Abstract
Purpose
To investigate intraocular pressure (IOP) control and corneal graft survival rates in eyes with glaucoma drainage device (GDD) implantation and penetrating keratoplasty (PK) and 5 years of follow-up data.
Design
Retrospective review.
Methods
We performed a review of records of all patients who underwent both GDD placement and PK at our institution between January 1, 1988 and December 31, 2003. Twenty-eight eyes of 27 patients were studied. Glaucoma outcome was assessed by postoperative IOP, number of glaucoma medications, and need for further glaucoma surgery. Corneal grafts were assessed for clarity.
Results
All eyes had GDD placement in the anterior chamber. The mean pre-GDD IOP was 28.8 ± 10.3 mm Hg on a mean of 2.6 ± 0.8 glaucoma medications. At 5-year follow-up, the mean IOP was 13.0 ± 5.9 mm Hg on a mean of 0.9 ± 1.0 glaucoma medications. GDD implantation successfully controlled glaucoma in 96%, 86%, 79%, 75%, and 71% of eyes at 1, 2, 3, 4, and 5 years, respectively. Grafts remained clear in 96%, 82%, 75%, 57%, and 54% of eyes at 1, 2, 3, 4, and 5 years, respectively. Failure of glaucoma outcome or graft survival was associated with prior intraocular surgeries.
Conclusions
Our data suggests that GDD placement can provide glaucoma control in a high percentage (71%) of eyes with PK even at 5 years. Furthermore, the success of PK in eyes with GDD remains reasonable (54%) at 5 years. IOP control and graft survival rates are comparable with earlier published studies with shorter follow-up or tube placement in the vitreous cavity.
Keywords: penetrating keratoplasty, corneal transplant, graft failure, glaucoma drainage device, incisional glaucoma surgery
Many patients with corneal disease requiring penetrating keratoplasty (PK) have concurrent glaucoma. Glaucoma is the leading cause of blindness after PK1 and may develop as a complication of keratoplasty.2 Alternately, previously controlled glaucoma may become uncontrolled after PK is performed.2–4
Irvine and Kaufman5 first reported a high incidence of elevated intraocular pressure (IOP) after PK. Although increased IOP after PK can develop in eyes with open or closed angles, peripheral anterior synechiae is present in up to 87% of patients post-PK.6 Distortion of the angle anterior and posterior to the trabecular meshwork has also been implicated as a cause for increased IOP after PK.7
Medical therapy alone is frequently insufficient for lowering IOPs in these eyes.2,4,8 Trabeculectomy performed after PK has had varying success rates of IOP control and graft survival.9,10 Another surgical option is the glaucoma drainage device (GDD).
Aqueous drainage tube implants are an useful alternative to medical therapy or trabeculectomy for the management of glaucoma in postkeratoplasty patients.11 The recent 3-year follow-up data from the multicenter Tube versus Trabeculectomy study may support an expanded role for GDDs in eyes with previous surgery.12 The number of eyes receiving GDD surgery in association with PK could increase in the future, therefore, it is relevant to gather insight on long-term follow-up after such surgery.
To date, the longest published follow-up data covering cornea and glaucoma outcomes in eyes with both GDD and PK is 3 to 4 years.13,14 We present data on glaucoma control and corneal graft survival at 5 years of follow up. In addition, all relevant clinical factors were evaluated for their association with glaucoma or corneal graft survival.
METHODS
The study protocol was approved by our university’s Institutional Review Board. A retrospective chart review was performed on all patients who underwent both GDD and PK at our tertiary care center between January 1, 1988 and December 31, 2003. The study window was selected to ensure a minimum of 5 years of available follow-up for identified patients. All surgeries were performed by faculty ophthalmologists on the Cornea and Glaucoma Services of our academic center.
Patients were identified through a record search based on Current Procedural Terminology codes for GDD (66180) and keratoplasty (65730, 65750, and 65755). Thirty eyes of 28 patients were studied in the final analysis. Study criteria required a patient age of 18 years or above at the time of first procedure, a minimum of 5-year follow-up for the later of the 2 surgeries, and that both procedures been performed in the same eye during the study period.
Collected data included demographic information, type of glaucoma, type of corneal pathology, previous ophthalmic surgeries, complications, preoperative and postoperative best-corrected visual acuity, preoperative and postoperative IOP, number and type of glaucoma medications, graft clarity, and episodes of graft rejection and decompensation, including graft thickness, presence of keratic precipitates, endothelial rejection lines, and infiltrates.
Success for glaucoma control was defined as no further glaucoma surgery and IOP between 5 and 18 mm Hg with or without glaucoma medications. IOP ≥19 mm Hg on 2 consecutive visits 3 months after the glaucoma surgery, or reoperation for glaucoma, was considered a failure of glaucoma outcome. Success for glaucoma control, in addition to the noted non-IOP factors, was also assessed using an upper IOP limit of 15 and 21 mm Hg to allow for comparison of IOP criteria with other reports.14,15 Combination medication eye drops were counted as 2 medications in data analyses. An oral carbonic anhydrase inhibitor was counted as 1 additional medication.
Success of the PK was defined as a clear graft without signs of rejection or failure. Graft failure or decompensation was diagnosed after persistent stromal edema lasting beyond a period of 1 month of intense steroid therapy, or by vascularization and opacification. Graft rejection episodes were diagnosed if there was increasing inflammation associated with corneal edema, vascularization, keratic precipitates, endothelial rejection lines, or subepithelial infiltrates 1 month after surgery but with a clinical response to steroid treatment occurring greater than 3 months before any eventual graft failure.
Surgical Technique and Postoperative Care
All GDDs used in this study were nonvalved, and included the Schocket-style, Baerveldt (350 mm2; Advanced Medical Optics, Santa Ana, CA), or double-plate Molteno (IOP, Inc, Costa Mesa, CA) devices. The technique of GDD implantation has been described earlier.13 Briefly, a conjunctival peritomy was performed at the limbus with blunt dissection down to bare sclera in 2 to 3 quadrants. Baerveldt implants were secured under the superior and lateral rectus muscles when possible. A 3-0 Supramid suture was passed into the tube lumen to function as a stent, and an 8-0 vicryl ligature suture was placed to form a watertight seal around the tube and stent. GDD plates were secured to sclera with 9-0 nylon sutures. A limbal tract was formed with a 23-gauge needle followed by prompt insertion of the tube through insertion forceps. Tubes were placed in the anterior chamber just overlying the iris without corneal touch. Two to 3 venting slits were placed in the tubes with an 8-0 TG-130-4 needle (Ethicon, Inc, Somerville, NJ) anterior to the ligature suture to allow slight aqueous egress. The externalized stent was removed as needed for IOP control 6 to 8 weeks postoperatively. A pericardial patch graft (Tutoplast; IOP, Inc, Costa Mesa, CA) was usually used to cover the tube and was secured to sclera with 8-0 vicryl sutures. The conjunctiva and Tenon’s tissue were closed in single layer with 8-0 vicryl sutures. All eyes were left leak free at the conclusion of GDD surgery.
For keratoplasty surgery, a full thickness trephination of the recipient cornea was performed 7.0 to 8.5 mm in diameter. A donor corneal button 0.25 to 0.5 mm larger than the recipient site was prepared and sutured into anatomic position with 16 interrupted 10-0 nylon sutures. Lensectomy, exchange or placement of an intraocular lens, anterior vitrectomy, or other concurrent procedures were performed as indicated.
Postoperatively, topical steroids were administered aggressively and tapered to a low dose over several months. Topical antibiotics were administered routinely. Nonsteroidal anti-inflammatory drops were given according to surgeon’s preference. Glaucoma medications were added as necessary to optimally control IOP. The grafts were managed with topical steroid prophylaxis, never less than twice daily, and occasionally oral or intravenous steroids for rejection episodes. Follow-up care intervals were determined by the postoperative course and surgeon’s preference, but usually included 1-day, 1-week, and 1-month visits in the initial postoperative period. After 1 year, patients were followed every 3 to 6 months.
Statistical Analysis
The preoperative patient characteristics of age, sex, race, IOP, number of glaucoma medications, glaucoma diagnosis, corneal diagnosis, lens status, number of prior ocular surgeries, preoperative visual acuity, and prior PK number were individually evaluated in a univariate manner.
Postoperative patient data analyzed include IOP, graft clarity and rejection episodes, number of glaucoma medications, need for subsequent glaucoma surgeries, relative timing of the PK and GDD surgery, and number and type of complications.
Subanalyses were performed when possible based on glaucoma diagnosis, including primary open angle glaucoma, chronic angle closure glaucoma, congenital, and traumatic glaucoma; and prekeratoplasty corneal diagnosis, including pseudophakic bullous keratopathy (PBK), aphakic bullous keratopathy (ABK), Fuchs endothelial dystrophy, and failed prior PK. Grouped analyses involving preoperative lens status, including aphakia, the presence of the natural lens, anterior chamber intraocular lens, or posterior chamber intraocular lens, including sulcus placement, were performed when possible.
When comparing 2 groups, analysis was performed using paired Student t test or a χ2 test where appropriate. Univariate analysis was performed using the log-rank test for comparing Kaplan-Meier survival curves. A P value less than 0.05 was considered statistically significant. The statistical analyses were performed using statistical analysis software (WinSTAT, R. Fitch Software, Krozingen, Germany) LIFETEST for the Kaplan-Meier curves and log-rank test.
RESULTS
A total of 28 eyes of 27 patients were included in the final analysis. The patient demographic data are shown in Table 1. Of the 28 eyes studied with both GDD and PK surgery, there were no cases of simultaneous GDD and PK surgery. All 28 eyes had the GDD implanted in the anterior chamber.
TABLE 1.
Patient Demographic Data
| N (%) | |
|---|---|
| Age range (mean ± SD), y | 21–89 (63.6 ± 18.0) |
| Sex | |
| Male | 10/28 (35.7) |
| Female | 18/28 (64.3) |
| Race | |
| White | 23/28 (82.1) |
| African-American | 5/28 (17.9) |
| Glaucoma type | |
| Primary open angle | 19/28 (67.9) |
| Chronic angle closure | 4/28 (14.3) |
| Congenital | 3/28 (10.7) |
| Traumatic | 2/28 (7.1) |
| Pre-GDD lens status | |
| Aphakic | 7/28 (25.0) |
| Phakic | 7/28 (25.0) |
| ACIOL | 2/28 (7.1) |
| PCIOL | 12/28 (42.9) |
| Pre-PK lens status | |
| Aphakic | 7/28 (25.0) |
| Phakic | 8/28 (28.6) |
| ACIOL | 4/28 (14.3) |
| PCIOL | 9/28 (32.1) |
| Ocular surgeries before GDD and PK | |
| Mean ± SD | 1.9 ± 1.2 (25/28, 89.3) |
| Range | 0–4 |
| PK before GDD and PK | |
| Mean ± SD | 0.2 ± 0.5 (5/28, 17.9) |
| Range | 0–2 |
| Ocular surgeries before GDD and PK | |
| Trabeculectomy | 9 (32.1) |
| Phaco/PCIOL | 6 (21.4) |
| PK | 5 (17.9) |
| ICCE | 5 (17.9) |
| GDD | 3 (10.7) |
| GDD revision | 3 (10.7) |
| ICCE/ACIOL | 2 (7.1) |
| ECCE/ACIOL | 2 (7.1) |
| ACIOL exchange | 2 (7.1) |
| Pas plana vitrectomy | 2 (7.1) |
| ECCE/PCIOL/PK | 1 (3.6) |
| PK/ACIOL exchange | 1 (3.6) |
| Trabeculectomy revision | 1 (3.6) |
| Phaco/PCIOL/trabeculectomy | 1 (3.6) |
| ACIOL placement | 1 (3.6) |
| ACIOL repositioning | 1 (3.6) |
| ACIOL removal | 1 (3.6) |
| Pars plana lensectomy | 1 (3.6) |
| Scleral buckle procedure | 1 (3.6) |
| Ruptured globe repair | 1 (3.6) |
| Diode cyclophotocoagulation | 1 (3.6) |
| Argon laser trabeculoplasty | 1 (3.6) |
| Surgeries performed with GDD | |
| Pars plana vitrectomy | 1 (3.6) |
| Surgeries performed with PK | |
| Phaco/PCIOL | 5 (17.9) |
| ECCE/PCIOL | 2 (7.1) |
| ECCE/ACIOL | 1 (3.6) |
| Sutured PCIOL, removal of ACIOL | 1 (3.6) |
ACIOL indicates anterior chamber intraocular lens; ECCE, extracapsular cataract extraction; GDD, glaucoma drainage device; ICCE, intracapsular cataract extraction; PCIOL, posterior chamber intraocular lens; PK, penetrating keratoplasty.
Glaucoma Outcome
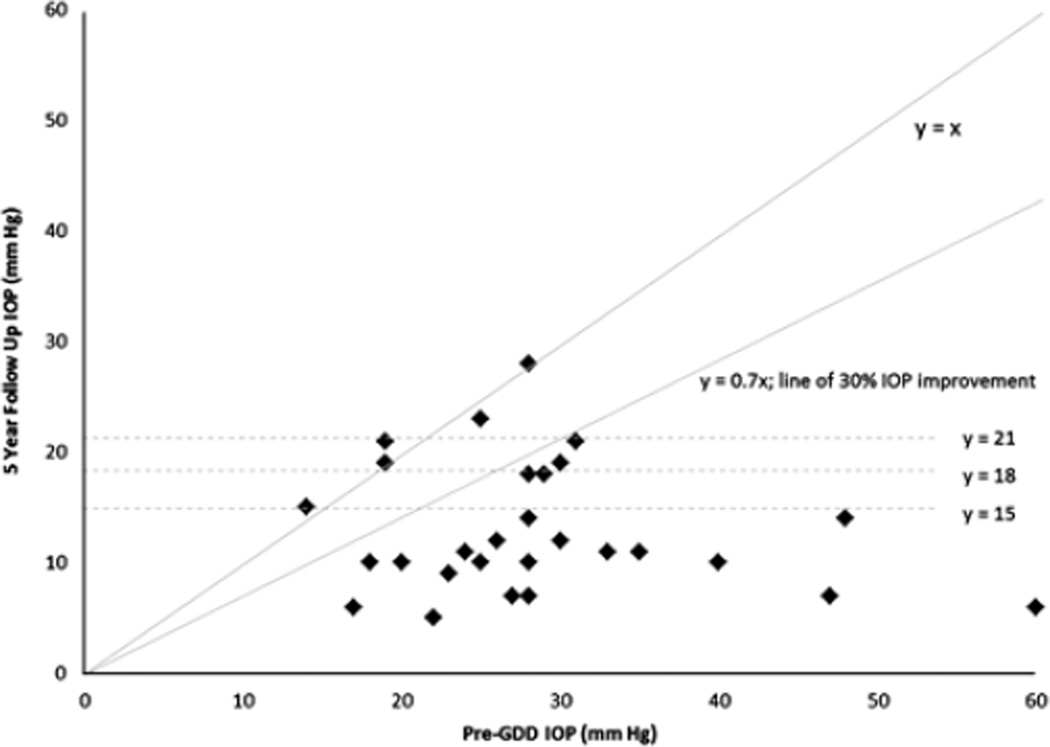
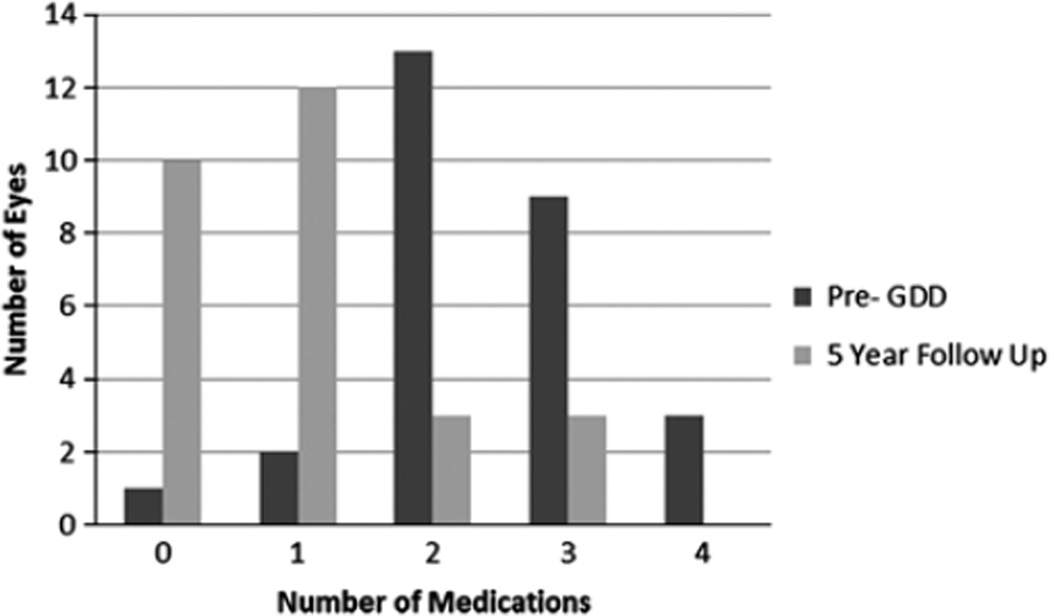
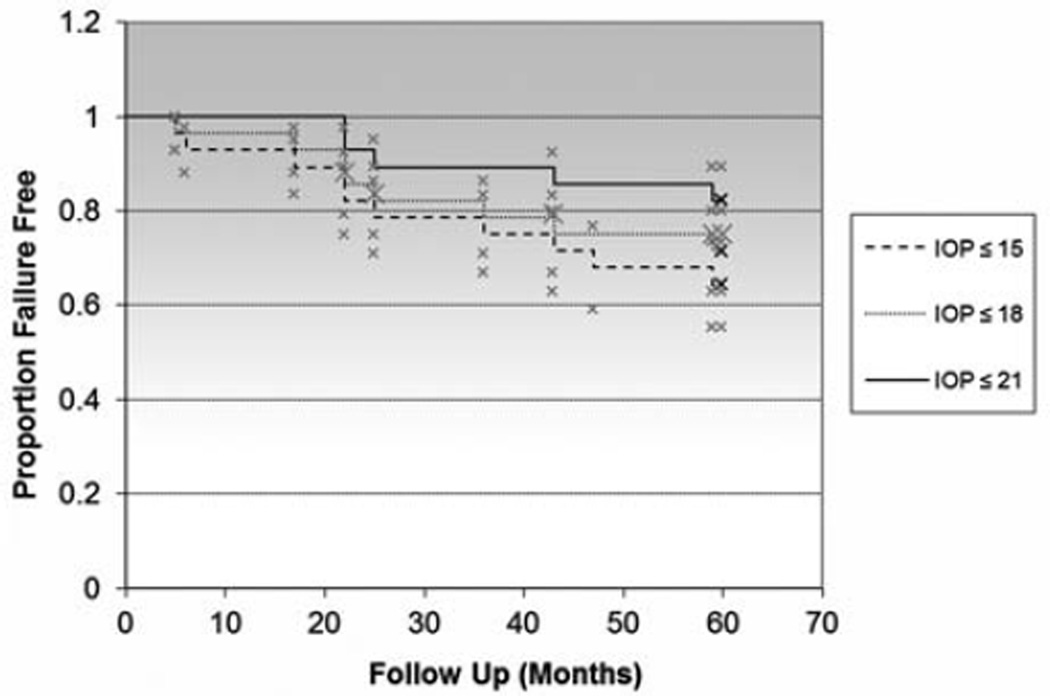
All GDDs implanted were Schocket-style, Baerveldt (350 mm2), or double-plate Molteno devices, based on surgeon’s preference and date of implantation at our institution (Table 2). The mean pre-GDD IOP was 28.8 ± 10.3 mm Hg on a mean of 2.6 ± 0.8 glaucoma medications. At 5-year follow-up, the mean IOP was 13.0 ± 5.9 mm Hg on a mean of 0.9 ± 1.0 glaucoma medications (Table 3; Figs. 1, 2). GDD implantation successfully controlled glaucoma in 96%, 86%, 79%, 75%, and 71% of eyes at 1, 2, 3, 4, and 5 years, respectively, as defined by the criteria noted above. IOP results for Schocket-style, Baerveldt (350 mm2), and double-plate Molteno devices were 13.8 ± 6.2, 11.8 ± 5.3, and 15.6 ± 6.9 mm Hg, respectively, and these differences were not statistically significant (P = 0.35). Type of GDD (ie, Schocket, Baerveldt, or Molteno) used did not correlate with glaucoma failure. Failure of glaucoma control was associated with prior intraocular surgeries. Preoperative glaucoma diagnosis (primary open angle glaucoma, chronic angle closure glaucoma, congenital, and traumatic glaucoma) did not correlate with glaucoma failure. There were no cases of hypotony (IOP < 5 mm Hg) causing failure of glaucoma outcome. The overall survival of glaucoma outcome was analyzed with a Kaplan-Meier curve (Fig. 3) using an upper IOP limit of 15, 18, and 21 mm Hg for comparison of IOP definition criteria, although IOP up to and including 18 mm Hg was the primary upper IOP limit endpoint used in the study.
TABLE 2.
Type of Glaucoma Drainage Device
| Type | No. Eyes (%) |
|---|---|
| Schocket | 4 (14.3) |
| Molteno (double plate) | 7 (25.0) |
| Baerveldt (350 mm2) | 17 (60.7) |
TABLE 3.
Postoperative Data
| Pre-GDD IOP (mm Hg) | Mean ± SD | 28.8 ± 10.3 |
| Range | 14–60 | |
| IOP at 5-year follow-up (mm Hg) | Mean ± SD | 13.0 ± 5.9 |
| Range | 5–28 | |
| Pre-GDD glaucoma medications (number) | Mean ± SD | 2.6 ± 0.8 |
| Range | 1–4 | |
| Glaucoma medications at 5-year follow-up (number) | Mean ± SD | 0.9 ± 1.0 |
| Range | 0–4 |
GDD indicates glaucoma drainage device; IOP, intraocular pressure.
FIGURE 1.
Scatter plot of pre-glaucoma drainage device (GDD) and 5-year follow-up intraocular pressure (IOP).
FIGURE 2.
Number of pre-glaucoma drainage device (GDD) and 5-year follow-up medications.
FIGURE 3.
Overall Kaplan-Meier survival curve of glaucoma success. IOP indicates intraocular pressure.
Cornea Outcome
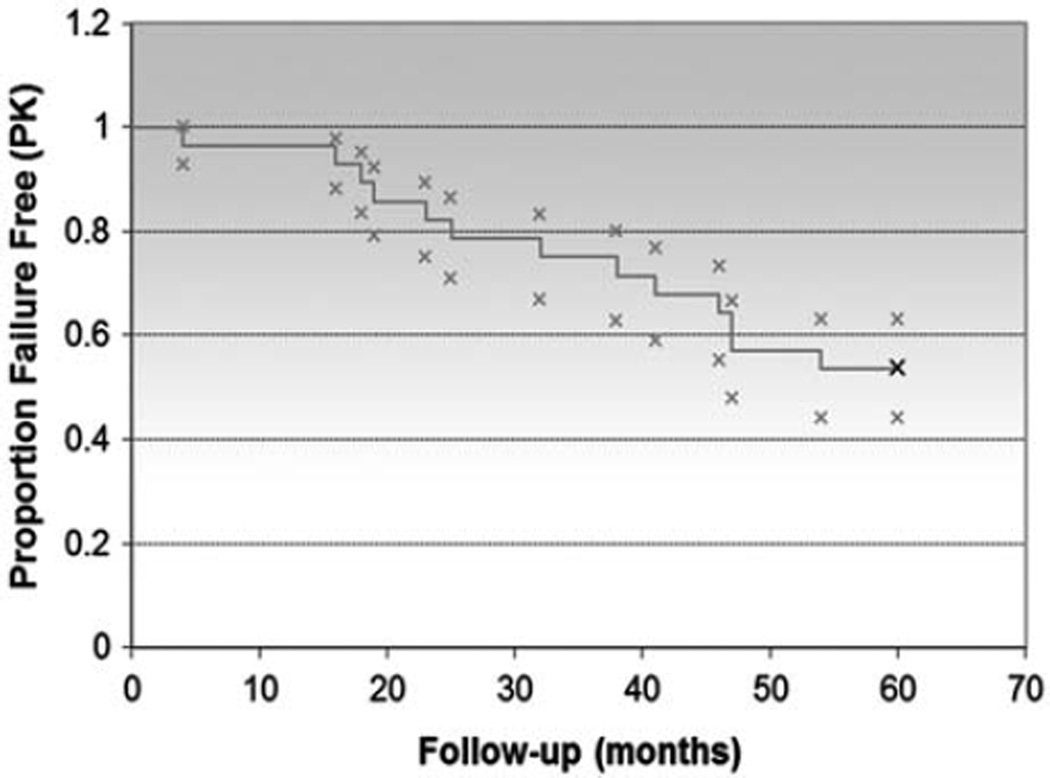
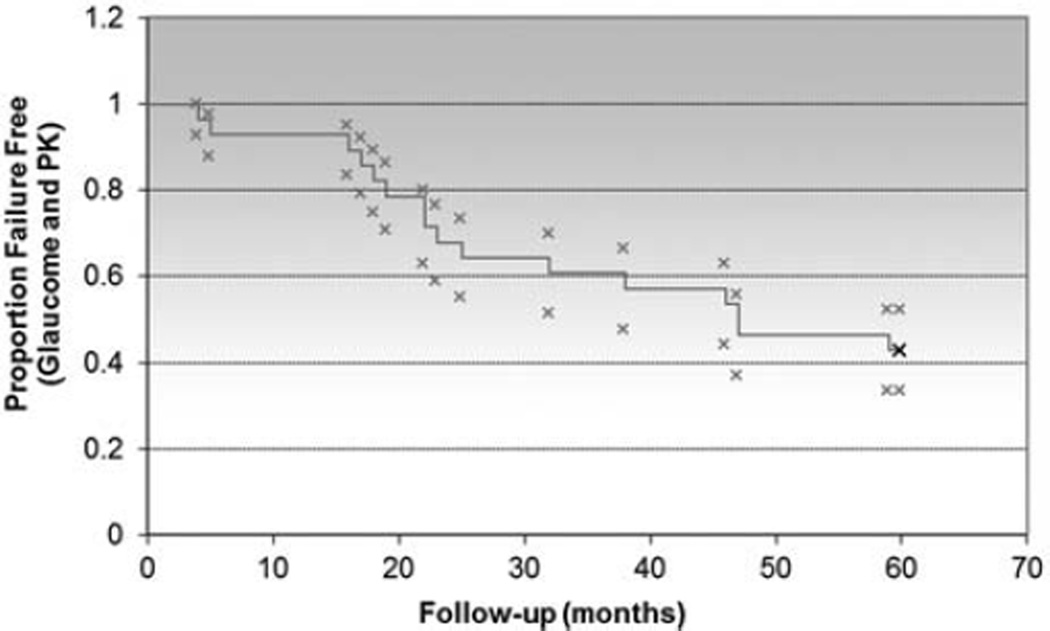
Grafts remained clear in 96%, 82%, 75%, 57%, and 54% of eyes at 1, 2, 3, 4, and 5 years, respectively. Prekeratoplasty corneal diagnoses are noted in Table 4. Failure of the PK was associated with prior intraocular surgeries and failure of glaucoma control. Correlation between preoperative lens status (aphakic, phakic, anterior chamber intraocular lens, or posterior chamber intraocular lens) and corneal diagnosis (including prior PK) with graft success did not attain statistical significance. Thirteen of 28 eyes (46.4%) had at least 1 episode of graft rejection, including 8 that eventually experienced graft failure in the study period. The overall survival of graft success was analyzed with a Kaplan-Meier curve (Fig. 4).
TABLE 4.
Pre-PK Corneal Diagnoses
| Pre-PK Corneal Diagnosis | No. Eyes (%) |
|---|---|
| PBK | 9 (32.1) |
| ABK | 6 (21.4) |
| Fuchs endothelial dystrophy | 4 (14.3) |
| Failed PK (all from PBK) | 3 (10.7) |
| Stromal scar | 2 (7.1) |
| Corneal ulcer | 1 (3.6) |
| Corneal blood stain | 1 (3.6) |
| Lattice dystrophy | 1 (3.6) |
| Keratoconus | 1 (3.6) |
ABK indicates aphakic bullous keratopathy; PBK, pseudophakic bullous keratopathy; PK, penetrating keratoplasty.
FIGURE 4.
Overall Kaplan-Meier survival curve of penetrating keratoplasty (PK) success.
Overall Glaucoma and Cornea Outcome
In 10 of 28 (35.7%) eyes studied, the GDD was placed before PK surgery. PK was performed first in 18 of 28 (64.3%) eyes (Table 5). For eyes receiving a GDD first, an average of 27 months elapsed until the PK was performed. For eyes receiving a PK first, an average of 50 months elapsed before the GDD was placed. The effect of the relative timing of the GDD and PK surgery was analyzed and did not show a significant difference in graft survival based on which procedure was performed first (P = 0.25). The overall combined survival of glaucoma and graft outcome was analyzed with a Kaplan-Meier curve (Fig. 5).
TABLE 5.
Timing of GDD and PK
| First | No. Eyes (%) |
|---|---|
| GDD | 10 (35.7) |
| PK | 18 (64.3) |
GDD indicates glaucoma drainage device; PK, penetrating keratoplasty.
FIGURE 5.
Overall Kaplan-Meier survival curve of glaucoma (intraocular pressure ≤ 18 mm Hg) and graft success. PK indicates penetrating keratoplasty.
The types of glaucoma reintervention and other surgical complications are noted in Table 6. Corneal ulcers occurred in 3 of 28 eyes (10.7%). There were no cases of endophthalmitis in the 5-year follow-up period.
TABLE 6.
Additional Glaucoma Procedures and Complications
| N (%) | |
|---|---|
| Additional glaucoma procedures | |
| Revision of existing GDD | 4 (14.3) |
| Implantation of additional GDD | 2 (7.1) |
| Diode laser cyclophotocoagulation | 3 (10.7) |
| Anterior vitrectomy for plugged GDD | 1 (3.6) |
| Other complications | |
| Corneal ulcer | 3 (10.7) |
| YAG pupillomembranectomy | 1 (3.6) |
| Dislocated IOL | 1 (3.6) |
| Retinal detachment | 1 (3.6) |
GDD indicates glaucoma drainage device; IOL, intraocular lens; YAG, yttriumaluminumgarnet.
DISCUSSION
GDD implantation has been established as a viable surgical option for controlling IOP in patients with corneal transplants.14,15 Earlier studies have reported varying levels of glaucoma and graft success in this patient population as shown in Table 7.16 Notably, no earlier study has a mean follow-up of greater than 38 months.14 Our data indicates that GDD placement can provide glaucoma control in a high percentage (71%) of eyes with PK even at 5 years. Moreover, the success of PK in eyes with GDD remains reasonable (54%) at 5 years. By comparison, the Cornea Donor Study Investigator Group reported a 5-year follow-up failure rate of 11% in post-PK eyes without glaucoma and 20%, 29%, and 58% failure if glaucoma had been treated with medications alone, surgery alone, or both, respectively.24
TABLE 7.
Studies of Glaucoma Drainage Device in Postkeratoplasty Glaucoma
| Author | No. Eyes | Mean Follow-up (mo) |
Success Rate for IOP Control (%) |
Graft-survival Rate (%) |
|---|---|---|---|---|
| Kirkness et al2 | 20 | 26 | 80 | 80 |
| Beebe et al17 | 35 | 25 | 86 | 49 |
| Sherwood et al10 | 26 | 22 | 96 | 58 |
| Rapuano et al18 | 46 | 23 | 96 | 65 |
| Coleman et al19 | 31 | 20 | 52 | 62 |
| McDonnell et al8 | 17 | 13 | 71 | 71 |
| Arroyave et al3 | 72 | 12 | 92 | 57 |
| Joos et al20 (only vitreous cavity) | 9 | 17 | 100 | 89 |
| Kwon et al15 | 55 | 36 | 82 | 55 |
| Sidoti et al21 (only vitreous cavity) | 34 | 12 | 85 | 61 |
| Alvarenga et al22 | 40 | 24 | 63 | 26 |
| Al-Torbak23 | 25 | 25 | 82 | 55 |
| Witmer et al14 (only vitreous cavity) | 51 | 38 | 82 | 41 |
| Current study | 28 | 60 | 71 | 54 |
IOP indicates intraocular pressure.
Adapted from Eye (Lond). 2009;23:1972–1979.
The preoperative patient characteristics in our study population resemble those reported elsewhere.3,25,26 In our study, 15 of 28 eyes had a prekeratoplasty diagnosis of ABK or PBK (Table 4), a finding consistent with the earlier noted studies. The incidence of glaucoma with PK varies with the indication for PK. Glaucoma is more common after PK for ABK and PBK and less common for eyes with corneal dystrophies.25 Foulks25 reported a series of 502 corneal transplants, 18% of which developed glaucoma, including 39% of all aphakes. Kirkness and Ficker6 noted that of 1122 full-thickness keratoplasties, 14% developed glaucoma, including 29% of all patients with PBK. Those authors noted that only 3% of corneal dystrophy patients developed glaucoma after PK. More recently, Ing et al26 reported that 21% of 394 corneal transplants developed glaucoma, including 44% of all patients with PBK.
All eyes in this study received large surface area nonvalved glaucoma drainage devices. Earlier studies have noted similar final IOP outcomes whether eyes received Schocket, Baerveldt (350 mm2), or douple-plate Molteno implants.27,28 In our study, there were no significant IOP differences noted between the 3 different types of glaucoma tubes, although the number of eyes studied allows only the determination of large differences.
The optimal location of tube tip placement remains controversial14,21 The anterior chamber is the most common location for GDD placement and allows ready observation of the tube tip, although the tube proximity to the corneal endothelium may potentiate graft decompensation.14 In our study, all 28 eyes had tubes placed in the anterior chamber, based on surgeon’s preference at our institution. No eyes experiencing graft failure in the study period had a tube position in the anterior chamber that was felt to be directly contributing to corneal decompensation. Another location for GDD tube placement is in the vitreous cavity. Implantation of the tube into the posterior segment through the pars plana was first reported to be an effective therapy for neovascular glaucoma by Lloyd et al.29 Entry of the tube through the pars plana requires a complete vitrectomy, usually through a coordinated surgery with a vitreoretinal surgeon. Pars plana tube placement, however, has been suggested as safer for the corneal endothelium.14,20 Recently, Witmer et al14 reported 51 eyes with a pars plana Baerveldt implant and keratoplasty. Mean follow-up was 38 months with IOP control in 82%, but graft survival only in 41% of eyes at final analysis.
A third, although less common, option for tube placement is in the ciliary sulcus, a location that does not require a vitrectomy and may be safer for the cornea.30 Sulcus placement of the tube, however, may be technically more challenging and may result in difficulty ascertaining the final tube position in the eye. Rumelt and Rehany30 reported 3 eyes with sulcus tube placement with a prior PK and noted that all grafts remained clear for the follow-up period of 18 months with successful IOP control. Prata et al31 studied 5 eyes with previous PK and concurrent sulcus GDD. All 5 eyes had successful IOP control and 4 of 5 had clear grafts at final follow-up.
Although the optimal location for tube placement remains controversial, our study of 28 eyes with anterior chamber tubes compares favorably (IOP control in 71% and graft survival in 54%) with studies using predominantly pars plana tubes (IOP control in 62% to 100% and graft survival in 41% to 48%).3,14,21
The relative timing of the GDD and PK is another factor to consider, as eyes that receive a GDD followed by a subsequent PK may be inherently different than eyes that receive a PK followed by a GDD. Earlier studies have reported no association between graft survival and timing of the GDD.3,19,22 Two reports noticed a nonsignificant trend toward decreased graft survival when the keratoplasty was performed first.17,18 However, Kwon et al15 found eyes with the GDD inserted before PK were 3.8 to 4.7 times more likely to have graft failure than when they were performed simultaneously or when the PK was performed first, respectively. In our study, there was a trend toward decreased graft survival when the PK was performed first, although this was not statistically significant.
Our study provides useful data on long-term outcomes of eyes with both GDD and PK surgery. Expected medical comorbidities and availability of follow up in patients with such sick eyes limited the number of eyes (28) completing the requisite of 5 years of data collection, although our findings seem to corroborate reported data in similar eyes with shorter follow-up.10,14,15 We also note that none of the eyes we studied had valved GDDs, although such eyes have been earlier studied with shorter follow-up.32,33 In addition, defining glaucoma control primarily from an IOP standpoint has inherent limitations, although this endpoint has been used in earlier studies.
Considerations for future studies must include a look at the growing role of endothelial keratoplasty, a technique of corneal transplantation being increasingly performed in many patients who may have otherwise received a PK in the past. Allan et al34 suggest that endothelial keratoplasty patients may have a lower risk of postkeratoplasty glaucoma compared with PK, as these surgeries may cause less-angle distortion and are associated with a smoother postoperative course. The optimal location of the GDD tube in endothelial keratoplasty eyes has not yet been specifically studied, and may be a future research consideration. In addition, longer follow-up of eyes with both GDD and PK (up to 10 y) would provide even more useful data to the glaucoma and corneal surgeon.
In summary, our study’s final outcomes show glaucoma control and graft survival comparable with earlier published studies with shorter follow-up. Furthermore, there is no significantly greater graft failure rate over 5 years with tube placement in the anterior chamber compared with published data on tubes in the vitreous cavity. GDD placement can provide excellent glaucoma control and reasonable graft survival rates up to 5 years in patients with PK.
Acknowledgments
This manuscript was supported in part by an unrestricted grant from Research to Prevent Blindness.
Footnotes
Financial disclosures: none.
Contribution of authors: Each author certifies that they have made a direct and substantial contribution to the study reported in the manuscript by participating in at least the following 3 areas: (1) conceiving and designing the study or analyzing and interpreting the data; (2) writing the manuscript or providing critical revisions that are important for the intellectual content; and (3) approving the final version of the manuscript.
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Ayyala RS. Penetrating keratoplasty and glaucoma [review] Surv Ophthalmol. 2000;45:91–105. doi: 10.1016/s0039-6257(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 2.Kirkness CM, Ling Y, Rice NS. The use of silicone drainage tubing to control post-keratoplasty glaucoma. Eye. 1988;2:583–590. doi: 10.1038/eye.1988.109. [DOI] [PubMed] [Google Scholar]
- 3.Arroyave CP, Scott IU, Fantes FE, et al. Corneal graft survival and intraocular pressure control after penetrating keratoplasty and glaucoma drainage device implantation. Ophthalmology. 2001;108:1978–1985. doi: 10.1016/s0161-6420(01)00803-x. [DOI] [PubMed] [Google Scholar]
- 4.Beebe WE. Management of glaucoma in penetrating keratoplasty patients [review] Refract Corneal Surg. 1991;7:67–69. [PubMed] [Google Scholar]
- 5.Irvine AR, Kaufman HE. Intraocular pressure following penetrating keratoplasty. Am J Ophthalmol. 1969;68:835–844. doi: 10.1016/0002-9394(69)94577-2. [DOI] [PubMed] [Google Scholar]
- 6.Kirkness CM, Ficker LA. Risk factors for the development of postkeratoplasty glaucoma. Cornea. 1992;11:427–432. doi: 10.1097/00003226-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Olson RJ, Kaufman HE. A mathematical description of causative factors and prevention of elevated intraocular pressure after keratoplasty. Invest Ophthalmol Vis Sci. 1977;16:1085–1092. [PubMed] [Google Scholar]
- 8.McDonnell PJ, Robin JB, Schanzlin DJ, et al. Molteno implant for control of glaucoma in eyes after penetrating keratoplasty. Ophthalmology. 1988;95:364–369. doi: 10.1016/s0161-6420(88)33187-8. [DOI] [PubMed] [Google Scholar]
- 9.Gilvarry AME, Kirkness CM, Steele ADM, et al. The management of post-keratoplasty glaucoma by trabeculectomy. Eye. 1989;3:713–718. doi: 10.1038/eye.1989.110. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood MB, Smith MF, Driebe WT, Jr, et al. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthalmic Surg. 1993;24:185–189. [PubMed] [Google Scholar]
- 11.Doyle JW, Smith MF. Glaucoma after penetrating keratoplasty [review] Semin Ophthalmol. 1994;9:254–257. doi: 10.3109/08820539409060024. [DOI] [PubMed] [Google Scholar]
- 12.Gedde SJ, Heuer DK, Parrish RK., II Tube versus trabeculectomy study group. Review of results from the Tube Versus Trabeculectomy Study [review] Curr Opin Ophthalmol. 2010;21:123–128. doi: 10.1097/ICU.0b013e3283360b68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd MA, Baerveldt G, Heuer DK, et al. Initial clinical experience with the Baerveldt implant in complicated glaucomas. Ophthalmology. 1994;101:640–650. doi: 10.1016/s0161-6420(94)31283-8. [DOI] [PubMed] [Google Scholar]
- 14.Witmer MT, Tiedeman JS, Olsakovsky LA, et al. Long-term intraocular pressure control and corneal graft survival in eyes with a pars plana Baerveldt implant and corneal transplant. J Glaucoma. 2010;19:124–131. doi: 10.1097/IJG.0b013e3181a98cc1. [DOI] [PubMed] [Google Scholar]
- 15.Kwon YH, Taylor JM, Hong S, et al. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001;108:272–278. doi: 10.1016/s0161-6420(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 16.Banitt M, Lee RK. Management of patients with combined glaucoma and corneal transplant surgery. Eye (Lond) 2009;23:1972–1979. doi: 10.1038/eye.2008.377. [Epub January 16, 2009]. [DOI] [PubMed] [Google Scholar]
- 17.Beebe WE, Starita RJ, Fellman RL, et al. The use of Molteno implant and anterior chamber tube shunt to encircling band for the treatment of glaucoma in keratoplasty patients. Ophthalmology. 1990;97:1414–1422. doi: 10.1016/s0161-6420(90)32393-x. [DOI] [PubMed] [Google Scholar]
- 18.Rapuano CJ, Schmidt CM, Cohen EJ, et al. Results of alloplastic tube shunt procedures before, during, or after penetrating keratoplasty. Cornea. 1995;14:26–32. [PubMed] [Google Scholar]
- 19.Coleman AL, Mondino BJ, Wilson MR, et al. Clinical experience with the Ahmed Glaucoma Valve implant in eyes with prior or concurrent penetrating keratoplasties. Am J Ophthalmol. 1997;123:54–61. doi: 10.1016/s0002-9394(14)70992-4. [DOI] [PubMed] [Google Scholar]
- 20.Joos KM, Lavina AM, Tawansy KA, et al. Posterior repositioning of glaucoma implants for anterior segment complications. Ophthalmology. 2001;108:279–284. doi: 10.1016/s0161-6420(00)00521-2. [DOI] [PubMed] [Google Scholar]
- 21.Sidoti PA, Mosny AY, Ritterband DC, et al. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108:1050–1058. doi: 10.1016/s0161-6420(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 22.Alvarenga LS, Mannis MJ, Brandt JD, et al. The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol. 2004;138:200–205. doi: 10.1016/j.ajo.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Al-Torbak AA. Outcome of combined Ahmed glaucoma valve implant and penetrating keratoplasty in refractory congenital glaucoma with corneal opacity. Cornea. 2004;23:554–559. doi: 10.1097/01.ico.0000122704.49054.95. [DOI] [PubMed] [Google Scholar]
- 24.Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116:1023–1028. doi: 10.1016/j.ophtha.2008.12.050. [Epub April 23, 2009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foulks GN. Glaucoma associated with penetrating keratoplasty. Ophthalmology. 1987;94:871–874. doi: 10.1016/s0161-6420(87)33542-0. [DOI] [PubMed] [Google Scholar]
- 26.Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105:1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 27.Smith MF, Doyle JW, Sherwood MB. Comparison of the Baerveldt glaucoma implant with the double-plate Molteno drainage implant. Arch Ophthalmol. 1995;113:444–447. doi: 10.1001/archopht.1995.01100040060027. [DOI] [PubMed] [Google Scholar]
- 28.Smith MF, Sherwood MB, McGorray SP. Comparison of the double-plate Molteno drainage implant with the Schocket procedure. Arch Ophthalmol. 1992;110:1246–1250. doi: 10.1001/archopht.1992.01080210064026. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd MA, Heuer DK, Baerveldt G. Combined Molteno implantation in pars plana vitrectomy for neovascular glaucoma. Ophthalmology. 1991;98:1401–1405. doi: 10.1016/s0161-6420(91)32120-1. [DOI] [PubMed] [Google Scholar]
- 30.Rumelt S, Rehany U. Implantation of glaucoma drainage implant tube into the ciliary sulcus in patients with corneal transplants. Arch Ophthalmol. 1998;116:685–687. doi: 10.1001/archopht.116.5.685. [DOI] [PubMed] [Google Scholar]
- 31.Prata TS, Mehta A, De Moraes CG, et al. Glaucoma implant in the ciliary sulcus: midterm follow-up. J Glaucoma. 2010;19:15–18. doi: 10.1097/IJG.0b013e3181a2fc2d. [DOI] [PubMed] [Google Scholar]
- 32.Al-Torbak A. Graft survival and glaucoma outcome after simultaneous penetrating keratoplasty and Ahmed glaucoma valve implant. Cornea. 2003;22:194–197. doi: 10.1097/00003226-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Hollander DA, Giaconi JA, Holland GN, et al. Graft failure after penetrating keratoplasty in eyes with Ahmed valves. Am J Ophthalmol. 2010;150:169–178. doi: 10.1016/j.ajo.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Allan BD, Terry MA, Price FW, Jr, et al. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26:1039–1042. doi: 10.1097/ICO.0b013e31812f66e5. [DOI] [PubMed] [Google Scholar]