race was analyzed by ANOVA.
Weight normalization and allometric scaling using a scaling factor of 0.75 were utilized to analyze the association of sirolimus clearance with age in order to assess for maturational effects on sirolimus clearance29-31.
RESULTS
At the time of the study there were a total of 50 patients enrolled. Data from 44 patients were analyzed, with 6 patients excluded due to age > 19 years (Figure 1). The mean age was 8.4 years (SD 4.5, range 3-18), with a mean weight of 29.8 kg (SD 16.7, range 12-85.8) (Table 1). The IT2B estimated mean sirolimus clearance was 11.84 L/hr in the study population and 35% higher than the initial population value (Table 2). For the 44 patients analyzed, a total of 545 sirolimus concentrations were obtained as part of the PK guided dosing over a two-year trial period (Figure 3). Of note, actual measured concentrations frequently were below the 10 ng/mL target as the time after the last dose often exceeded 12 hours (e.g. 14-16h post dose trough due to clinic hours) or because of missed doses; with the predicted 12h trough on target. Target concentration attainment at month 3 was achieved in 23/44 (52%) patients. Mean time to target concentration was 81 days (SD 52). The mean therapeutic dose was 2.0 mg/m2 twice daily (SD 0.72, range 0.77-3.9), which is 2.5 times greater than the sirolimus starting dose in the clinical trial. When allometrically scaled, the mean therapeutic dose was 0.14 (mg/kg)0.75 (SD 0.05, range 0.06-0.29). Concurrent medications and sirolimus concentrations were evaluated for potential drug-drug interactions with sirolimus therapy. No clinically relevant interactions were observed in this small cohort. No sirolimus concentrations were below the limit of quantification.
Figure 1.
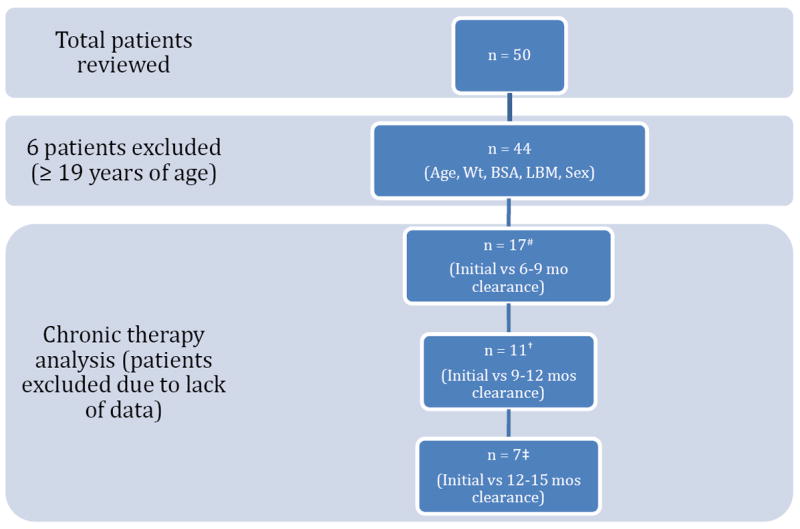
# 27 patients excluded due to no concentration-time data at 6-9 months of therapy
† 33 patients excluded due to no concentration-time data at 9-12 months of therapy
‡ 37 patients excluded due to no concentration-time data at 12-15 months of therapy
Table 1.
| Baseline Demographics
| |
|---|---|
| Age, years, mean (SD) | 8.4 (4.5) |
|
| |
| Sex | |
| • Male, n (%) | 29 (66) |
| • Female, n (%) | 15 (34) |
|
| |
| Race | |
| • White, n (%) | 33 (75) |
| • Black, n (%) | 3 (7) |
| • Asian, n (%) | 3 (7) |
| • Not reported, n (%) | 5 (11) |
|
| |
| Ethnicity | |
| • Hispanic, n (%) | 5 (11) |
| • Non-Hispanic, n (%) | 35 (80) |
| • Not reported, n (%) | 4 (9) |
|
| |
| Weight (kg), mean | 29.8 (16.7) |
|
| |
| Height (cm), mean | 125.4 (23.3) |
|
| |
| BSA (m2), mean | 1.0 (0.36) |
|
| |
| LBM (kg), mean | 28 (14.9) |
Abbreviations: LBM, lean body mass; BSA, body surface area
Table 2.
| Population Results | |||
|---|---|---|---|
| Mean (SD) | Median (range) | Interquartile range | |
| Clearance (L/hr) | 11.8 (4.6) | 11.6 (2.2-24.1) | 8.5-14.4 |
| Clearance (L/hr/70kg) | 0.17 (0.07) | 0.17 (0.03-0.34) | 0.12-0.21 |
| Clearance (L/hr/pop median wt) | 0.54 (0.2) | 0.53 (0.10-1.11) | 0.39-0.66 |
| Clearance (L/hr/1.85m2) | 6.4 (2.5) | 6.27 (1.2-13.0) | 4.63-7.78 |
| Therapeutic Dose (mg/m2/dose) | 2.0 (0.7) | 1.9 (0.77-3.9) | 1.52-2.41 |
| Therapeutic Dose (mg/kg/dose) | 0.08 (0.04) | 0.07 (0.02-0.19) | 0.06-0.09 |
| Therapeutic dose ((mg/kg)ˆ0.75) | 0.14 (0.05) | 0.13 (0.06-0.29) | 0.11-0.16 |
Figure 3.
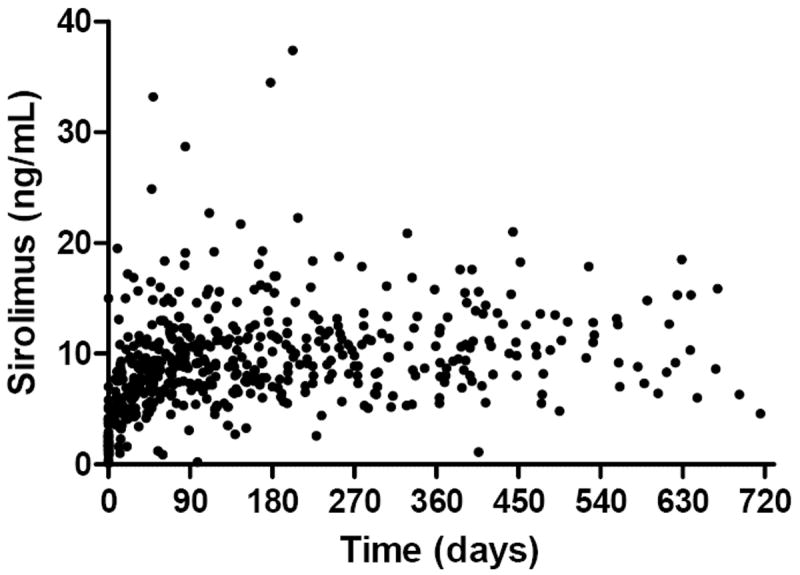
Observed sirolimus concentrations (n=545) as part of the PK guided dosing in 44 patients with NF1 during the two-year trial period. Note: actual measured concentrations frequently were below the 10 ng/mL target as the time after the last dose often exceeded 12 hours or because of missed doses.
Age and body size (weight and BSA) were significantly associated with sirolimus clearance (r2 = 0.55, 0.65, and 0.67, respectively) (Figure 2). The correlation of age and weight-normalized sirolimus clearance was less robust (r2=0.25). When clearance was allometrically scaled, weight-normalized sirolimus clearance was not related to age in this cohort (Figure 2B). Sirolimus clearance was also associated with height and lean body mass (height r2=0.66, LBM r2=0.61).
Figure 2.
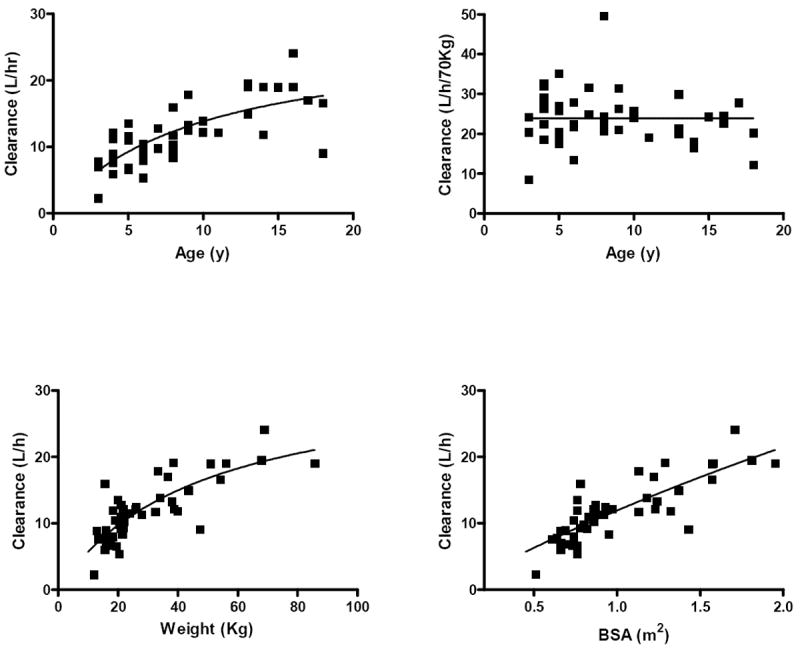
A. sirolimus clearance (L/h) vs. age (y), R2 = 0.55; B. allometrically scaled sirolimus clearance (L/hr/70kg) vs. age (y), R2 = 0.25; C. sirolimus clearance (L/h) vs weight (kg), R2 = 0.65; D. sirolimus clearance (L/h) vs. body surface area (BSA, m2), R2 = 0.67; Solid lines indicate mean line of fit.
There was no statistically significant association of sex (n=44; p=0.15), ethnicity (n=40; p=0.65), or race (n=39; p=0.67) with sirolimus clearance in the study population. Additionally, there was no statistically significant change in sirolimus clearance with long-term (up to 15 months) sirolimus therapy [initial vs. 1) 6-9mo, n = 17, p = 0.21; 2) 9-12mo, n = 11, p = 0.53; 3) 12-15mo, n = 7, p = 0.50]. Mean sirolimus clearance at 0-3mo, 6-9mo, 9-12mo, and 12-15mo was 0.5 (SD 0.11), 0.46 (SD 0.18), 0.48 (SD 0.25), and 0.44 (SD 0.26) L/hr/kg, repectively.
DISCUSSION
Published sirolimus pharmacokinetic data in pediatrics are limited, especially in patients with NF1. As in other reports19,20,32, sirolimus clearance in this study population when normalized to body weight (mean CL = 0.44 ± 0.15 L/kg) was much higher than in published adult data (mean CL = 0.21 ± 0.1 L/hr/kg). Previous studies have also reported higher rates of sirolimus clearance in children, and the use of twice daily dosing was employed based on these previous findings19,33. The reason for increased sirolimus clearance in children is most likely related to difference in body size. After allometric scaling, a mean clearance of 24.2 L/hr/70kg was observed for children older than 4 years (Figure 2), which approaches adult values (approximately 28 L/hr)23, 25, 34. The mean therapeutic dose in this study (2.0 mg/m2 BID) was higher than the starting sirolimus dose (0.8 mg/m2 BID) used in the clinical trial, likely owing to scaling doses from older patients without consideration of sirolimus clearance differences as well as applying higher target concentrations in pediatric patients with NF1. Based on these findings, it is reasonable to consider higher initial dosing in future studies of sirolimus in pediatric patients with NF1. Of note, the recommendation for higher initial dosing may not extend to those patients less than 3 years of age.
There were several limitations to this preliminary pharmacokinetic study. Volume of distribution could not be robustly estimated in these analyses, and could not be evaluated as an independent pharmacokinetic parameter. Secondly, the age range of the study cohort did not include patients younger than 3 years. When clearance was normalized to allometrically scaled weight, its relationship with age was no longer significant, suggesting that at age > 3-4 years maturation is complete and does not contribute meaningfully to sirolimus clearance in this cohort. This finding may not extend to patients under 3 years of age, as clearance may relate to age in the very young due to developmental and maturational changes. Unpublished data from a similar ongoing PK-guided study of sirolimus in complicated vascular anomalies show lower clearance in patients 0-3 years of age35.
Ethnicity, race, and chronic therapy analyses included small sample sizes; therefore, the influence of these factors on sirolimus clearance could not be determined. Body surface area was most highly correlated with sirolimus clearance, among all covariates analyzed in this study (r2=0.67). Despite BSA-based initial dosing, high variability in sirolimus clearance remained, which supports the continued need for therapeutic drug monitoring.
CONCLUSION
To our knowledge, this is the first sirolimus pharmacokinetic study in pediatric patients with NF1. These preliminary data are vital for trial design to further evaluate sirolimus dose individualization in children. These data will also be a foundation for a dose refinement model that will allow estimation of a therapeutic sirolimus dose using clinical data and a single concentration or abbreviated AUC measurement, thus optimizing the therapeutic drug monitoring process.
Acknowledgments
Part of this work was supported by Department of Defense grant W81XWH-05-615, Neurofibromatosis Clinical Trials Consortium (B.W., M.F., B.W., J.P.P., A.V.V) and National Institutes of Health grant 5K24HD050387 (A.V.V.). Special thanks to the colleagues and staff of the STOPN study performed through the NF1 Clinical Trials Consortium.
Footnotes
Conflict of interest disclosure: none
References
- 1.Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009 Jan;123(1):124–133. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]
- 2.Theos A, Korf BR. Pathophysiology of neurofibromatosis type 1. Ann Intern Med. 2006 Jun 6;144(11):842–849. doi: 10.7326/0003-4819-144-11-200606060-00010. [DOI] [PubMed] [Google Scholar]
- 3.Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999 Mar 26;89(1):31–37. doi: 10.1002/(sici)1096-8628(19990326)89:1<31::aid-ajmg7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Korf BR. Malignancy in neurofibromatosis type 1. Oncologist. 2000;5(6):477–485. doi: 10.1634/theoncologist.5-6-477. [DOI] [PubMed] [Google Scholar]
- 5.Needle MN, Cnaan A, Dattilo J, et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974-1994. J Pediatr. 1997 Nov;131(5):678–682. doi: 10.1016/s0022-3476(97)70092-1. [DOI] [PubMed] [Google Scholar]
- 6.Berven LA, Willard FS, Crouch MF. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp Cell Res. 2004 Jun 10;296(2):183–195. doi: 10.1016/j.yexcr.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006 Jun 1;66(11):5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Li F, Cardelli JA, Martin KA, Blenis J, Huang S. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene. 2006 Nov 9;25(53):7029–7040. doi: 10.1038/sj.onc.1209691. [DOI] [PubMed] [Google Scholar]
- 9.Namba R, Young LJ, Abbey CK, et al. Rapamycin inhibits growth of premalignant and malignant mammary lesions in a mouse model of ductal carcinoma in situ. Clin Cancer Res. 2006 Apr 15;12(8):2613–2621. doi: 10.1158/1078-0432.CCR-05-2170. [DOI] [PubMed] [Google Scholar]
- 10.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signallingcontrols tumour cell growth. Nature. 2006 May 25;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 11.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005 Apr;16(4):525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 12.Wan X, Shen N, Mendoza A, Khanna C, Helman LJ. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 2006 May;8(5):394–401. doi: 10.1593/neo.05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005 Apr 1;65(7):2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 14.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003 Dec 9;2003(212):re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 16.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005 Jan;37(1):19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 17.Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003 Feb;4(2):117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 18.Dansirikul C, Morris RG, Tett SE, Duffull SB. A Bayesian approach for population pharmacokinetic modelling of sirolimus. Br J Clin Pharmacol. 2006 Oct;62(4):420–434. doi: 10.1111/j.1365-2125.2005.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schachter AD, Meyers KE, Spaneas LD, et al. Short sirolimus half-life in pediatric renal transplant recipients on a calcineurin inhibitor-free protocol. Pediatr Transplant. 2004 Apr;8(2):171–177. doi: 10.1046/j.1399-3046.2003.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schachter AD, Benfield MR, Wyatt RJ, et al. Sirolimus pharmacokinetics in pediatric renal transplant recipients receiving calcineurin inhibitor co-therapy. Pediatr Transplant. 2006 Dec;10(8):914–919. doi: 10.1111/j.1399-3046.2006.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anglicheau D, Le Corre D, Lechaton S, et al. Consequences of genetic polymorphisms for sirolimus requirements after renal transplant in patients on primary sirolimus therapy. Am J Transplant. 2005 Mar;5(3):595–603. doi: 10.1111/j.1600-6143.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 22.Aspeslet LJ, Yatscoff RW. Requirements for therapeutic drug monitoring of sirolimus, an immunosuppressive agent used in renal transplantation. Clin Ther. 2000;22(Suppl B):B86–92. doi: 10.1016/s0149-2918(00)89025-6. [DOI] [PubMed] [Google Scholar]
- 23.Djebli N, Rousseau A, Hoizey G, et al. Sirolimus population pharmacokinetic/pharmacogenetic analysis and bayesian modelling in kidney transplant recipients. Clin Pharmacokinet. 2006;45(11):1135–1148. doi: 10.2165/00003088-200645110-00007. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(Suppl B):B101–121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman JJ, Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol. 1997 May;37(5):405–415. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]
- 26.Korf B, et al. A Phase II Study of the mTOR inhibitor Sirolimus in Neurofibromatosis Type 1 Related Plexiform Neurofibromas. Bethesda: National Library of Medicine; 2012. ClinicalTrials.gov identifier: NCT00634270. [Google Scholar]
- 27.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006 Mar;59(3):490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 28.Proost JH, Meijer DK. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med. 1992 May;22(3):155–163. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 29.Anderson BJ, Holford NH. Mechanistic basis ofusing body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 30.Kleiber M. Body size and metabolic rate. Physiol Rev. 1947 Oct;27(4):511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- 31.Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996 May;30(5):329–332. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 32.Tejani A, Alexander S, Ettenger R, et al. Safety and pharmacokinetics of ascending single doses of sirolimus (Rapamune, rapamycin) in pediatric patients with stable chronic renal failure undergoing dialysis. Pediatr Transplant. 2004 Apr;8(2):151–160. doi: 10.1046/j.1399-3046.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- 33.Schubert M, Venkataramanan R, Holt DW, et al. Pharmacokinetics of sirolimus and tacrolimus in pediatric transplant patients. Am J Transplant. 2004 May;4(5):767–773. doi: 10.1111/j.1600-6143.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 34.Le Meur Y, Djebli N, Szelag JC, et al. CYP3A5*3 influences sirolimus oral clearance in de novo and stable renal transplant recipients. Clin Pharmacol Ther. 2006 Jul;80(1):51–60. doi: 10.1016/j.clpt.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Adams D, et al. Safety and Efficacy Study of Sirolimus in Complicated Vascular Anomalies. Bethesda (MD): 2012. ClinicalTrials.gov identifier: NCT00975819. [Google Scholar]


