Abstract
The goal of this study was to determine if a multi-mineral natural product derived from red marine algae, could reduce colon polyp formation in mice on a high fat diet. C57BL/6 mice were maintained for up to 18 months either on a high-fat “Western-style” diet or on a low-fat diet (AIN 76A), with or without the multi-mineral-supplement. To summarize, colon polyps were detected in 22 of 70 mice (31%) on the high-fat diet, but in only 2 of 70 mice (3%) receiving the mineral-supplemented high-fat diet (p<0.0001). Colon polyps were detected in 16 of 70 mice (23%) in the low-fat group; not significantly different from high-fat group but significantly higher than the high-fat-supplemented group (p=0.0006). This was in spite of the fact that the calcium level in the low-fat diet was comparable to the level of calcium in the high-fat diet containing the multi-mineral-product. Supplementation of the low-fat diet reduced the incidence to 8 of 70 mice (11% incidence). Taken together, these findings demonstrate that a multi-mineral natural product can protect mice on a high-fat diet against adenomatous polyp formation in the colon. These data suggest that increased calcium alone is insufficient to explain the lower incidence of colon polyps.
Keywords: Calcium, multi-mineral natural product, trace elements, lanthanoid, colon polyps, adenocarcinoma, adenoma, chemoprevention
INTRODUCTION
Past epidemiological (1–6) and interventional (7,8) studies in human subjects have demonstrated the capacity of calcium to reduce colon polyp formation and reduce colon cancer risk. In vitro studies have demonstrated the growth-regulating activity of calcium, and have provided insights into growth-suppressing mechanisms (9–12). Although calcium has demonstrable chemopreventive activity against colon polyp formation, protection provided by calcium alone can be described as modest. It is estimated that under conditions of optimal use, a reduction in polyp incidence of 20–22% might be achieved (13). Additional interventions are needed.
In a series of studies, Newmark and colleagues (14–16) demonstrated that mice fed a high-fat “Western-style” diet (HFWD) developed colon polyps at a higher rate than mice fed a standard low-fat rodent chow diet. The HFWD, along with its high content of saturated fat and carbohydrate, has several additional features that may contribute to the formation of colon polyps. These include a reduction in folic acid, a reduction in methyl group donors, a lower content of fiber and a reduction in calcium. Replacement studies demonstrated that supplementation with calcium reduced polyp formation (though not to background levels) (16). Replacing the missing folic acid, fiber or methyl group donors had little effect. In the present study we have assessed colon polyp formation in C57BL/6 mice fed a high fat diet in the absence and presence of a multi-mineral-containing natural product derived from the skeletal remains of the red marine algae, Lithothamnion calcareum. The multi-mineral-containing product dramatically reduced polyp formation in the high-fat – fed mice (i.e., from 31% incidence to 3% incidence). In contrast, mice fed a low-fat diet without the multi-mineral-containing product but with a comparable level of calcium to that provided by the multi-mineral product had a colon polyp incidence of 23%. These data confirm and extend a previous small scale study in which the multi- mineral product protected mice from colonic polyps on a high-fat diet. In the pilot study, colon polyps were seen in 4 of 20 mice (20% incidence) on the HFWD mice versus 0% incidence in mice on the supplemented HFWD (17).
MATERIALS AND METHODS
Multi-mineral natural product
The multi-mineral natural product used in this study is obtained from the skeletal remains of the red marine algae, Lithothamnion calcareum (Pallas), also known as Phymatolithon calcareum (Pallas) (18). The algae thrive in the cold Atlantic waters off the southwest coast of Ireland and northwest coast of Iceland. Minerals from sea water are accumulated in the algae fronds over the lifespan of the organism. Eventually, the mineralized fronds break off of the living organism and fall to the ocean floor, from where they are harvested. The mineralized fronds are separated from extraneous materials, sterilized, dried, and milled under ISO and HACCP certification. The final product contains approximately12% calcium(wt/wt), 1% magnesium, and measurable levels of 72 other trace minerals - essentially all of the minerals that the algae are able to accumulate from the sea water. The product is sold as a food supplement under the name Aquamin® (GRAS 000028) and is used in various products for human consumption in Europe, Asia, Australia, and North America [Marigot Ltd, Cork, IR]. The mineral composition of the algae product can be found in Supplement Table 1.
Diet groups
Inbred C57BL/6 mice were obtained from Charles River, Portage, MI at three weeks of age. The animals were started within one week of arrival on either a standard low-fat rodent chow diet (AIN 76A), to serve as a control or a high-fat, Western-style diet (HFWD), prepared according to the formulation of the Newmark stress diet (14). The diet is a modification of AIN 76A, and is designed to mimic the diet consumed by many individuals in Western society (19–21). The HFWD contains 20 gram% fat from corn oil as compared to 5 gram% in AIN 76A (Supplement Table 2). The percentage of calories from fat in this diet is 37.8% as compared to 11.5% in the AIN 76A diet. Although sucrose is reduced in the HFWD relative to the AIN 76A control diet, the overall calories provided in the HFWD is 4764 kcal/kg versus 3902 kcal/kg in AIN 76A. In addition to its high fat content, the HFWD has additional modifications. Methionine is replaced with cysteine, amounts of fiber, folic acid and choline are reduced, and the calcium level is reduced from 5.25 gm/kg to 0.41 gm/kg. Both the HFWD and AIN 76A contain a mix of essential trace elements including potassium, magnesium, manganese, chromium, copper, iron, selenium and zinc. For half of the animals on the HFWD, the diet was supplemented with the multi-mineral natural product at 62 g/1.062 kg, resulting in a calcium concentration comparable to that in AIN 76A. The concentration of calcium is 1.34 mg/kcal in low-fat (AIN 76A) diet, 3.24 mg/kcal in supplemented AIN 76A, 0.086 mg/kcal in the HFWD and 1.64 mg/kcal in the HFWD supplemented with the multi-mineral product. Diets were formulated and provided by Research Diets Incorporated (New Brunswick, NJ). The multi-mineral-rich supplement was compounded into the two diets, which were fed to the animals as solid food pellets. The composition of each diet is presented in Supplement Table 2.
Treatment protocol and necropsy
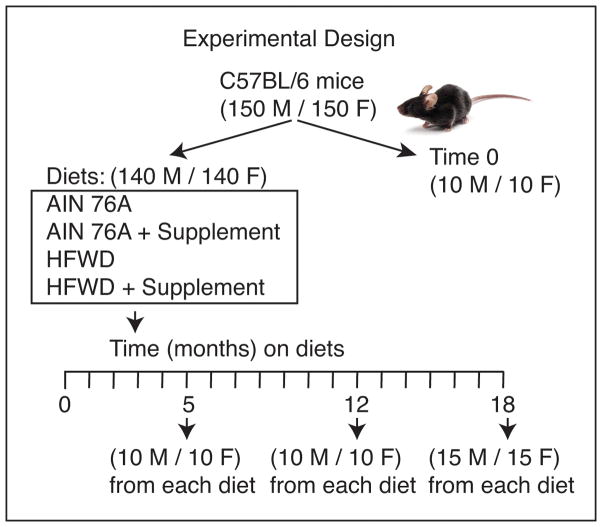
Separate cohorts of male and female C57BL/6 mice were housed five mice to a cage, and maintained for 5, 12 or 18 months on their respective diet as summarized in the flow chart (Figure 1). For the 5 and 12 month periods, there were 10 male and 10 female mice per diet group. For the 18 month period, there were 15 mice of each sex per diet group. Food was provided ad libitum. Animals were monitored at 2-day intervals throughout the maintenance phase and were weighed every two weeks. Mice on the HFWD diet gained more weight than did animals on the low-fat diet, but the multi-mineral supplement had no effect on average weight gain for either male or female mice in either diet. Between 0–5 months, there were no premature deaths. Between 6–12 months, five animals died and between 13 and 18 months, a total of 25 mice died or were euthanized. The majority of these deaths occurred in months 16 and 17. Of the animals that died prematurely, two were on unsupplemented AIN 76A, 3 were on mineral-supplemented AIN 76A, 17 were on the unsupplemented HFWD and 8 were on the mineral-supplemented HFWD. A complete autopsy was done on all animals that died prematurely. The most common cause of premature death was euthanasia for ulcerative dermatitis, condition that is well-known to occur in C57BL/6 mice in long-term studies (22). Liver tumors were observed in three mice and mouse urologic syndrome was observed in four male mice. In the remaining animals, there, was no obvious abnormality. Some of these mice died spontaneously without any cause and tissue could not be collected because of autolysis. Animals that died prematurely were included in their respective groupings.
Figure 1.
Experimental design.
Whether the animals died prematurely or were euthanized at the respective three time-points, they were autopsied as follow: After the abdominal cavity was opened, the gastrointestinal tract was removed from the stomach to the rectum and flushed with saline. The entire length was opened longitudinally, fixed in 2% formalin, stained with methylene blue and examined under a stereoscopic dissecting microscope. Visible raised tumors were identified in this manner and counted. Each observable mass was placed in 2% buffered formalin, and prepared for histology. Tissue was stained with hematoxylin and eosin and examined at the light microscopic level by a board-certified veterinary pathologist (I.B). Lesions were classified according to the recently revised, standardized guidelines established by the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND) project (23). This project represents consensus criteria for histopathological lesions in rodents as established by the North American, European, British, and Japanese Societies of Toxicologic Pathology. Proliferative lesions were classified as hyperplasias, adenomas or adenocarcinomas.
Blood was obtained at the time of necropsy from each animal. Serum calcium levels were determined using the Vet-Test Dry Chemistry Analyzer (Idexx Laboratories, Westbrook, ME). Calcium levels in long bones were determined in parallel. Long bones (one femur and one tibia from each animal) were carefully separated from the surrounding connective tissue. Bones from all animals in each group were “pooled” in order to obtain a sufficient quantity of bone, and the bone tissue converted to ash. Calcium levels were then determined by atomic absorption spectroscopy (Advanced Laboratories, Inc., Salt lake City, UT).
All of the procedures involving animals were reviewed and approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan.
Statistical evaluation
Differences in polyp incidence were assessed for statistical significance using the Fisher Exact Test (two-tailed). Differences were considered significant at the p<0.05 level.
RESULTS
Colon polyp incidence in mice with or without the multi-mineral natural product as a dietary supplement
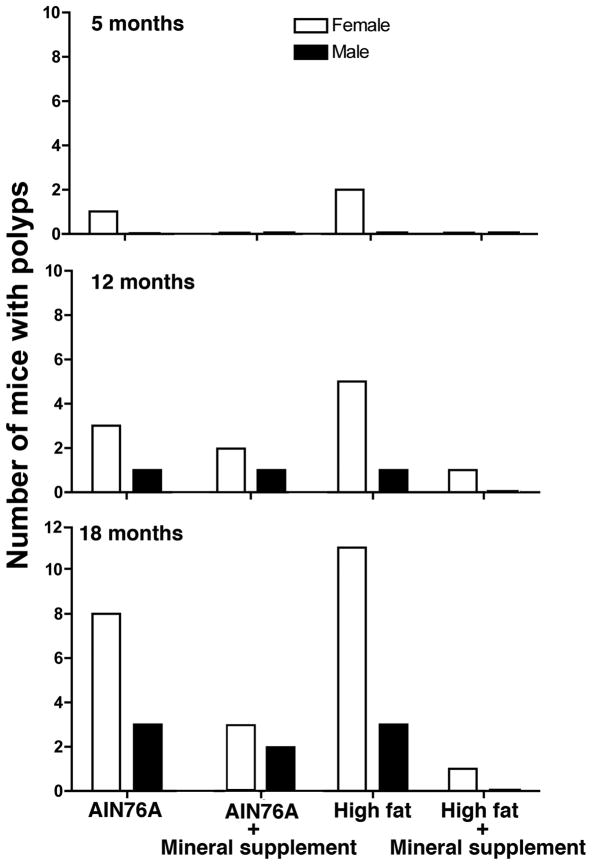
In this study, 20 animals per group (10 males and 10 females) were sacrificed at 5 months and at 12 months and 30 animals per group (15 males and 15 females) were sacrificed at 18 months. The incidence of colon polyp formation by time, gender and diet group is shown in Figure 2. A total of 22 out of 70 mice on the high fat diet without the mineral supplement had at least one visible polyp at necropsy (31% incidence). In contrast, only 2 out of 70 mice on the high-fat diet with the supplement had a visible polyp (3% incidence) (p<0.0001 by Fisher Exact Test). A control group of mice on the (low-fat) AIN 76A diet had detectable polyps in 16 of 70 animals (23% incidence). The number of animals with polyps in the AIN 76A group was not statistically different from the number of mice on the HFWD alone, but was statistically higher than the number of mice with polyps on the HFWD with supplementation (p=0.0006 by Fisher Exact Test). This was in spite of the fact that mice in this group received a comparable amount of dietary calcium to animals in the high-fat group with the mineral supplement. When the multi-mineral product was included in the diet of mice on the low-fat diet, polyps were seen in 8 of 70 mice (11% incidence). When data from the high-fat and low-fat diets were combined, overall polyp incidence was reduced from 27% in mice on un-supplemented diets to 7% in mice on supplemented diets (p<0.0001 by Fisher Exact Test).
Figure 2. Colon polyp formation in mice; Effects of time, gender and presence or absence of the multi-mineral natural product.
Data are based on stereo-microscopic identification. At 5 and 12 months, there were 10 males and 10 females per group. At 18 months, the total number of animals per group was 15 males and 15 females. When findings from the three time-points were analyzed together (Fisher Exact Test), polyp formation was greater in female mice than in males (p<0.0001) and the inclusion of the multi-mineral supplement in the HFWD lowered polyp formation (p<0.0001) relative to the HFWD alone and relative to the AIN 76A diet (p=0.0006).
Overall, the incidence of polyp formation was strongly weighted toward females. Of the total number of animals with polyps (48 in all), 37 were females and 11 were males (p<0.0001 by Fisher Exact Test). Not surprisingly, the incidence of polyp formation increased with age. A total of 3 polyps were observed at 5 months, 14 polyps at 12 months and 31 polyps at 18 months. Among animals on the high fat diet for 18 months (15 males and 15 females), there were a total of 14 mice with polyps (47%) while in mice on the HFWD with the mineral supplementation (15 males and 15 females), the incidence dropped to 3% (p= 0.0002 by Fisher Exact Test). Thus, the most significant protection was observed at the longest time-point.
Table 1 shows the location of the polyps within the colon. Virtually all of the polyps developed in the cecum or at the ileocecal junction (39 total) while the remaining polyps were scattered throughout the proximal colon (7 polyps) and distal colon (2 polyps). There were no significant differences between females and males in polyp location. Likewise, no diet-specific effect on polyp location was seen. The cecum (especially near the ileocecal junction) is known to be the site at which most spontaneous tumors arise in conventional-raised rodents (24).
Table 1.
Distribution of polyps within the colon
| Location | Number of polyps | |||
|---|---|---|---|---|
| AIN 76A | HFWD | |||
| Unsuppl. | + Suppl. | Unsuppl. | + Suppl. | |
| Cecum | 15 | 6 | 17 | 1 |
| Proximal colon | 1 | 1 | 4 | 1 |
| Distal colon | 0 | 1 | 1 | 0 |
Distribution of polyps within the colon was based on the examination under a stereoscopic dissecting microscope. This is based on combined data for polyps at all-time points and from both genders. There were a total 35 males and 35 females present in each diet group including all-time points.
Histological findings
Of the 48 lesions identified by stereomicroscopy, a total of 43 lesions were confirmed histologically. Ten lesions were classified as foci of hyperplasia, 20 as adenomas and 13 as adenocarcinomas. The remaining 5 lesions (all very small) were missed during histological processing, possibly due to the difficulty in orientation of such small lesions. The histological distribution of the lesions across the different diet groups is shown in Table 2.
Table 2.
Histological classification of lesions within the colon
| Lesion type | Histological classification | |||
|---|---|---|---|---|
| AIN 76A | HFWD | |||
| Unsuppl. | + Suppl. | Unsuppl. | + Suppl. | |
| Hyperplasia | 2 (1F/1M) | 2 (2F/0M) | 5 (4F /1M) | 1 (1F/0M) |
| Adenoma | 8 (8F/0M) | 3 (1F/2M) | 9 (9F/0M) | 0 |
| Adenocarcinoma | 6 (3F/3M) | 3 (2F/1M) | 4 (4F/0M) | 0 |
Histological classification was done according to the internationally standardized guidelines for the assessment of rodent tumors (23).
Values represent total number by category. Values in parenthesis reflect females and males.
The five macroscopic lesions that were not confirmed histologically were all very tiny lesions. Three of these were in high-fat males, one was in high-fat female and the fifth was in a high-fat female with the mineral supplement.
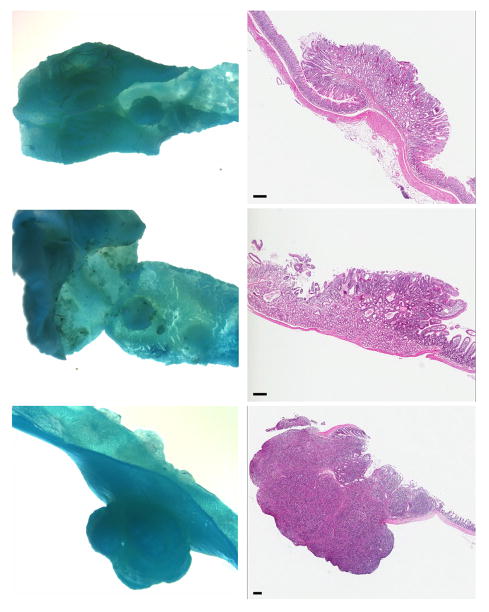
Foci of hyperplasia consisted of elongated crypts with maintenance of normal glandular structure and without compression of adjacent mucosa. The adenomas consisted of sessile (flat) or pedunculated masses of elongated crypts with piling of basal epithelium and compression of adjacent mucosa. Herniation of glands into the submucosa was sometimes present but the basement membranes appeared to be intact. Adenocarcinomas consisted of dysplastic epithelial glands with invasion into the submucosa and loss of a well-demarcated basement membrane. Intravascular or intralymphatic foci were not seen and there was no gross evidence of metastasis. Figure 3 shows stereoscopic pictures and corresponding histology of a normal colonic mucosa (upper panels), a representative adenoma (middle panels) and an adenocarcinoma (lower panels).
Figure 3. Stereomicroscopic and histological appearance of colon.
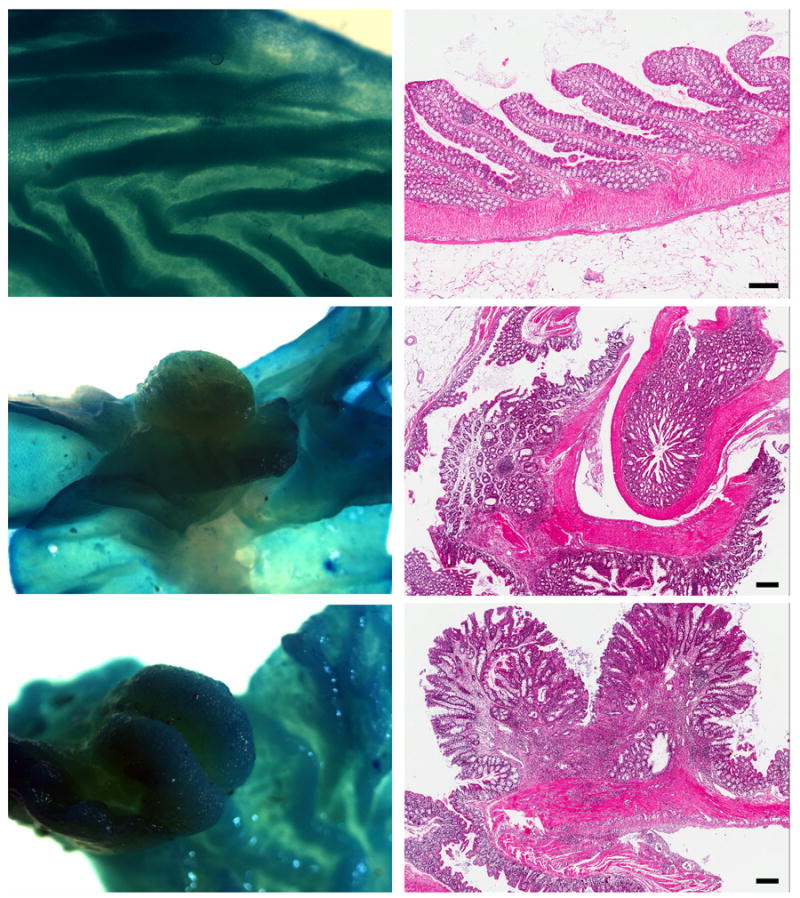
Upper panels: Normal colonic mucosa; Middle panels: mucosal adenoma; Lower Panels: Adenocarcinoma of colon. Both tumors were from female mice on HFWD. (Histology: hematoxylin and eosin; bar = 100 μm).
Tumors in the small intestine and stomach
As part of the study, the stomach, duodenum, jejunum and ileum were also examined stereoscopically for tumors. A total of 16 overt masses were identified. Of these, 14 were on the serosal surface and when examined histologically, proved to be dense clusters of lymphoid cells – i.e., either lymphomas or gut associated lymphoid tissue (GALT). Lymphoma of the gastrointestinal system is a common finding in aged C57BL/6 mice (25). Lymphoid lesions were equally divided among the diet groups. The two lesions on the mucosal surface were identified histologically as adenomas – one in the stomach of a male mouse on the high-fat diet and one in the duodenum of a female mouse on the mineral-supplemented high-fat diet (Figure 4). With so few lesions, nothing can be said concerning dietary influence.
Figure 4. Stereomicroscopic and histological appearance of three extra-colonic lesions.
Upper panels: mucosal polyp (adenoma) in stomach. Middle panels: mucosal polyp (adenoma) in duodenum. Lower panels: Inflammatory cell nodule (lymphoma) on the serosal surface of ileum. (Histology: hematoxylin and eosin; bar = 200 μm).
Systemic and bone calcium levels in mice on the high fat diet with and without the multi-mineral natural product
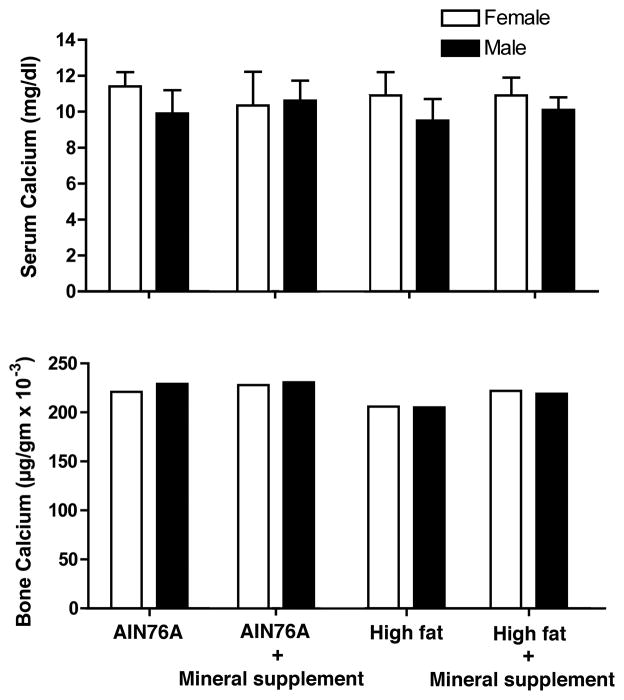
Serum calcium levels were assessed in mice sacrificed at the 18-month time-point. Values are shown in Figure 5. Female mice had a slightly higher serum calcium level than males but differences were not significant. Diet appeared to have no effect on serum calcium levels for either female or male mice. Since bone serves as a reservoir for calcium in the body, bone calcium content was assessed. Differences were slight. Supplementation increased bone calcium compared to the respective control groups by 2–7% (Figure 5). Taken together, these findings suggest no major changes in systemic calcium levels in mice given the multi-mineral supplement.
Figure 5. Calcium levels in serum and bone.
Serum calcium values are means and standard deviations. For bone calcium measurements, bones from all mice in a group were pooled to provide a single value.
DISCUSSION
The data presented here demonstrate a virtually complete inhibition of colon polyp formation in mice on a HFWD supplemented with a natural product from the red marine algae, Lithothamnion calcareum. The natural product consists of the inorganic minerals accumulated from seawater in the algae fronds. Calcium is the major constituent, but in addition, there is a high level of magnesium and detectable levels of 72 additional trace elements (see Supplement Table 1 for the complete analysis). What minerals in the algae product contribute to colon polyp prevention and the mechanism(s) underlying their chemopreventive activity are not fully understood. Calcium is, undoubtedly, critical, but it is unlikely that calcium alone is responsible for all of the activity in the natural product. The level of protection seen with the multi-mineral product was greater than that reported previously for calcium alone (16). Furthermore, protection was greater than seen in our control group of mice on the low-fat rodent diet, AIN 76 A, which had a comparable amount of calcium to that provided in the multi-mineral material. Both epidemiological studies (1–6) and interventional studies (7,8) in humans have demonstrated that calcium has the capacity to reduce polyp formation in the colon. However, its effectiveness is far from complete. A multi-mineral approach to polyp prevention may have efficacy not observed with calcium alone. There may be another benefit to a multi-mineral – based approach. A recent meta-analysis of the calcium supplementation literature came to the conclusion that calcium supplement use was associated with an increase in cardiovascular events (26). The inclusion of multiple minerals along with calcium in a supplement could, perhaps, reduce the calcium concentration required for efficacy. Only controlled clinical studies will be able to address this issue. The multi-mineral algae product used here is a GRAS-listed agent and is already present in a number of products sold for human consumption in Asia, Australia, Europe and North America. A small scale clinical study for osteoarthritis symptom-relief has been conducted (27). A clinical study focused on colon polyp reduction could be carried out and could provide valuable additional information on the potential clinical benefit of this product with respect to the colon.
The multi-mineral natural product contains a number of cationic metals (in addition to calcium) that may contribute to reduced polyp formation in the colon – either alone or in conjunction with calcium. Among these, magnesium may be especially important. A recent study demonstrated that while magnesium supplementation by itself had little chemopreventive activity, the ratio of calcium : magnesium was as important as the level of calcium itself (28). Other minerals present in the natural product, including copper, chromium, manganese, molybdenum, selenium and zinc have all been shown to reduce tumor formation or suppress other types of tissue injury in the gastrointestinal tract (29–32). Several of these are components of anti-oxidant enzymes (33) and thought to function by reducing oxidant-driven mutations that lead to dis-regulated growth. Each of these elements might exert some degree of protection against polyp formation by itself, or they might function synergistically with one another or with calcium. It is unlikely, however, that any one of these components is the major sole contributor to protection for the simple reason that all of these essential trace elements are present in both the high-fat diet and the AIN 76A diet used here (see footnote in Supplement Table 2). It is hard to envision how the additional amounts contributed in the algae product could provide a benefit not seen with the same dietary minerals already present.
The rare earth (lanthanoid) minerals comprise another component of the multi-mineral natural product that may be beneficial. The lanthanoids are cationic metals with atomic numbers between 57 and 71. With the exception of promethium, which is a man-made element, all of the other lanthanoids are present in detectable levels in the natural product (Supplement Table 1). These closely related elements have an ionic radius similar to that of calcium, but with a higher overall charge density (34,35). Lanthanoid ions bind to calcium-binding sites on proteins, and in some cases the binding affinity is greater than that of calcium itself (36). The extracellular calcium-sensing receptor (CaSR) is a lanthanoid-responsive protein in epithelial cells (37–40). Lanthanoids also impact the functioning of calcium channels, including receptor-activated channels, voltage-gated channels and those responsive to mechanical stress (41–43). Other important regulatory molecules influenced by lanthanoids include calcineurin and certain calcium-dependent ATPases (44). With multiple regulatory molecules affected by lanthanoid ions, it is not unlikely that cellular activity would be altered in their presence. A past study (45) demonstrated that gadolinium, one of the lanthanoid metals, was able to activate certain features of the differentiation program in human colon epithelial cells. In a recent study (46), we demonstrated epithelial cell growth inhibition with several individual lanthanoid metals (including gadolinium) under conditions in which other major cationic mineral components of the multi-mineral natural product were ineffective. Finally, we have also recently found that micromolar amounts of gadolinium synergize with calcium to induce apoptosis in colon carcinoma cells under conditions in which either alone is ineffective (unpublished). Based on these observations, we hypothesize that the lanthanoid metals in the multi-mineral natural product contribute to reduced colon polyp formation by modulating calcium effects on epithelial cell growth and differentiation.
This is not to suggest that other minerals in the multi-mineral rich product are inert. This is also not to suggest that a direct effect on colonic epithelial cell growth and differentiation is the only possible mechanism for the multi-mineral supplement. Rather, there are likely to be multiple individual minerals contributing to growth control in a number of different ways. Among possibilities for ways in which alterations in dietary minerals could alter the carcinogenesis process is pH buffering to foster a change in the microbial content of the gut (47) and reduce inflammation (48). Bile salt chelation is another possibility (49). Finally, alterations in dietary minerals might directly influence the transit time through the gastrointestinal tract and lead to differential exposure to carcinogens in the diet. All of these possibilities will need to be examined separately.
It is of interest that the majority of colon polyps in both control and high-fat diets occurred in female mice with a preponderance occurring in aged animals. The increased incidence of colonic polyps in female mice as compared to males, and in aged female mice as compared to the younger cohort may suggest the possible role of estrogen as an inhibitory factor in polyp development and the loss of estrogen during menopause as a contributor to colon cancer development. While mice do not undergo a precipitous drop in estrogen levels at menopause, there is a gradual decline in estrogen production beginning at about 12 months of age (50), such that by 16 months, circulating estrogen levels are essentially zero. In support of this, estrogen receptors have been identified in gastrointestinal epithelial cells (51). Additionally, the Women’s Health Initiative trial of estrogen plus progestin in post-menopausal women identified a reduced risk of colon cancer in association with the hormone replacement therapy (52). Finally, a small-scale study in rodents found that estrone in conjunction with soy reduced tumor formation in azoxymethane-treated animals more effectively than soy alone (53). At this point, it is not clear how mineral supplement might overcome the loss of estrogen in the aged mice.
In conclusion, this study shows that a multi-mineral natural product obtained from marine algae is able to reduce colon polyp formation in C57BL/6 mice both on a high-fat diet and a low-fat diet. Based on the results presented here, we suggest calcium alone cannot explain the protective effects of the multi-mineral supplement, and that a multi-mineral approach to colon polyp chemoprevention may prove to be more efficacious than an approach based on the use of calcium alone. Our past studies showing that the same multi-mineral natural product also reduces bone loss in female mice (54) and our recent studies showing reduced liver tumor formation (55) suggest that the beneficial effects of the multi-mineral supplement may extend beyond the colon.
Supplementary Material
Acknowledgments
This study was supported in part by grant CA140760 from the National Institutes of Health, Bethesda, MD, and by grant 11-0577 from the Association for International Cancer Research, St. Andrews, Fife, Scotland.
The authors would like to acknowledge Ron Craig (Histomorphometry Core) for his ScanScope services and Mark Deming (The Pathology Imaging Laboratory) for his help with stereomicroscopy and imaging. The core laboratories are supported by the Department of Pathology at the University of Michigan. The authors would also like to thank Marigot, Ltd. (Cork, Ireland) for providing the multi-mineral supplement (Aquamin®) as a gift.
References
- 1.McCullough ML, Robertson AS, Rodriguez C, Jacobs EJ, Chao A, et al. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States) Cancer Causes Control. 2003;14:1–12. doi: 10.1023/a:1022591007673. [DOI] [PubMed] [Google Scholar]
- 2.Flood A, Peters U, Chatterjee N, Lacey JV, Jr, Schairer C, et al. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev. 2005;14:126–132. [PubMed] [Google Scholar]
- 3.Wakai K, Hirose K, Matsuo K, Ito H, Kuriki K, et al. Dietary risk factors for colon and rectal cancers: a comparative case-control study. J Epidemiol. 2006;16:125–135. doi: 10.2188/jea.16.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, et al. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- 5.Kampman E, Giovannucci E, van ‘t Veer P, Rimm E, Stampfer MJ, et al. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am J Epidemiol. 1994;139:16–29. doi: 10.1093/oxfordjournals.aje.a116931. [DOI] [PubMed] [Google Scholar]
- 6.Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States) Cancer Causes Control. 2000;11:459–466. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- 7.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 8.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 9.Tu CL, Oda Y, Bikle DD. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J Invest Dermatol. 1999;113:340–345. doi: 10.1046/j.1523-1747.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- 10.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Behrens J, Vakaet L, Friis R, Winterhager E, Roy FV, et al. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, et al. Essential role of BCL9-2 in the switch between β-catenin’s adhesive function and transcriptional functions. Genes & Development. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostick RM, Goodman M, Sidelnikov E. Calcium and vitamin D. In: Potter JD, Lindor NM, editors. Genetics of Colorectal Cancer. New York, NY: Springer Science + Business Media, LLC; 2009. pp. 273–294. [Google Scholar]
- 14.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57BI/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 15.Yang K, Kurihara N, Fan K, Newmark H, Rigas B, et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 16.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, et al. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57BI/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Ther. 2010;9:93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adey WH, McKibbin DL. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo. Botanical Marina. 1970;13:100–106. [Google Scholar]
- 19.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN. Eating patterns and risk of colon cancer. Am J Epid. 1998;148:4–16. doi: 10.1093/aje/148.1.4-a. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 21.Potter JD. Risk factors for colon neoplasia – epidemiology and biology. Eur J Cancer. 1995;31:1033–1038. doi: 10.1016/0959-8049(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 22.Kastenmayer RJ, Fain MA, Perdue KA. A restrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Amer Assoc Lab Animal Sci. 2006;45:8–12. [PubMed] [Google Scholar]
- 23.Betton GR, Whiteley LO, Anver MR, Brown R, Deschl U, et al. Gastrointestinal tract. Springer; Berlin Heidelberg, New York: 2001. International Classification of Rodent Tumors, the Mouse; pp. 54–56. [Google Scholar]
- 24.Greaves P. Digestive System, Histopathology of Preclincial Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation. 4. New York: Academic Press-Elsevier; 2011. p. 403. [Google Scholar]
- 25.Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, Body Weight, and Neoplasia in Ad Libitum-Fed and Diet-Restricted C57BL6 Mice Fed NIH-31 Open Formula Diet. Toxicol Pathol. 1995;23(5):570–82. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 26.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. Brit Med J. 2010;341:C3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frestedt JL, Walsh M, Kuskowski MA, Zenk JL. A natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized controlled pilot trial. Nutr J. 2008;7:9. doi: 10.1186/1475-2891-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr. 2007;86(3):743–51. doi: 10.1093/ajcn/86.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis CD, Feng Y. Dietary copper, manganese and iron affect the formation of aberrant crypts in colon of rats administered 3,2′-dimethyl-4-aminobiphenyl. J Nutr. 1999;129:1060–1067. doi: 10.1093/jn/129.5.1060. [DOI] [PubMed] [Google Scholar]
- 30.Alwahaibi N, Mohamed J, Alhamadani A. Supplementation of selenium reduces chemical hepatocarcinogenesis in male Sprague-Dawley rats. J Trace Elements Biol Med. 2010;24:119–123. doi: 10.1016/j.jtemb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Chen W-Y, Chen C-J, Liao J-W, Mao FC. Chromium attenuates hepatic damage in a rat model of chronic cholestasis. Life Sci. 2009;84:606–614. [PubMed] [Google Scholar]
- 32.Kang X, Zhong W, Liu J, Song Z, McClain CJ, et al. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–83. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- 34.Evans CH, editor. Biochemistry of the Elements. New York: Plenum Press; 1990. Biochemistry of the lanthanides. [Google Scholar]
- 35.Gschneidner KA Jr, Eyring L, editors. Elsevier Science & Technology Books, editor. Handbook on the Physics and Chemistry of Rare Earths. Amsterdam, The Netherlands: 2000. [Google Scholar]
- 36.Pidcock E, Moore GR. Structural characteristics of protein binding sites for calcium and lanthanide ions. J Biol Inorg Chem. 2001;6:479–489. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, et al. Multiple Ca(2+) -binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry. 2009;48(2):388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273(23):14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- 39.McLarnon SJ, Riccardi D. Physiological and pharmacological agonists of the extracellular Ca2+ -sensing receptor. Eur J Pharmacol. 2002;447(2–3):271–278. doi: 10.1016/s0014-2999(02)01849-6. [DOI] [PubMed] [Google Scholar]
- 40.Riccardi D, Maldonado-Perez D. The calcium-sensing receptor as a nutrient sensor. Biochem Soc Trans. 2005;33:316–320. doi: 10.1042/BST0330316. [DOI] [PubMed] [Google Scholar]
- 41.Estacion M, Mordan LJ. Competence induction by PDGF requires sustained calcium influx by a mechanism distinct from storage-dependent calcium influx. Cell Calcium. 1993;14(6):439–454. doi: 10.1016/0143-4160(93)90003-o. [DOI] [PubMed] [Google Scholar]
- 42.Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J Gen Physiol. 1990;95(4):679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol. 1998;275:C619–621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 44.Palasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47(4):1107–1114. [PubMed] [Google Scholar]
- 45.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: Promotion of E-cadherin expression and suppression of β-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 46.Jenkins W, Perone P, Walker K, Bhagavathula N, Aslam MN, et al. Fibroblast Response to Lanthanoid Metal Ion Stimulation: Potential Contribution to Fibrotic Tissue Injury. Biol Trace Elem Res. 2011;144:621–35. doi: 10.1007/s12011-011-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newmark HL, Lupton JR. Determinants and consequences of colonic luminal pH: implications for colon cancer. Nutr Cancer. 1990;14(3–4):161–73. doi: 10.1080/01635589009514091. [DOI] [PubMed] [Google Scholar]
- 48.Itzkowitz SH, Yio X. Inflammation and Cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 49.Azuma N, Suda H, Iwasaki H, Yamagata N, Saeki T, Kanamoto R, Iwami K. Anti-tumor effects of several food proteins in a rat model with colon cancer and their reverse correlation with plasma bile acid concentration. J Nutr Sci Vitaminol. 2000;46:91–96. doi: 10.3177/jnsv.46.91. [DOI] [PubMed] [Google Scholar]
- 50.vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproduction aging in humans, laboratory rodents and other selected species. In: Knobil E, Neill JD, editors. Physiology of reproduction. 2. Raven Press; New York: 1994. pp. 1215–1314. [Google Scholar]
- 51.Wada-Hiraike Osamu, Imamov Otabek, Hiraike Haruko, Hultenby Kjell, Schwend Thomas. Role of estrogen receptor β in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103(8):2959–64. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–92. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo J-Y, Li X, Browning JD, Rottinghaus GE, Lubahn DB, et al. Dietary soy isoflavones and Estrone protect ovariectomized ERαKO and wild-type mice from carcinogen-induced colon cancer. J Nutr. 2004;134:179–182. doi: 10.1093/jn/134.1.179. [DOI] [PubMed] [Google Scholar]
- 54.Aslam MN, Kreider JM, Paruchuri T, Bhagavathula N, DaSilva M, et al. A Mineral-Rich Extract from the Red Marine Algae Lithothamnion calcareum Preserves Bone Structure and Function in Female Mice on a Western-Style Diet. Calcif Tissue Int. 2010;86:313–324. doi: 10.1007/s00223-010-9340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aslam MN, Bergin I, Naik M, Hampton A, Allen R, et al. A Multi-Mineral Natural Product Inhibits Liver Tumor Formation in C57BL/6 Mice. Biol Trace Elem Res. 2012;147:267–274. doi: 10.1007/s12011-011-9316-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.