SUMMARY
Germline mutations in LKB1 (STK11) are associated with the Peutz-Jeghers syndrome (PJS), which includes aberrant mucocutaneous pigmentation, and somatic LKB1 mutations occur in 10% of cutaneous melanoma. By somatically inactivating Lkb1 with K-Ras activation (±p53 loss) in murine melanocytes, we observed variably pigmented and highly metastatic melanoma with 100% penetrance. LKB1 deficiency resulted in increased phosphorylation of the SRC family kinase (SFK) YES, increased expression of WNT target genes, and expansion of a CD24+ cell population, which showed increased metastatic behavior in vitro and in vivo relative to isogenic CD24− cells. These results suggest that LKB1 inactivation in the context of RAS activation facilitates metastasis by inducing an SFK-dependent expansion of a prometastatic, CD24+ tumor subpopulation.
INTRODUCTION
The LKB1 (or STK11) gene encodes a serine/threonine kinase that phosphorylates and activates several targets, including AMPK and the AMPK-related kinases (Alessi et al., 2006). LKB1 regulates cancer-relevant cell biologic phenotypes, including migration, invasion, metabolism, and polarity (Alessi et al., 2006; Shah et al., 2008). Germline mutations in LKB1 cause the Peutz-Jeghers syndrome (PJS; OMIM 175200), an autosomal, dominant disorder characterized by hamartomatous polyps of the gastrointestinal tract and increased mucocutaneous pigmentation (Jeghers et al., 1949). Patients with PJS are tumor prone, demonstrating a significantly increased risk for several cancers (e.g., of colon, pancreas, breast, ovary, and testis) (Giardiello et al., 2000; Lim et al., 2004; Sanchez-Cespedes, 2007). Somatic LKB1 mutations also are common in sporadic cancers: most notably lung adenocarcinoma (~30%; Ji et al., 2007; Weir et al., 2007), cervical carcinoma (~15%; Forbes et al., 2011; Wingo et al., 2009), and melanoma (~10%; Forbes et al., 2011; Guldberg et al., 1999; Rowan et al., 1999).
In addition to the finding of frequent somatic inactivation in melanoma, several lines of evidence suggest that LKB1 plays an important role in melanocyte biology and limits melanocyte transformation. Patients with PJS demonstrate hyperpigmentation of the lips, oral mucosa, hands, and feet, which are comprised of atypical epidermal melanocytes. Although pathognomonic for PJS, the pathologic basis for these macular lesions is unknown. Moreover, recent reports have suggested that LKB1 is functionally inactivated by activating mutations of B-RAF (Esteve-Puig et al., 2009; Zheng et al., 2009), which are found in approximately 50% of human melanoma, suggesting that functional compromise of LKB1 is common in melanoma.
A role for LKB1 in regulating tumor differentiation and metastasis has been suggested in epithelial cancers. For example somatic inactivation of Lkb1 combined with activation of K-Ras in genetically engineered murine models (GEMMs) of lung cancer results in tumors with an expanded spectrum of differentiation and considerably augmented metastasis compared to K-Ras-driven tumors lacking p53 or Ink4a/Arf (Ji et al., 2007). LKB1 mutation is associated with advanced stage and metastasis in human patients with aerodigestive carcinomas (Guervos et al., 2007; Matsumoto et al., 2007). Loss of LKB1 has been reported to promote metastatic behaviors (e.g., resistance to anoikis, increased invasiveness) in a variety of epithelial cell types in vitro through diverse mechanisms including inhibition of SIK1 (Cheng et al., 2009) or AMPK (Taliaferro-Smith et al., 2009) as well as activation of EMT, focal adhesion, and SRC family kinases (SFKs) (Carretero et al., 2010). Given these observations suggesting a role for LKB1 in melanocyte biology and transformation, we sought to determine the effects of LKB1 inactivation in human cell lines and GEMMs of melanoma.
RESULTS
Lkb1 Deletion in Melanocytes In Vitro and In Vivo
We intercrossed an established 4-hydroxytamoxifen (4-OHT)-inducible melanocyte-specific CRE allele (Tyr-CRE-ERT2, abbreviated “T”; Bosenberg et al., 2006) and three conditional alleles: Lox-Stop-Lox-(LSL)-KrasG12D (abbreviated “K”; Johnson et al., 2001); Lkb1L/L (Bardeesy et al., 2002); and p53L/L (Jonkers et al., 2001). To investigate the effect of Lkb1 on melanocyte growth and proliferation, we isolated murine melanocytes from neonatal mice of these defined genotypes. Melanocytic origin of the cells was confirmed by immunofluorescence staining for the expression of tyrosinase and tyrosinase-related protein 1 (see Figure S1A available online). Cells were treated with 4-OHT in vitro to allow CRE activation and induce allelic recombination, which was confirmed by PCR (data not shown). Although wild-type (WT), TK, TLkb1L/L, and 4-OHT-untreated control melanocytes grew poorly in vitro, 4-OHT-treated, primary melanocytes from TKLkb1L/L and TKp53L/L; Lkb1L/L mice demonstrated robust in vitro proliferation without growth arrest over 2 months (Figure 1A; data not shown). Given that Ink4a/Arf-deficient melanocytes are immortal in culture (Sviderskaya et al., 2002), we assessed Ink4a/Arf expression in cultured melanocytes with and without Lkb1. In accord with prior studies by Bardeesy et al. (2002) and Ji et al. (2007), Ink4a/Arf expression was significantly attenuated in TKLkb1L/L versus TK melanocytes (Figure S1B). These findings suggest that Lkb1 loss leads to melanocyte immortalization by compromising Ras-mediated Ink4a/Arf activation.
Figure 1. Lkb1 Restrains Melanocytic Hyperproliferation Induced by K-Ras Activation.
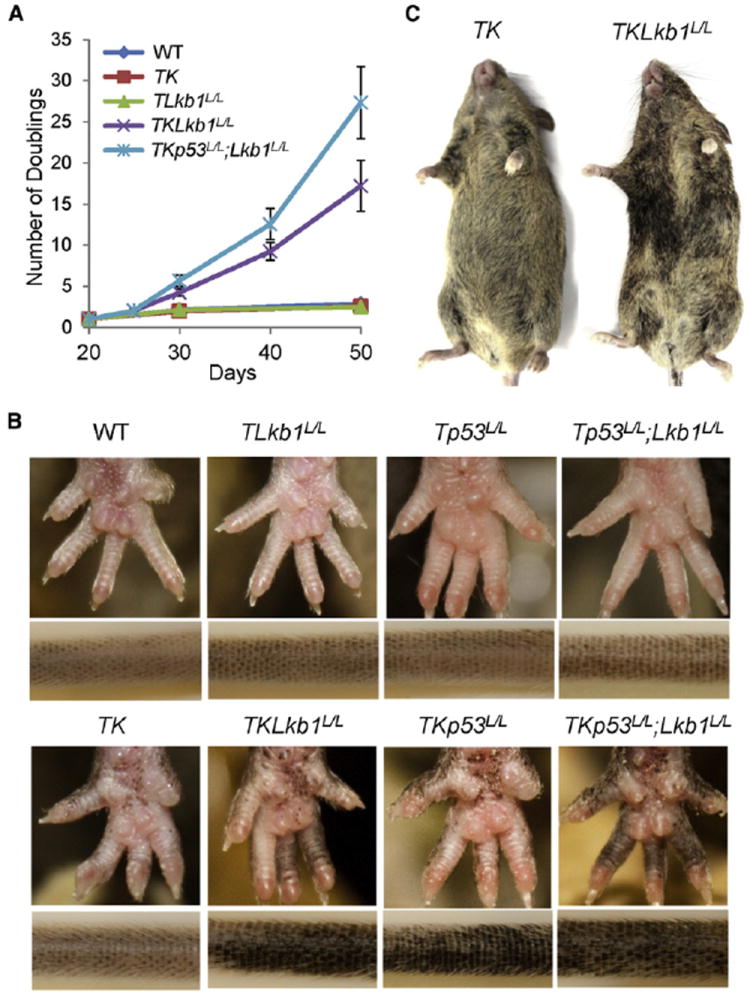
(A) Growth curves of primary melanocyte cultures from mice of indicated genotypes are illustrated. Cells were treated with or without 4-OHT at 20 days postisolation to activate CRE recombinase, and cell numbers were counted during serial passage. At least three primary lines were generated for each group, and representative results are shown. Error bars show SD.
(B) Changes in pigmentation of representative 8-week-old adult mice of the indicated melanocyte-specific genotypes are shown. Mice without K-Ras activation exhibit normal pigmentation, whereas K-Ras-expressing cohorts showed pigmented macules on the paws and tails, with increasing hyperpigmentation noted with concomitant Lkb1 and/or p53 loss.
(C) Representative mice with melanocyte-specific loss of Lkb1 and K-Ras activation show increased and heterogeneous coat color pigmentation compared to Lkb1-intact mice.
See also Figure S1.
To examine the role of Lkb1 in melanocytes in vivo, neonatal mice were topically treated with 4-OHT to activate CRE and induce recombination as previously described by Bosenberg et al. (2006) and Monahan et al. (2010). Within 4 weeks of 4-OHT treatment, mice from K-Ras-expressing cohorts (TK, TKLkb1L/L, TKp53L/L, and TKp53L/L; Lkb1L/L) developed melanocytic hyperproliferation and exhibited pigmented cutaneous macules not seen in WT or 4-OHT-untreated littermates (Figure 1B; data not shown). These effects were stronger in TKLkb1L/L and TKp53L/L cohorts than in the TK cohort, and the most pronounced effects were observed in TKp53L/L; Lkb1L/L mice. Accompanying the obvious melanocytic hyperproliferation in the tails and paws, coat color was more heterogeneous and darker when K-Ras activation was combined with Lkb1 loss (Figure 1C; data not shown). Interestingly, the skin and coat color from K-Ras WT cohorts (TLkb1L/L, Tp53L/L, and Tp53L/L; Lkb1L/L) appeared normal (Figure 1B). In aggregate these in vitro and in vivo data show that homozygous Lkb1 inactivation is not sufficient to induce melanocytic hyperproliferation in isolation but potently cooperates with somatic K-Ras activation in this regard.
Lkb1 Loss Promotes Melanoma Formation and Metastasis
We next followed these cohorts for melanoma formation. In accord with our prior results, tumors were not observed in TK mice, or in animals of any genotype without K-Ras activation (TLkb1L/L, Tp53L/L, or Tp53L/L; Lkb1L/L) when followed to 70 weeks (Figure 2A). Combined somatic Lkb1 loss and K-Ras activation, however, led to melanoma formation with 100% penetrance and latencies ranging from 24 to 56 weeks (median of 38.5). As previously reported by Monahan et al. (2010), concomitant somatic p53 deletion combined with K-Ras activation also potently facilitated tumorigenesis, with a penetrance and latency similar to that seen in the TKLkb1L/L mice. Despite suggestions that Lkb1 loss compromises p53 function (Jones et al., 2005; Karuman et al., 2001), we nonetheless noted strong cooperation between deletion of Lkb1 and p53 in the context of K-Ras activation (TKp53L/L; Lkb1L/L), with a sharp reduction of median tumor latency to 11 weeks. Therefore, Lkb1 and p53 independently restrain Ras-mediated melanomagenesis.
Figure 2. Lkb1 Inactivation Promotes Melanoma Formation and Metastasis.
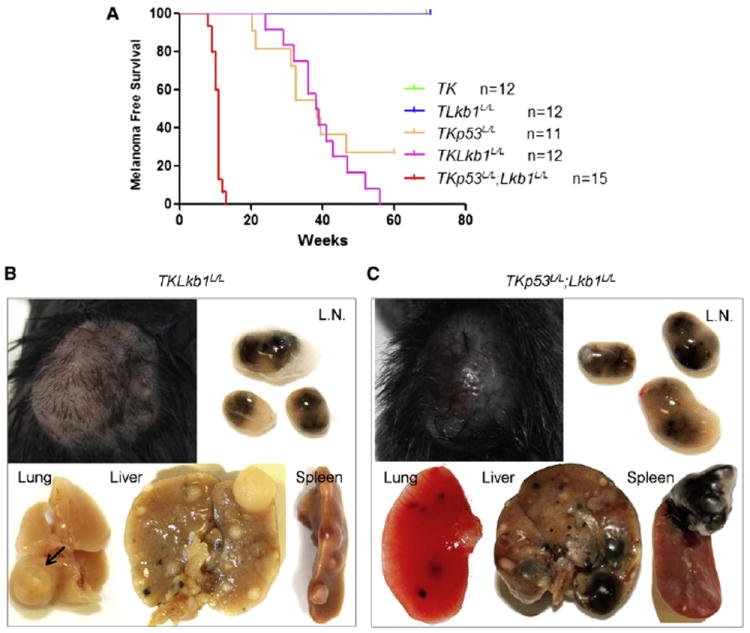
(A) Kaplan-Meier analysis of melanoma-free survival of cohorts of indicated genotypes is presented.
(B and C) Representative primary tumors and metastases exhibiting variable pigmentation from TKLkb1L/L (B) and TKp53L/L; Lkb1L/L (C) mice are shown. L.N., lymph node. Arrow in (B) indicates a hypopigmented lung metastasis.
See also Table S1 and Figure S2.
Although metastasis is seen with multicopy N-Ras and c-Met transgenic alleles (Ackermann et al., 2005; Scott et al., 2011), metastasis is not a feature of melanoma models driven by a multi-copy H-Ras transgenic allele (Chin et al., 1997; Scott et al., 2011) or endogenous expression of mutant K-Ras (Monahan et al., 2010). Likewise, we have not observed hematogenous or lymph node metastases in K-Ras-driven melanoma models with intact Lkb1 function, including TKp16L/L, TKp53L/L, and TKp53L/L; p16L/L mice (Table S1; see also Monahan et al., 2010). Against this prior experience, we were surprised to note high-volume metastasis in 100% of tumor-bearing mice with somatic K-Ras activation and Lkb1 loss (TKLkb1L/L and TKp53L/L; Lkb1L/L). In these mice, metastases were found in lymph node, lung, liver, and spleen, but not in kidney or brain (Figures 2B and 2C; Table S1). Because we do not observe metastasis in Lkb1-intact tumors induced by activated H- or K-Ras (e.g., with combined Ink4a/Arf or p53 loss), we concluded that the strong enhancement of metastasis in this model resulted from Lkb1 inactivation.
Although the primary melanomas in both TKLkb1L/L and TKp53L/L; Lkb1L/L mice were unpigmented or hypopigmented, metastases found in lymph node, lung, liver, and spleen contained both unpigmented and deeply pigmented lesions (Figures 2B and 2C). These results are reminiscent of prior findings in lung cancer by Ji et al. (2007), where Lkb1 loss both promoted metastasis as well as an extended spectrum of tumor differentiation (e.g., adenocarcinoma versus squamous carcinoma). Therefore, loss of Lkb1 appears to promote melanoma metastasis in the context of increased differentiation potential, consistent with an effect of Lkb1 on a tumor-initiating compartment with augmented multipotency.
The YES SRC Family Kinase Is Activated by LKB1 Loss and Results in Enhanced Metastatic Properties
In order to understand the mechanism whereby Lkb1 regulates metastasis, we studied the effects of Lkb1 on cell migration and invasion in vitro. Toward that end, we generated tumor cell lines from mice of defined genotypes with and without Lkb1. We observed a strong effect of Lkb1 loss on the in vitro wound healing or scratch assay. Compared to melanoma cells with WT Lkb1, including TKp53L/L; p16L/L and Tyr-Ras; Ink4a/Arf−/− (“TRIA”; Chin et al., 1997) cells, Lkb1-deficient melanoma cells migrated more rapidly to fill an in vitro wound (Figure 3A; Movies S1 and S2). Likewise, loss of Lkb1 increased tumor invasiveness as quantified using the Matrigel invasion assay (Figure 3B), whereas proliferation in 2D culture or soft agar was not influenced by Lkb1 status (Figure S3). To confirm that these effects reflected Lkb1 function, Lkb1 null melanoma cells were transduced with WT Lkb1 or kinase-dead Lkb1 (Lkb1-KD), and Lkb1 expression was knocked down in Lkb1 intact melanoma cell lines by transducing a small hairpin RNA (shRNA) targeting Lkb1 (Figure 3C). In scratch assays and Matrigel invasion, Lkb1 restoration in Lkb1 null tumor cells inhibited cell migration and invasion, which was dependent on the kinase activity of Lkb1. Likewise, partial knockdown of Lkb1 in TKp53L/L; p16L/L cell lines significantly promoted cell migration and invasion (Figures 3D and 3E). These data demonstrate that loss of Lkb1 promotes melanoma cell migration and invasion in vitro.
Figure 3. Loss of Lkb1 Promotes Melanoma Cell Migration and Invasion In Vitro.
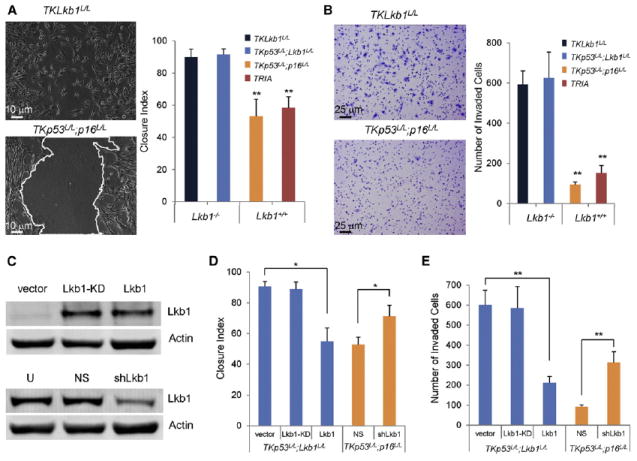
(A) Cells of indicated genotypes were subjected to in vitro wound healing or scratch assay. Representative photomicrographs of TKLkb1L/L cells and TKp53L/L; p16L/L are shown on the left. Mean closure index, determined as described in the Supplemental Experimental Procedures, is graphed by genotype on the right (n = 3 replicates per genotype).
(B) Cells of indicated genotypes were subjected to Matrigel invasion assay. Representative photomicrographs of cells that have invaded through Matrigel are shown on the left and mean values graphed for cells of indicated genotypes on the right (n = 3 replicates per genotype).
(C) Western analysis of TKp53L/L; Lkb1L/L cells transduced with nonfunctional Lkb1-KD (“kinase dead”) or Lkb1, and TKp53L/L; p16L/L cells transduced with nonspecific shRNA (NS) or shRNA targeting Lkb1 (shLkb1) is presented. U, untreated.
(D and E) Isogenic cells with and without Lkb1 as indicated were subjected to in vitro scratch assay (D) and Matrigel invasion assay (E) as in (A) and (B), respectively. Error bars show SD. *p < 0.05; **p < 0.01.
See also Movies S1 and S2, and Figure S3.
Unbiased proteomic analysis has revealed that Lkb1 loss activates SFKs in lung tumors (Carretero et al., 2010), and therefore, we examined the effect of Lkb1 function on SFK phosphorylation, which correlates with SFK activation, in melanoma cells. Lkb1 knockdown led to increased phosphorylation of SFKs in murine TKp53L/L; p16L/L melanoma cells using a pan-SFK phospho-specific antibody (Figure 4A). We further examined the phosphorylation states of individual SFK members that are abundantly expressed in melanoma, including Src, Fyn, and Yes, by immunoprecipitation of each protein with an SFK-specific antibody followed by immunoblotting with an antibody to a shared phospho-tyrosine site (Y416). Although Src and Fyn phosphorylation was not significantly changed by Lkb1 knockdown, Yes phosphorylation was significantly increased by Lkb1 knockdown (Figure 4A). These results suggest that Yes activity, at least in part, reflects Lkb1 function in melanoma.
Figure 4. Lkb1 Loss Results in SFK Activation.
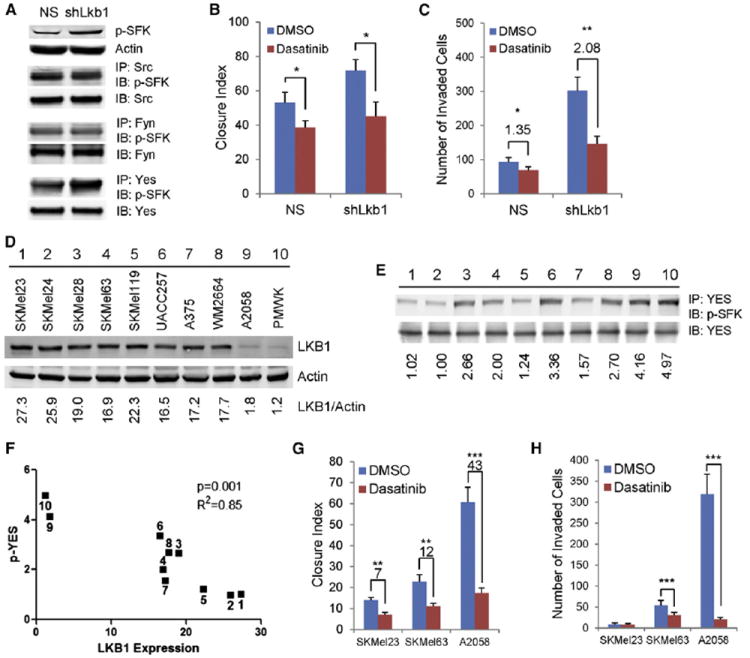
(A) Representative western analysis of TKp53L/L; p16L/L cells with or without Lkb1 knockdown is shown. Cell lysates were either directly immunoblotted (IB) with antibody against p-SFKs (Y416) or immunoprecipitated (IP) first with indicated antibodies against Src, Fyn, or Yes. NS, nonspecific shRNA.
(B) TKp53L/L; p16L/L melanoma cells with or without LKB1 knockdown were grown in media containing vehicle (DMSO) or dasatinib (30 nM). Closure index was measured 12 hr after wounding.
(C) TKp53L/L; p16L/L melanoma cells with or without LKB1 knockdown were subjected to Matrigel invasion assay with and without treatment with pan-SFK inhibitor, dasatinib (30 nM).
(D) Representative western analysis of indicated human melanoma cells with LKB1 quantification by LICOR analysis is shown.
(E) YES phosphorylation in indicated human melanoma cells with phospho-YES level quantification by LICOR analysis is presented. Cells were labeled with the same number as in (D).
(F) Correlation analysis of LKB1 and phospho-YES expression in human melanoma cells is illustrated. Cells were labeled with the same number as in (D).
(G and H) SKMel23, SKMel63, and A2058 cells were treated with DMSO or 30 nM dasatinib and subjected to in vitro scratch assay (G) or Matrigel invasion assay (H).
Error bars show SD. *p < 0.05; **p < 0.01; ***p < 0.001.
See also Figure S4.
To test whether increased SFK activity is involved in the effect of Lkb1 loss on melanoma cells, we treated the TKp53L/L; p16L/L melanoma cells with or without Lkb1 knockdown with the pan-SFK inhibitor dasatinib. Dasatinib treatment inhibited melanoma cell proliferation (Figure S4A); however, this effect was independent of Lkb1 knockdown. In contrast whereas treatment with dasatinib resulted in a modest decrease (14%) in cell migration in Lkb1-intact cells, the effect was enhanced (27%) in melanoma cells with Lkb1 knockdown (Figure 4B). A similar Lkb1-dependent effect of dasatinib on cell invasion was noted in Matrigel invasion (Figure 4C). These observations suggest that the activation of SFKs due to Lkb1 loss contributes to melanoma cell migration and invasion, but not proliferation.
To confirm the effects of LKB1 loss and SFK activity across species, we turned to the study of human melanoma cell lines. We examined LKB1 expression in a panel of human melanoma cell lines by western analysis and immunohistochemistry on a cell line tissue microarray (TMA). LKB1 expression was heterogeneous in these human melanoma cell lines, with an ~30-fold range of expression observed (Figures 4D and S4B). Two lines with heterozygous LKB1 mutations (A2058 and PMWK) demonstrated lowest expression of functional protein. No correlation between LKB1 expression and RAS/RAF status was observed (Figures 4D and S4B). Further examination revealed that phospho-YES level anticorrelated with LKB1 expression in these human melanoma cell lines (Figures 4E and 4F). To investigate cell motility, invasion, and dasatinib sensitivity in these cells, we focused on lines SKMel23, SKMel63, and A2058 with high, medium, and low LKB1 expression, respectively. As shown in Figures 4G and 4H, LKB1 expression anticorrelated with cell motility, invasion, and sensitivity to dasatinib treatment. In accord with prior studies showing a potent effect of Lkb1 haploinsufficiency on tumorigenesis (Ji et al., 2007), these data show that complete loss of LKB1 is not required to promote malignant growth because even reduced levels of functional LKB1 protein promote phospho-YES expression and invasive behavior in vitro.
To study the relationship of LKB1 and SFK activity in isogenic lines, we knocked down LKB1 expression in A2058 cells (Figure S5A) and analyzed the phosphorylation status of all SFKs using an 8-plex Luminex assay. In accord with the murine results (Figure 4A), LKB1 knockdown in human A2058 cells resulted in an increase in YES phosphorylation as well as a more modest but significant effect on FYN phosphorylation (Figure 5A). The activity of all the other SFK members was not changed by LKB1 knockdown (Figure 5A). To assess the role of individual SFKs in mediating the effects of LKB1 loss, we knocked down the expression of individual SFK members by transfecting A2058 cells with siRNAs specifically targeting SRC, FYN, or YES (Figure 5B). We noted that LKB1 knockdown in A2058 cells had a similar effect on wound healing and Matrigel invasion to that seen in murine melanoma (Figures 5C and 5D). This effect of LKB1 inactivation was reverted by knockdown of YES, but not FYN or SRC (Figures 5C and 5D). Of note the proliferation of these cells was not affected by knockdown of YES (Figure S5B). The effect of LKB1 inactivation on YES phosphorylation, cell motility, and invasion was also observed in a second human melanoma cell line, SKMel28, with relatively high expression of LKB1 (Figures S5C–S5F). Therefore, as opposed to lung cancer where greater effect of LKB1 loss is on SRC (Carretero et al., 2010), the effects of increased SFK activity on cell migration and invasion associated with LKB1 loss in melanoma cells are predominantly mediated by YES.
Figure 5. The Effects of LKB1 Loss on Melanoma Cells Are Mediated by the YES Kinase.
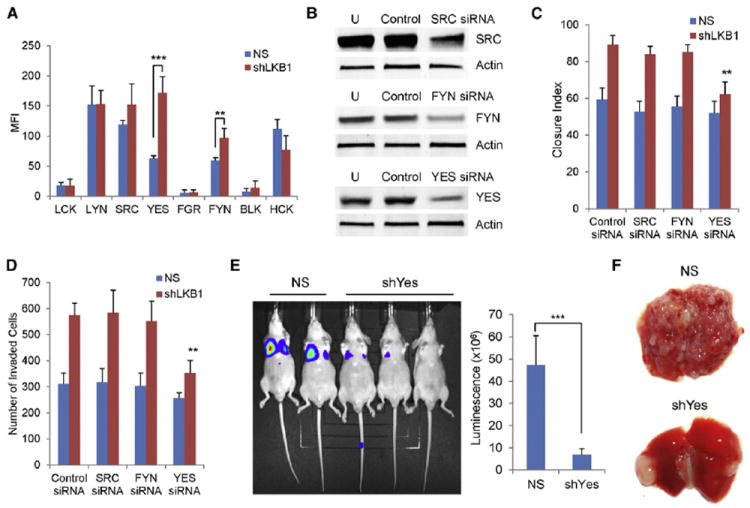
(A) Tyrosine phosphorylation status of SFK members in A2058 cells with or without LKB1 knockdown is illustrated. Mean values of three replicates per kinase are graphed. MFI, median fluorescence intensity. NS, nonspecific shRNA.
(B) A2058 cells expressing shLKB1 were transfected with scrambled control siRNAs or siRNAs targeting SRC, FYN, or YES. Cell lysates were immunoblotted with indicated antibodies 48 hr after transfection. U, untreated.
(C and D) A2058 cells with or without LKB1 knockdown were transfected with indicated SFK siRNAs. Cells were subjected to in vitro scratch assay (C) or Matrigel invasion assay (D) 48 hr after siRNA transfection.
(E and F) Luciferase-expressing TKp53L/L; Lkb1L/L melanoma cells with or without Yes knockdown were injected into nude mice via tail vein. Mice were examined by luciferase imaging (E) and dissection (F). Luminescence was quantified (E).
Error bars show SD. **p < 0.01; ***p < 0.001.
See also Figure S5.
To determine the role of Yes activity in metastasis of Lkb1-deficient melanoma in vivo, we employed the tail vein metastasis assay. Targeting of Yes by shRNA was used to knock down expression in TKp53L/L; Lkb1L/L melanoma cells (Figure S5G), which did not affect cellular proliferation (Figure S5H). Cells with or without Yes knockdown were injected into nude mice via the tail vein, and lung metastases were examined 3 weeks later. As shown in Figures 5E and 5F, TKp53L/L; Lkb1L/L melanoma cells were highly metastatic in vivo, whereas knockdown of Yes resulted in a 7-fold decrease of tumor metastasis. In contrast, dasatinib treatment (50 mg/kg/day orally) did not significantly inhibit tumor metastasis in vivo (Figures S5I and S5J). This result is in contrast to the in vitro effects of dasatinib treatment (Figures 4B and 4C) as well as previous studies in lung cancer models by Carretero et al. (2010). Given this discrepancy, we investigated the potency of dasatinib in cell-based assays against SFKs. In accord with previous in vitro kinase and cell-based assays by Deguchi et al. (2008) and Konecny et al. (2009), Src and Fyn were inhibited by lower doses of dasatinib than those needed to inhibit Yes (Figure S5K, ~10-fold difference in the EC50 for Src versus Yes). Therefore, whereas dasatinib is adequately potent for in vitro studies (Figures 4B, 4C, 4G, and 4H), in vivo inhibition of Yes activity appears to require greater doses of dasatinib than those needed to inhibit SRC, the more important SFK in LKB1-deficient lung cancer. These data suggest that an SFK inhibitor with greater potency against YES may exhibit better antimelanoma efficacy in humans.
Microarray Analysis of Lkb1-Regulated Transcript
To provide a mechanistic understanding of how LKB1 loss promotes melanoma metastasis, we performed RNA expression profiling on 20 melanoma cell lines and 9 primary tumors with or without functional Lkb1. The transcriptional effects of Lkb1 inactivation were large: a total of 2,767 and 15,795 genes were differentially associated with Lkb1 competence in cell lines and primary tumors, respectively (false discovery rate [FDR] <5%) (Table S2). To better understand pathways regulated by Lkb1 in melanoma cells, we constructed an “overlap” list of transcripts that were differentially regulated by Lkb1 inactivation in both melanoma cell lines and primary tumors. Toward that end, we picked the top 1,000 upregulated and downregulated transcripts associated with Lkb1 loss in cell lines and primary tumors and identified all transcripts appearing on both lists. This winnowed overlap list contained 55 upregulated genes, and 55 downregulated genes (Figures S6A and S6B). Gene Set Enrichment Analysis (GSEA) of this overlap list indicated a significant enrichment for several pathways (Table S3), with targets of mir27 and Lef1 being particularly highly represented (Figures S6A and S6B). Lef1 is a critical transcriptional activator responsive to WNT/β-catenin, and this observation is consistent with recent work suggesting that mir27 expression promotes metastasis via activation of β-catenin signaling (Zhang et al., 2011). Moreover, we noted a more than 2-fold increased expression of other validated WNT targets (Axin2, Nkd1, Lgr5, and Bmp4, FDR <1%) in Lkb1-deficient tumors. Therefore, these transcriptional analyses suggest that increased expression of targets of mir27 and Wnt/β-catenin is associated with melanoma metastasis.
LKB1 Inactivation Induces an SFK-Dependent Expansion of a Prometastatic, CD24+ Tumor Subpopulation
The association of Lkb1 loss with increased Lef1 expression has been previously noted in lung cancer by Ji et al. (2007), and therefore, we looked for other transcripts associated with Lkb1 loss in both tissues. Although we also noted increased expression of Nedd9 and Vegf-c in both tumor types (data not shown), the most reliably upregulated gene associated with LKB1 loss in cell lines and primary tumors from both tumor types was CD24 (“CD24a” in the mouse, Figure S6A; Ji et al., 2007). We considered this protein of interest for further study given its role as a known modulator of advanced disease and metastasis (Baumann et al., 2005; Kristiansen et al., 2003; Lee et al., 2011; Senner et al., 1999) and marker of stem-progenitor cells in several tumor types (Al-Hajj et al., 2003; Gao et al., 2010; Lee et al., 2011). Prior work has demonstrated heterogeneous expression of CD24 in human melanoma (Shields et al., 2007; Stuelten et al., 2010), and expression is not uniform on all cells within a given cell line. Instead, consistent with its ability to mark tumor-initiating fractions, CD24 expression is noted on a tumor subfraction, with expression ranging from <1% to 13% of cells in melanoma lines.
We therefore investigated the relationship of Lkb1 status and CD24 expression. Cell lines derived from murine melanomas with intact Lkb1 function exhibited a low fraction (<3%) of CD24+ cells. Remarkably, inactivation of Lkb1 was associated with a marked expansion of the CD24+ population ranging from 10% to more than 30% of cells (Figures 6A, 6B, S6C, and S6D). Correspondingly, restored expression of Lkb1 in Lkb1 null melanoma cells suppressed CD24 expression within 6 days of transduction, which was dependent on the kinase activity of Lkb1 (Figures 6B and S6E). Likewise, Lkb1 null (TKLkb1L/L) tumors contained an increased CD24+ subpopulation compared to Lkb1-competent (TRIA) tumors in vivo (Figure S6F). These data demonstrate a highly dynamic, 3- to 10-fold effect of Lkb1-kinase activity on expression of cell surface CD24, a known facilitator of metastasis.
Figure 6. Lkb1 Loss Expands a Prometastatic CD24+ Cell Population.
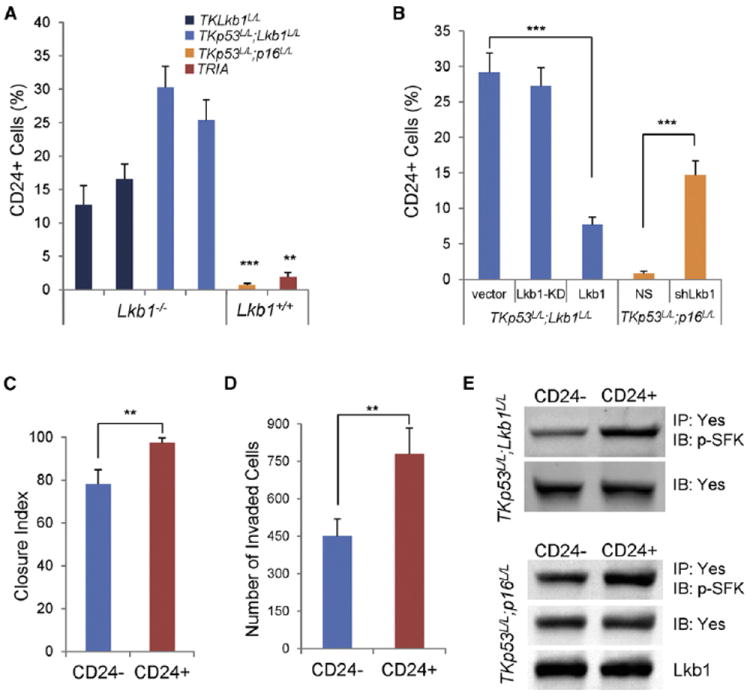
(A) CD24 expression of melanoma cells of indicated genotypes was examined by flow cytometry.
(B) TKp53L/L; Lkb1L/L cells were transduced with Lkb1-KD or Lkb1. TKp53L/L; p16L/L cells were transduced with NS or shLkb1. Cells with and without Lkb1 function as indicated were examined for CD24 expression by flow cytometry.
(C and D) CD24+ cells and CD24− cells were isolated from TKp53L/L; Lkb1L/L cells by FACS. Sorted cells were subjected to scratch assay (C) and Matrigel invasion assay (D).
(E) CD24+ and CD24− cells were isolated from TKp53L/L; Lkb1L/L and TKp53L/L; p16L/L cells. Cell lysates were subjected to direct immunoblotting or immuno-blotting following immunoprecipitation as indicated.
Error bars show SD. **p < 0.01; ***p < 0.001.
See also Figure S6 and Tables S2 and S3.
Given that CD24 expression (both increased and decreased) has been associated with functional heterogeneity and tumor-initiating cells in other cancer types, we examined the in vitro properties of CD24+ versus CD24− cells in melanoma cell lines. CD24+ and CD24− cells were isolated from TKp53L/L; Lkb1L/L cells by fluorescence-activated cell sorting (FACS) (Figure S6G), and the separated populations were assessed for proliferation, migration, and invasion. Although no difference was observed in the proliferation of CD24+ versus CD24− cells (Figure S6H), CD24+ cells showed significantly increased cell migration and invasion (Figures 6C and 6D). Correspondingly, Yes phosphorylation was higher in CD24+ cells than in CD24− cells from both Lkb1-competent and -deficient cell lines (Figure 6E). These results suggest that CD24 expression is associated with increased metastatic behavior in vitro as well as Yes phosphorylation and that increased Yes activation is a common feature of the CD24+ subpopulation regardless of Lkb1 status in melanoma.
To confirm the effects of LKB1 loss on CD24 expression in human cells, we examined CD24 expression in a large panel of human melanoma cell lines. CD24 expression varied over a 28-fold range and anticorrelated with LKB1 expression (Figures 7A and 7B). In A2058 cells, shRNA targeting of LKB1 led to a marked and rapid increase in CD24 expression (from 4% to 36% with LKB1 knockdown) (Figure 7C), and a similar effect was also observed in SKMel28 cells (Figure S7A). Expression of CD44, another commonly used “tumor stem cell” marker, was not modulated by LKB1 knockdown within this time frame (Figure S7B). As in murine cells, the activity of YES and total SFKs was predominantly observed in CD24+ compared to CD24− cells (Figures S7C and S7D). Moreover, the level of phospho-ERK was also increased by LKB1 knockdown, with a greater effect in CD24+ versus CD24− cells, whereas no effect of LKB1 knockdown was noted on phospho-AKT levels (Figure S7E). The increase in CD24 mRNA and protein expression due to LKB1 loss was suppressed in a dose-dependent fashion by transiently treating cells with dasatinib in both human and murine melanoma cells (Figures 7D, 7E, and S7F), with CD24 mRNA sharply decreasing with as little as 12 hr of dasatinib treatment. In accord with the in vitro motility and invasion results (Figures 5C and 5D), the effect of LKB1 loss on CD24 expression was rescued by siRNA to YES, but not SRC or FYN (Figure 7F). These data show that the ability of LKB1 loss to induce expansion of the prometastatic CD24+ compartment requires the activity of YES kinase.
Figure 7. Expansion of the CD24+ Fraction in Response to LKB1 Inactivation Requires YES Kinase.
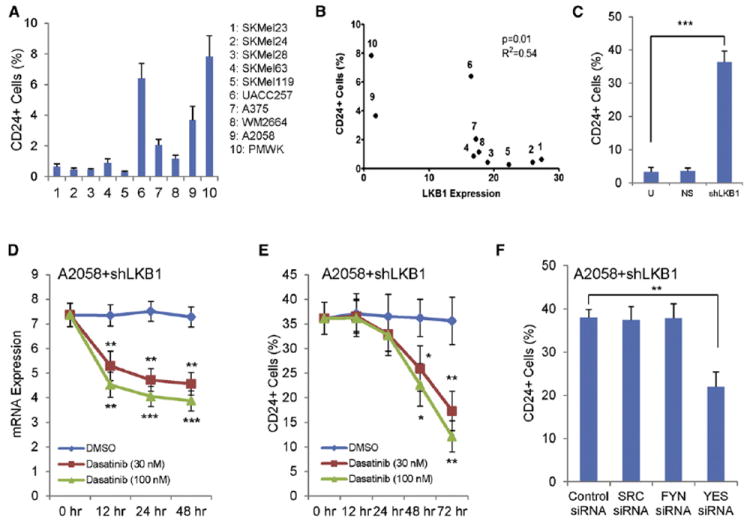
(A) Percentage of CD24+ cells in indicated human melanoma cells determined by flow cytometry is illustrated.
(B) Correlation analysis of LKB1 and CD24 expression in human melanoma cells is presented. LKB1 expression was quantified in Figure 4D. Cells were labeled with the same number as in (A) and Figure 4D.
(C) CD24 expression in A2058 human melanoma cells increases with LKB1 knockdown (shLKB1). U, untreated. NS, nonspecific shRNA.
(D and E) A2058 cells with LKB1 knockdown were treated with the indicated concentrations of dasatinib and harvested for analysis at the indicated times. The expression of CD24 mRNA (D) was measured by quantitative RT-PCR and calculated as relative expression to A2058 cells with NS-shRNA. The expression of CD24 protein (E) was measured by flow cytometry (n = 3 replicates).
(F) A2058 cells with LKB1 knockdown were transfected with indicated SFK siRNAs. CD24 expression was measured by flow cytometry 72 hr after the transfection (n = 3 replicates).
Error bars show SD. *p < 0.05; **p < 0.01; ***p < 0.001.
See also Figure S7.
We next performed an analysis of the relationship between CD24 expression and tumor formation. More colony-forming cells (CFCs) were noted in the CD24+ fraction from both Lkb1-deficient (TKp53L/L; Lkb1L/L) and Lkb1-competent (TKp53L/L; p16L/L) cell lines (Figure S8A). We investigated the in vivo tumor growth of isolated CD24+ and CD24− cells (Figure S8B) by xenograft transplantation. Although all mice developed tumors, CD24+ cells grew more rapidly whether they were derived from Lkb1-defective or -competent melanomas (Figure S8C). FACS-sorted CD24+ and CD24− cells were injected into nude mice via tail vein, with in vivo metastases examined 3 weeks later. CD24+ cells from both Lkb1-deficient and Lkb1-competent melanomas exhibited greater metastatic ability than CD24− cells (Figure 8). Examination of CD24 expression detected both CD24+ and CD24− cells in tumor metastases formed by either CD24+ or CD24− cells (Figures 8C and 8F). Yes knockdown significantly attenuated the size of the CD24+ subpopulation in metastases formed after tail vein injection of TKp53L/L; Lkb1L/L (Figure S8D). These data indicate that CD24+ melanoma cells exhibit a qualitative increase in metastatic propensity regardless of Lkb1 status and suggest that Lkb1 inactivation predominantly promotes tumor progression by leading to a marked expansion of this CD24+ fraction.
Figure 8. CD24+ Cells Exhibit Higher Metastatic Potential than CD24– Cells In Vivo.
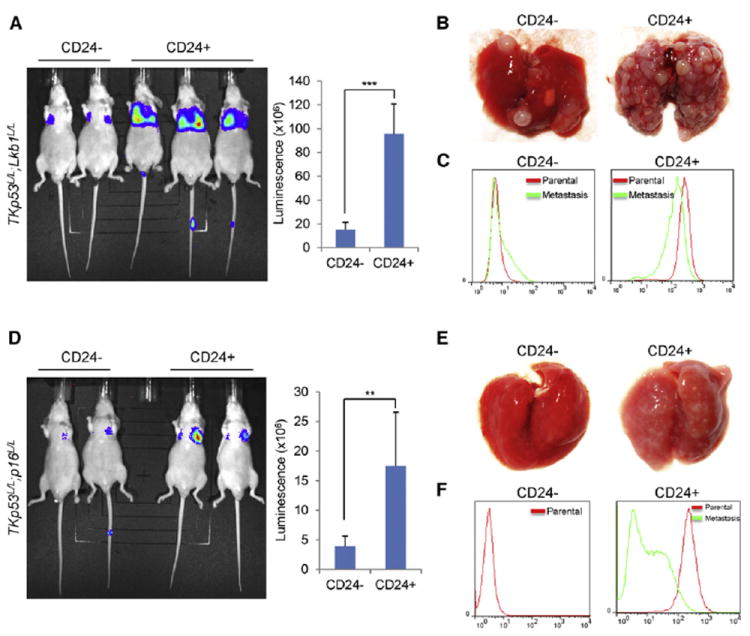
(A–F) CD24+ and CD24− cells were isolated from luciferase-expressing Lkb1-deficient (TKp53L/L; Lkb1L/L, A–C) and Lkb1-competent (TKp53L/L; p16L/L, D–F) melanoma cells and injected into nude mice via tail vein (n = 6 mice for each group). Three weeks after tail vein injection, mice were examined by luciferase imaging (A and D) and dissection (B and E). Luminescence was quantified, and statistical analysis was performed. Error bars show SD. **p < 0.01; ***p < 0.001. Tumor cells were examined by flow cytometry for CD24 expression (C and F) prior to tail vein injection (parental) or 3 weeks later after metastatic growth in the lung (metastasis). No metastasis sample was retrievable from the CD24− fraction of TKp53L/L; p16L/L cells. See also Figure S8.
DISCUSSION
In this work we show that mice with melanocyte-specific Lkb1 loss and K-Ras activation develop highly metastatic melanomas. Lkb1-deficient melanoma cells increased invasive behavior in vitro compared to isogenic Lkb1-competent melanoma cells. Further investigation revealed that LKB1 deficiency resulted in activation of SFKs, particularly YES, and expansion of a CD24+ cell population that showed increased invasive behavior in vitro and in vivo. Inhibition of YES activity suppressed CD24 expression and decreased metastatic behavior. Collectively, these results demonstrate that LKB1 functions as a strong suppressor of melanoma metastasis by regulating YES activity, which determines the size of a prometastatic CD24+ tumor subpopulation.
Of interest with regard to the phenotypic expression of PJS, we showed that combined melanocyte-specific Lkb1 loss and K-Ras activation results in increased melanocyte proliferation and in vivo hyperpigmentation. The excess melanocytic proliferation in TKLkb1L/L (and TKLkb1L/+), but not in TLkb1L/L, mice, suggests that melanocytic hyperproliferation seen in patients with PJS may reflect sporadic secondary events that activate regulators of proliferation (e.g., RAS) rather than loss of the second copy of LKB1. Therefore, TKLkb1L/L mice serve as a model to study this poorly understood feature of PJS. A weakness of this model is the reliance on K-RAS mutation, which is ~10-fold less common than that of N-RAS in human melanoma. To date, we have not been able to generate tumors with an N-RasLSL allele featuring a codon 12 mutation (Haigis et al., 2008), even when combined with inactivation of several different tumor suppressor genes. The explanation for this difference in the melanoma-promoting effects of the various RAS isoforms is an area of active ongoing investigation.
In addition to altered pigmentation, TKLkb1L/L and TKp53L/L; Lkb1L/L mice exhibited highly metastatic melanoma. Although metastasis has been reported in a small number of autochthonous murine tumor models (Ackermann et al., 2005; Guy et al., 1992; Scott et al., 2011), these models feature lower volumes of metastatic disease with variable penetrance and rely on supraphysiologic expression of oncogenes. In contrast the present model couples melanocyte-specific, somatic single-copy K-Ras activation under the control of its endogenous promoter with homozygous Lkb1 deletion to produce 100% penetrance of metastasis with a high burden of metastatic disease. For example several tumor-bearing TKLkb1L/L and TKp53L/L; Lkb1L/L mice exhibited >50% involvement of the liver, lung, and/or spleen with multifocal metastasis (Figures 2B, 2C, and S2). We believe that the high burden and penetrance of metastases in this model address a significant unmet need in cancer research for experimentally tractable, highly metastatic, autochthonous tumor models. Moreover, in murine models of both lung cancer (Carretero et al., 2010; Ji et al., 2007) and melanoma, Lkb1-deficient tumors demonstrate increased histomorphometric heterogeneity and more frequent metastasis compared to tumors lacking p53 or Ink4a/Arf. Because metastasis is not noted in TKp53L/L, TKp16L/L, or TRIA mice (Table S1; Monahan et al., 2010), the effects of Lkb1 loss on metastasis are not explained by the effects of Lkb1 on expression of p16INK4a, Arf, or p53.
Of interest, Dankort et al. have recently reported melanoma metastasis, albeit with lower tumor burdens, in 4-OHT-treated Tyr-CRE-ERT2B-RafLSL/+PtenL/L mice (Dankort et al., 2009). This result is consistent with the notion that B-Raf mutation (Esteve-Puig et al., 2009; Zheng et al., 2009) induces a partial compromise of Lkb1 function. More recently, this group has shown that WNT/β-catenin activation strongly enhances metastasis in this model (Damsky et al., 2011), which matches our findings of increased expression of β-catenin/Lef1 targets in Lkb1-deficient tumors (Figures S6A and S6B). It is not clear from our data if the YES-dependent expansion of the prometastatic, CD24+ melanoma subfraction requires expression of β-catenin/LEF1 targets, but both events appear to result from LKB1 inactivation.
SFK activity has been shown to closely associate with melanoma metastasis (Homsi et al., 2009; Putnam et al., 2009), and prior work in particular has suggested a prominent role for YES among the SFKs in melanoma pathogenesis. For example the kinase activity of YES, but not SRC, is significantly higher in most melanoma cell lines than in melanocytes (Loganzo et al., 1993). Likewise, activation of YES, but not FYN or SRC, appears essential to mediate the increased invasiveness of human melanoma cells in response to stimulation with gangliosides or neurotrophins (Hamamura et al., 2011; Marchetti et al., 1998). Although the mechanism whereby loss of LKB1 kinase activity induces YES activation is not clear, the present data suggest YES as a promising therapeutic target in melanoma lacking LKB1 function. Dasatinib exhibits modest antimelanoma activity in human patients (Kluger et al., 2011). Because we and others (Deguchi et al., 2008; Konecny et al., 2009) have noted an ~10-fold decrease in the potency of dasatinib for YES compared to SRC and other SFKs, these results suggest that an SFK inhibitor with greater potency against YES inhibitor would exhibit greater in vivo antimelanoma efficacy.
Increased YES activity in turn leads to an expansion of a tumor subpopulation that is characterized by increased cell motility and invasion, as well as CD24+ expression. Surprisingly, although LKB1 function is inhibited in most or all of the cells, the activation of YES and expression of CD24 in response to LKB1 inactivation is limited to a minority (~10%–30%) of cells, which exhibit enhanced metastatic properties. A CD24+ population of cells is present, albeit at lower frequency, in LKB1-competent melanoma cells, and loss of LKB1 kinase activity appears to induce an expansion of this prometastatic fraction. The prometastatic properties of CD24+ cells were increased relative to isogenic CD24− cells regardless of whether the CD24+ cells were derived from LKB1-deficient or -competent cell lines. This observation is consistent with evidence that CD24 expression is associated with advanced disease and increased metastasis in glioma and epithelial cancers (Baumann et al., 2005; Kristiansen et al., 2003; Lee et al., 2011; Senner et al., 1999). Therefore, our data are most consistent with the model that the principal effect of LKB1 inactivation with regard to metastasis is to markedly increase the frequency of this prometastatic subpopulation.
Importantly, whereas CD24 expression appears to play a direct role in facilitating tumor metastasis, it has also been observed to mark heterogeneous subpopulations (e.g., “tumor stem cells”) of a variety of cancers (Al-Hajj et al., 2003; Gao et al., 2010; Lee et al., 2011). Therefore, our data are consistent with the model that CD24 expression directly facilitates melanoma metastasis but also that CD24 expression merely serves as a marker of a tumor subpopulation with increased metastatic properties. With regard to the latter possibility, LKB1 loss led to an increase in a tumor subfraction with increased colony-forming activity and expanded tumor differentiation potential in vivo (as reflected by the variable degree of tumor pigmentation), which are properties of “tumor stem cells.” Although the concept of a tumor stem cell in melanoma is controversial, our results are compatible with the possibility that the increased tumor heterogeneity noted in the setting of LKB1 inactivation reflects an augmented tumor stem cell fraction.
In summary this work shows a prominent role for LKB1 in melanocyte biology and the suppression of melanoma metastasis. We observed that a principal effect of LKB1 loss on metastasis required expansion of a CD24+ prometastatic tumor subfraction that exhibited some properties of a tumor stem cell. Expansion of this compartment required the activity of YES kinase. These data suggest that a determination of LKB1 mutational status in patients with advanced melanoma will contribute to prognosis prediction and identify promising therapeutic targets (YES and CD24) in melanoma with compromised LKB1 function.
EXPERIMENTAL PROCEDURES
Mouse Colony
Mice were housed and treated in accordance with protocols approved by the institutional care and use committee for animal research at the University of North Carolina. Animals were generated and genotyped as previously described: T (Bosenberg et al., 2006); K-RasL/L (or “K”; Johnson et al., 2001); Lkb1L/L (Bardeesy et al., 2002); p53L/L (Jonkers et al., 2001); and TRIA (Chin et al., 1997). All cohorts reported in Figures 1 and 2 (TK, TLkb1L/L, Tp53L/L, Tp53L/L; Lkb1L/L, TKLkb1L/L, TKp53L/L, TKp53L/L; Lkb1L/L) were newly generated and contemporaneously housed for this work. Data from the TKp16L/L and TKp53L/L; p16L/L cohorts shown in Table S1 are a historical comparison from a prior study by Monahan et al. (2010). All cohorts were N1 in C57BL/6 and, where possible, compared to littermate controls. To induce CRE recombinase in vivo, pups were treated with 4-OHT as described by Dankort et al. (2009) and Monahan et al. (2010). In tumor survival cohorts, mice were monitored for tumors three times per week and sacrificed for tumor size (>1.3 cm) or morbidity (ulceration, weight loss). All sacrificed animals were analyzed for metastasis by necropsy. H&E of tumors after formalin fixation and paraffin embedding was performed, with analysis showing spindle-shaped melanoma with variable pigmentation (Figure S2). Melanocytic lineage was further confirmed by staining for melanocytic markers (Figure S2). Kaplan-Meier analysis of melanoma-free survival was determined using GraphPad Prism software.
Cell Lines and Cell Culture
Primary melanocyte cultures and murine tumor cell lines were generated as described by Bennett et al. (1989) and Sharpless et al. (2002). Human melanoma cells studied were immortalized cell lines and were IRB exempt. To induce CRE recombinase in vitro, primary melanocyte cultures were treated with 4-OHT at 20 days postisolation for 48 hr. Melanoma cells were maintained in DMEM containing 10% FBS. Dasatinib was from LC Laboratories (D-3307). Immunoprecipitation, immunoblotting, immunofluorescence, SFK 8-Plex, and quantitative RT-PCR assays are described in the Supplemental Experimental Procedures.
Cell Migration and Invasion Assays
The in vitro scratch (wound healing) assay was performed as described previously by Carretero et al. (2010). Matrigel invasion was determined using invasion chambers obtained from BD Biosciences, with assays performed according to the manufacturer’s instructions. See Supplemental Experimental Procedures.
Flow Cytometric Analysis and FACS
Cells were labeled with indicated antibodies, washed, resuspended, and strained (40 μm). Data were recorded with a CyAn ADP flow cytometer (Dako Cytomation) and analyzed by FlowJo software. Antibodies used were Anti-Human APC-CD24 (eBioscience), Anti-Mouse FITC-CD24 (eBioscience), Anti-Mouse PE-Cy5-CD24 (eBioscience), and Anti-Human FITC-CD44 (BD Biosciences). For CFC assay, single cells were FACS-sorted into individual wells of 96-well plates. CFCs were counted after culturing the cells for 3 weeks.
Tail Vein Metastasis Assay
Five to 6-week-old female nu/nu mice were maintained under pathogen-free conditions. Cells were transduced with a luciferase reporter gene prior to injection. CD24+ cells and CD24− cells were separated by FACS. A total of 0.5 × 105 cells were injected into nu/nu mice via tail vein (n = 6 per experimental group). Three weeks after the injection, mice were subjected to luciferase imaging and necropsy to determine lung metastasis. Luminescence was quantified using Living Image software (Caliper Life Sciences).
shRNA, siRNA, and Lentiviruses
shRNA constructs used for knocking down LKB1 expression in A2058 cells were described previously by Carretero et al. (2010). shRNA targeting Yes was from Thermo Scientific. For suppression of Src, Fyn, and Yes expression, cells were transfected with the appropriate antisense oligonucleotides using Lipofectamine RNAiMAX (Invitrogen). Src siRNA (sc-29228), Fyn siRNA (sc-29321), Yes siRNA (sc-29860), and scrambled control siRNA (sc-37007) were from Santa Cruz Biotechnology.
Supplementary Material
Significance.
Although LKB1 loss has been linked to metastasis in epithelial cancers, and LKB1 compromise occurs frequently in melanoma, the role of LKB1 inactivation in melanoma progression and metastasis is unknown. Here, we show that mice with Lkb1 loss and K-Ras activation develop highly penetrant melanomas that are extraordinarily metastatic. Further investigation revealed that LKB1 loss leads to expansion of a highly invasive and tumor-clonogenic subpopulation of cells expressing high levels of CD24, a modulator of metastasis and marker of stem-progenitor cells. The expansion of the CD24+ subpopulation in response to LKB1 inactivation requires the activity of the YES SRC family kinase. These data suggest a mechanism whereby LKB1 regulates metastasis and identify a promising therapeutic target, YES, in LKB1-deficient melanoma.
Acknowledgments
The authors wish to thank the UNC Lineberger Comprehensive Cancer Center Mouse Phase 1 Unit for assistance with animal handling and tumor assessment. We thank the UNC gastrointestinal histology and flow cytometry core facilities for assistance. This work was supported by the UNC University Cancer Research Fund, and grants from the NCI.
Footnotes
ACCESSION NUMBERS
Microarray data have been deposited at GEO with the accession number GSE34866.
SUPPLEMENTAL INFORMATION
Supplemental Information includes eight figures, three tables, two movies, and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.ccr.2012.03.048.
References
- Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Dexter TJ, Devlin LM, Heasman J, Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989;105:379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B, Nogueira C, Horner JW, 2nd, Depinho R, Chin L. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis. 2006;44:262–267. doi: 10.1002/dvg.20205. [DOI] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, McNamara KL, Brandstetter KA, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Liu P, Wang ZC, Zou L, Santiago S, Garbitt V, Gjoerup OV, Iglehart JD, Miron A, Richardson AL, et al. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci Signal. 2009;2:ra35. doi: 10.1126/scisignal.2000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, 2nd, DePinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D, Rimm DL, McMahon M, Bosenberg M. beta-Catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y, Kimura S, Ashihara E, Niwa T, Hodohara K, Fujiyama Y, Maekawa T. Comparison of imatinib, dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines. Leuk Res. 2008;32:980–983. doi: 10.1016/j.leukres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Esteve-Puig R, Canals F, Colome N, Merlino G, Recio JA. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS One. 2009;4:e4771. doi: 10.1371/journal.pone.0004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- Guervos MA, Marcos CA, Hermsen M, Nuno AS, Suarez C, Llorente JL. Deletions of N33, STK11 and TP53 are involved in the development of lymph node metastasis in larynx and pharynx carcinomas. Cell Oncol. 2007;29:327–334. doi: 10.1155/2007/635962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg P, thor Straten P, Ahrenkiel V, Seremet T, Kirkin AF, Zeuthen J. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene. 1999;18:1777–1780. doi: 10.1038/sj.onc.1202486. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamura K, Tsuji M, Hotta H, Ohkawa Y, Takahashi M, Shibuya H, Nakashima H, Yamauchi Y, Hashimoto N, Hattori H, et al. Functional Activation of Src Family Kinase Yes Protein Is Essential for the Enhanced Malignant Properties of Human Melanoma Cells Expressing Ganglioside GD3. J Biol Chem. 2011;286:18526–18537. doi: 10.1074/jbc.M110.164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi J, Cubitt CL, Zhang S, Munster PN, Yu H, Sullivan DM, Jove R, Messina JL, Daud AI. Src activation in melanoma and Src inhibitors as therapeutic agents in melanoma. Melanoma Res. 2009;19:167–175. doi: 10.1097/CMR.0b013e328304974c. [DOI] [PubMed] [Google Scholar]
- Jeghers H, Mc KV, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949;241:1031–1036. doi: 10.1056/NEJM194912292412601. [DOI] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Karuman P, Gozani O, Odze RD, Zhou XC, Zhu H, Shaw R, Brien TP, Bozzuto CD, Ooi D, Cantley LC, Yuan J. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7:1307–1319. doi: 10.1016/s1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Kluger HM, Dudek AZ, McCann C, Ritacco J, Southard N, Jilaveanu LB, Molinaro A, Sznol M. A phase 2 trial of dasatinib in advanced melanoma. Cancer. 2011;117:2202–2208. doi: 10.1002/cncr.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny GE, Glas R, Dering J, Manivong K, Qi J, Finn RS, Yang GR, Hong KL, Ginther C, Winterhoff B, et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer. 2009;101:1699–1708. doi: 10.1038/sj.bjc.6605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen G, Schluns K, Yongwei Y, Denkert C, Dietel M, Petersen I. CD24 is an independent prognostic marker of survival in nonsmall cell lung cancer patients. Br J Cancer. 2003;88:231–236. doi: 10.1038/sj.bjc.6600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Lim W, Olschwang S, Keller JJ, Westerman AM, Menko FH, Boardman LA, Scott RJ, Trimbath J, Giardiello FM, Gruber SB, et al. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004;126:1788–1794. doi: 10.1053/j.gastro.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Loganzo F, Jr, Dosik JS, Zhao Y, Vidal MJ, Nanus DM, Sudol M, Albino AP. Elevated expression of protein tyrosine kinase c-Yes, but not c-Src, in human malignant melanoma. Oncogene. 1993;8:2637–2644. [PubMed] [Google Scholar]
- Marchetti D, Parikh N, Sudol M, Gallick GE. Stimulation of the protein tyrosine kinase c-Yes but not c-Src by neurotrophins in human brain-metastatic melanoma cells. Oncogene. 1998;16:3253–3260. doi: 10.1038/sj.onc.1201877. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Iwakawa R, Takahashi K, Kohno T, Nakanishi Y, Matsuno Y, Suzuki K, Nakamoto M, Shimizu E, Minna JD, Yokota J. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene. 2007;26:5911–5918. doi: 10.1038/sj.onc.1210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KB, Rozenberg GI, Krishnamurthy J, Johnson SM, Liu W, Bradford MK, Horner J, Depinho RA, Sharpless NE. Somatic p16(INK4a) loss accelerates melanomagenesis. Oncogene. 2010;29:5809–5817. doi: 10.1038/onc.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam AJ, Schulz VV, Freiter EM, Bill HM, Miranti CK. Src, PKCalpha, and PKCdelta are required for alphavbeta3 integrin-mediated metastatic melanoma invasion. Cell Commun Signal. 2009;7:10. doi: 10.1186/1478-811X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A, Bataille V, MacKie R, Healy E, Bicknell D, Bodmer W, Tomlinson I. Somatic mutations in the Peutz-Jeghers (LKB1/STKII) gene in sporadic malignant melanomas. J Invest Dermatol. 1999;112:509–511. doi: 10.1046/j.1523-1747.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- Scott KL, Nogueira C, Heffernan TP, van Doorn R, Dhakal S, Hanna JA, Min C, Jaskelioff M, Xiao Y, Wu CJ, et al. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell. 2011;20:92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senner V, Sturm A, Baur I, Schrell UH, Distel L, Paulus W. CD24 promotes invasion of glioma cells in vivo. J Neuropathol Exp Neurol. 1999;58:795–802. doi: 10.1097/00005072-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Shah U, Sharpless NE, Hayes DN. LKB1 and lung cancer: more than the usual suspects. Cancer Res. 2008;68:3562–3565. doi: 10.1158/0008-5472.CAN-07-6620. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Alson S, Chan S, Silver DP, Castrillon DH, DePinho RA. p16(INK4a) and p53 deficiency cooperate in tumorigenesis. Cancer Res. 2002;62:2761–2765. [PubMed] [Google Scholar]
- Shields JM, Thomas NE, Cregger M, Berger AJ, Leslie M, Torrice C, Hao H, Penland S, Arbiser J, Scott G, et al. Lack of extracellular signal-regulated kinase mitogen-activated protein kinase signaling shows a new type of melanoma. Cancer Res. 2007;67:1502–1512. doi: 10.1158/0008-5472.CAN-06-3311. [DOI] [PubMed] [Google Scholar]
- Stuelten CH, Mertins SD, Busch JI, Gowens M, Scudiero DA, Burkett MW, Hite KM, Alley M, Hollingshead M, Shoemaker RH, Niederhuber JE. Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells. 2010;28:649–660. doi: 10.1002/stem.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskaya EV, Hill SP, Evans-Whipp TJ, Chin L, Orlow SJ, Easty DJ, Cheong SC, Beach D, DePinho RA, Bennett DC. p16(Ink4a) in melanocyte senescence and differentiation. J Natl Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28:2621–2633. doi: 10.1038/onc.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, Shimamura T, Miller DS, Sharpless NE, Bardeesy N, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu S, Shi R, Zhao G. miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer Genet. 2011;204:486–491. doi: 10.1016/j.cancergen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, Cantley LC. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


