Summary
Somatic progenitors suppress differentiation to maintain tissue self-renewal. The mammalian SWI/SNF chromatin-remodeling complex regulates nucleosome packaging to control differentiation in embryonic and adult stem cells. Catalytic Brg1 and Brm subunits are required for these impacts, however, roles for SWI/SNF regulatory subunits are not fully understood. Here we show that ACTL6a/BAF53A modulates the SWI/SNF complex to suppress differentiation in epidermis. Conditional loss of ACTL6a resulted in terminal differentiation, cell cycle exit and hypoplasia while ectopic expression of ACTL6a promoted the progenitor state. A significant portion of genes regulated by ACTL6a were found to also be targets of KLF4, a known activator of epidermal differentiation. Mechanistically, we show that ACTL6a prevents SWI/SNF complex binding to promoters of KLF4 and other differentiation genes, and that SWI/SNF catalytic subunits are required for full induction of KLF4 targets. Thus, ACTL6a controls the epidermal progenitor state by sequestering SWI/SNF to prevent activation of differentiation programs.
Keywords: Stem Cell, ACTL6a, Differentiation, KLF4, SWI/SNF
Introduction
Homeostasis of somatic tissues requires a sustainable pool of progenitor cells that can both proliferate to replenish themselves and that can also enter a differentiation pathway to enable their progeny to perform specified tissue functions, such as barrier formation in epidermis. Maintenance of this progenitor state requires tight suppression of differentiation genes because their premature expression can abolish proliferative capacity and trigger cell death (Melino et al., 1994). In the case of epidermis, a stratified epithelial tissue, in which basement membrane-adherent progenitors migrate outwards to undergo terminal differentiation, several epigenetic regulators have recently been found to contribute to differentiation gene repression in progenitors. Among these are DNA methyltransferase 1 (DNMT1) (Sen et al., 2010), histone methylation regulators that include JMJD3, Setd8, CBX4, Jarid2 and Polycomb proteins (Driskell et al., 2011; Ezhkova et al., 2009; Luis et al., 2011; Mejetta et al., 2011; Sen et al., 2008), as well as regulators of histone acetylation, HDAC1, HDAC2 and Sin3a (LeBoeuf et al., 2010; Nascimento et al., 2011; Reyes et al., 1998). These studies point to a role for epigenetic regulators from multiple classes in the repression of differentiation gene induction within somatic progenitor cells.
Among classes of epigenetic regulators, chromatin remodeling complexes are commonly composed of a catalytic ATPase subunit, which utilizes the energy from ATP to move or eject DNA-bound nucleosomes, along with regulatory subunits that modulate the conformation and activity of the entire complex. The mammalian SWI/SNF chromatin remodeling complex is composed of 11 subunits encoded by 20 genes (Wu et al., 2009). Its catalytic ATPase subunit is either Brg1 or Brm. Brg1 is required during embryonic development although Brm seems to be dispensable (Bultman et al., 2000; Reyes et al., 1998). Compared to Brg1 and Brm, there are less data on the function of the 18 genes that encoding the regulatory subunits of the SWI/SNF complex. It is notable, however, that mice with loss of either BAF250a or BAF155 display an even more severe phenotype than Brg1 knockout mice (Gao et al., 2008; Kim et al., 2001), indicating that these regulatory elements may mediate critical biological functions.
In addition to classical epigenetic regulators, recent studies reveal that many proteins long-considered as components of the cytoskeleton can actually impact transcription (Grummt, 2006). Various types of lamins directly interact with chromatin and organize the nuclear landscape (Dechat et al., 2008). Nuclear myosin associates with RNA polymerase I and II (Vreugde et al., 2006; Ye et al., 2008), whereas nuclear actin co-purifies with all three known RNA polymerases and multiple epigenetic regulating complexes (Visa and Percipalle, 2010). In addition, multiple actin-like genes, conserved from yeast to human, associate with different epigenetic regulators. It remains unclear whether these actin-like genes are required in the gene regulatory control of progenitor differentiation.
In a search for epigenetic repressors of differentiation that are required for epidermal progenitor maintenance, we identified ACTL6a (actin-like 6a), an actin-like protein also known as BAF53a/INO80K/Arp4. Here we show that ACTL6a expression is significantly down-regulated during epidermal differentiation. Conditional deletion of ACTL6A in mouse epidermis abolished epidermal progenitor function, leading to cell cycle exit, terminal differentiation and ultimately hypoplasia followed by tissue loss. In the human context, ACTL6a depletion exerts similar impacts, decreasing progenitor clonogenicity and inducing ectopic expression of differentiation genes whereas enforced ACTL6a expression suppressed differentiation. ACTL6a target gene characterization identified KLF4 as a key target of ACTL6a repression. KLF4 loss significantly compensated for the defects caused by ACTL6a depletion in progenitors. Of the three epigenetic regulatory complexes well-characterized to contain ACTL6a, namely the TIP60 and KAT2a histone acetyltransferase (HAT) complexes, and the SWI/SNF chromatin remodeling complex, depletion of only the ARID1A/BAF250a member of the Brg1/Brm-containing SWI/SNF complex recapitulated ACTL6a effects. Consistent with a role for ACTL6a in regulating SWI/SNF impacts on progenitor gene regulation, ACTL6a was necessary to impair Brg1/Brm binding to differentiation gene promoters. These data suggest that ACTL6a maintains the undifferentiated progenitor state by opposing SWI/SNF-enabled activation of KLF4 and other epidermal differentiation genes.
Results
ACTL6a is down-regulated during epidermal tissue differentiation
Analysis of actin-like gene expression during calcium-induced epidermal keratinocyte differentiation in vitro identified ACTL6a as one of the most down-regulated actin-like genes (Fig. 1A-C, Table S2). To examine ACTL6a mRNA expression within intact epidermal tissue, laser capture microdissection was used to separate the undifferentiated progenitor basal epidermal layer from suprabasal differentiating layers. In agreement with in vitro findings, ACTL6a mRNA levels were decreased in differentiated layers relative to the basal layer (Fig. 1C). This mRNA down-regulation was reflected in ACTL6a protein levels as well, which also decreased during differentiation (Fig. 1D). Within intact tissue, ACTL6a protein was likewise most strongly expressed in less differentiated cells adjacent to the epidermal basement membrane (Fig. 1E). ACTL6a expression is thus down-regulated in epidermal differentiation.
Figure 1. ACTL6a is down regulated during epidermal differentiation.
(A) Heat map representing mRNA profiling analysis of all known actin-like genes comparing their expression between undifferentiated (−) and calcium differentiated (+) human keratinocytes in vitro. (B) ACTL6a mRNA down-regulation during keratinocyte differentiation in vitro (p<0.001, ANOVA). Bars represent mean ± SD. (C) ACTL6a mRNA levels from human epidermal laser capture microdissection of the undifferentiated progenitor-containing basal layer and differentiating suprabasal layers (p<0.05, t-test). Bars represent mean ± SD. (D) ACTL6a protein down-regulation during keratinocyte differentiation in vitro. (E) ACTL6a protein localizes primarily to less differentiated layers in human epidermal tissue in vivo [ACTL6a=green; collagen VII basement membrane marker=red; nuclear stain with Hoechst 33342=blue; scale bar=50 microns; dotted line indicates epidermal tissue upper boundary]. See also Table S2.
Conditional epidermal ACTL6a deletion ablates progenitor function
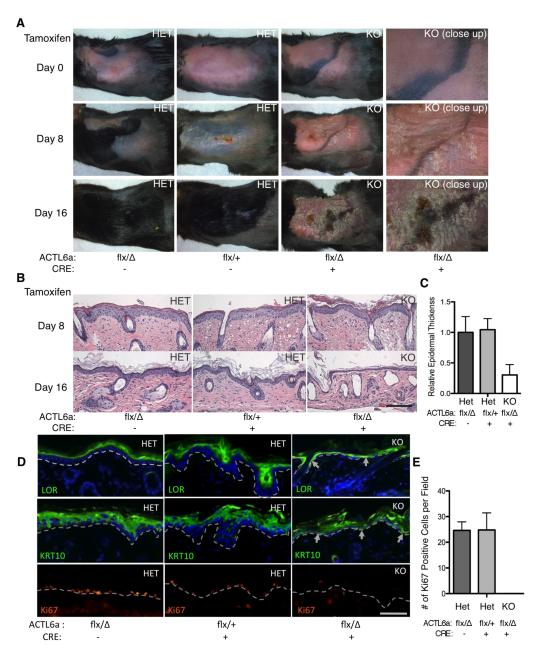
ACTL6a down-regulation during epidermal differentiation raised the possibility that ACTL6a may impact this process. To explore this, we undertook targeted ACTL6A deletion in mouse epidermis using Cre recombinase driven by the epidermal basal layer keratin 14 (K14) promoter (Huelsken et al., 2001). We first undertook conditional ACTL6A deletion in adult mouse epidermis using a K14-driven Tamoxifen-inducible Cre-estrogen receptor ligand domain fusion (Vasioukhin et al., 1999) (Fig. S1A). Cre activation via topical Tamoxifen application to a small region of mouse back skin was followed clinically by scaling then thinning, leading ultimately erosion and loss of epidermis by day 16 (Fig. 2A); this loss of surface epithelium was also visible in the peri-oral area where mice lick the topical agent (Fig. S1B). Over this time period, ACTL6a-deleted tissue underwent progressive epidermal hypoplasia (Fig. 2B,C; Fig. S1C) prior to a complete tissue failure. In concert with this, loss of ACTL6a was associated with induction of both early (keratin 1) and late (loricrin) differentiation protein expression in the basement membrane proximal basal progenitor layer where such proteins are normally never expressed (Fig. 2D). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay for apoptosis showed no significant difference in the interfollicular epidermal tissue between knockout and control animals (Fig. S1D), however, there was a profound loss of proliferation (Fig. 2D,E; Fig. S1F,H). We have further investigated the function of ACTL6a in embryonic epidermal tissue development using constitutive K14-Cre. Similar to adults, loss of ACTL6a during embryogenesis led to epidermal hypoplasia (Fig. S2B,D). Newborn ACTL6a KO animals die within only a few hours after birth, and are characterized by thin-appearing skin with epidermal erosions, and induction of differentiation proteins in the basal layer (Fig. S2A,B), without significant increase of apoptosis (Fig. S2E). Consistent with areas of epidermal erosion, the capacity of the epidermis to exclude dye is also significantly impaired in newborn KO animals compared to their littermates (Fig. S2A). ACTL6a deletion therefore leads to proliferative failure and premature terminal differentiation, leading to epidermal tissue loss in both embryonic development and adult tissue homeostasis.
Figure 2. Conditional epidermal ACTL6a deletion ablates progenitor function.
(A) Time course images of ACTL6a conditional knockout (KO) and heterozygous littermate control (HET) skin before (day 0) and after (day 8, day 16) tamoxifen induction of Cre-ER-mediated ACTL6a deletion in mouse epidermis. (B) Histology of mouse back skin sections from ACTL6a conditional knockout and littermates over the same time period [scale bar=50 microns]; note hypoplastic tissue collapse in KO epidermal tissue by day 16, after which point no epidermis could be detected. (C) Quantification of average epidermal tissue thickness (n=5). (p< 0.0001, ANOVA) Bars represent mean ± SD. (D) Differentiation protein expression [Loricrin=green; Keratin 1=green] and Ki67 expression [orange]; dotted line denotes the basement membrane. Arrowheads point out cells adjacent to the basement membrane expressing differentiation proteins in knockout tissue. (E) Mitotic indices calculated by the number of Ki67 positive cells per field in indicated genotypes. (p< 0.0001, ANOVA) Bars represent mean ± SD. See also Figure S1 and S2.
ACTL6a is required to repress premature progenitor differentiation
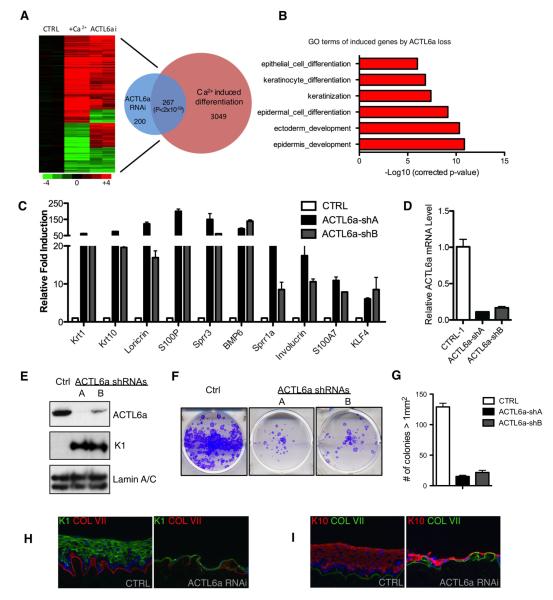
To further characterize the function of ACTL6a in epidermal homeostasis, we performed mRNA expression profiling on human epidermal keratinocytes treated with two independent ACTL6a shRNAs (Fig. 3). In undifferentiated keratinocytes, ACTL6a depletion altered a total of 467 differentiation genes (327 (70%) induced, 120 (30%) repressed, Tables S3 and S4). ACTL6a-regulated genes displayed a significant (p<2×10−59, Fisher’s Exact Test) overlap of 267 genes with the published calcium-induced epidermal keratinocyte differentiation profile (Sen et al., 2010), with most genes changing in the same direction (Fig. 3A). Genes that were up-regulated in undifferentiated keratinocytes by ACTL6a loss were significantly enriched with gene oncology (GO) terms relevant to epidermal differentiation (Fig. 3B); no specific GO terms were highly enriched in down-regulated genes (Fig. S3B). Dramatic de-repression of specific well characterized differentiation genes was confirmed with ACTL6a loss, at mRNA and protein levels (Fig. 3C-E). These data confirm that, in addition to the murine tissue context, ACTL6a is required to repress differentiation gene expression in the human setting as well.
Figure 3. ACTL6a is required to repress differentiation.
(A) Heat map (left) and Venn diagram (Rezai-Zadeh et al., 2003) illustrating the overlap between expression changes identified with ACTL6a loss and calcium-induced differentiation (p<2×10−59, Fisher’s Exact Test). Genes induced are colored in red, and repressed genes are colored in green. (B) GO (Gene Ontology) analysis demonstrating that ACTL6a loss induces differentiation-associated genes. (C) qRT-PCR verification of array data showing mRNA levels of differentiation associated genes during ACTL6a loss. Bars represent mean ± SD. (D) Verification of ACTL6a knockdown by duplicate independent shRNAs. Bars represent mean ± SD. (E) Immunoblots of ACTL6a-depleted keratinocytes demonstrating loss of ACTL6a protein as compared to empty vector controls. (F) Clonogenic assays of human keratinocytes with ACTL6a RNAi or empty vector controls. (p<0.005, ANOVA) (G) Colonies > 1mm2 in clonogenic assays are quantified (n=2/group) (p<0.001, ANOVA). Bars represent mean ± SD. (H) Organotypic human epidermal tissue comparing ACTL6a loss with empty vector control for K1 differentiation marker (green) comparing ACLT6a loss versus empty vector control and (I) K10 differentiation marker (red) comparing ACLT6a loss versus empty vector control. See also Figure S3, Table S3 and S4.
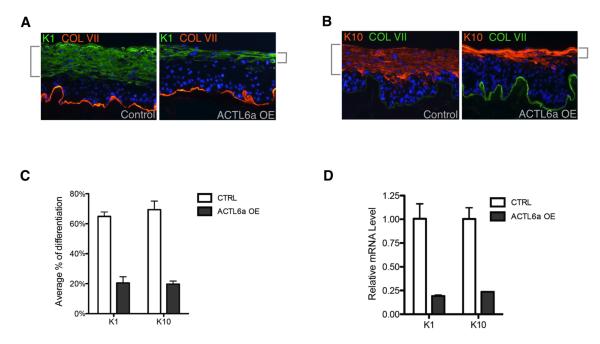
Consistent with a function for ACTL6a in maintaining the undifferentiated progenitor state, ACTL6a loss impaired clonogenic growth, with ACTL6a-depleted cells producing only an average of 14% of the colonies (> 1mm2) seen with control (Fig. 3E-G; Fig. S3F). We next tested the effects of altering ACTL6a function in organotypic human epidermal tissue, a setting that accurately recapitulates spatial patterns of epidermal gene expression (Truong et al., 2006). With striking similarity to conditional knockout mouse epidermis, organotypic human epidermal tissue displayed hypoplasia and ectopic differentiation occurring at the normally undifferentiated basal layer (Fig. 3H,I). Further supporting the notion that ACTL6a represses differentiation to maintain epidermal progenitor function, enforced expression of ACTL6a in all layers of regenerating human epidermal tissue impaired differentiation gene expression (Fig. 4; Fig S4A). ACTL6a is thus required to sustain epidermal self-renewal and to prevent premature progenitor differentiation.
Figure 4. ACTL6a over-expression suppresses differentiation.
(A) K1 differentiation marker (green) with enforced ACTL6a expression throughout the epidermis versus control. Note the decreased expanse of expression of K1 in less differentiated regenerating epidermal layers (brackets) in ACTL6a-overexpressing tissue compared to control. (B) K10 expression comparing ACTL6a overexpression versus control. (C) Quantification of differentiation marker K1 and K10 by percentage of relative thickness of K1/K10 expressing cells in tissue sections (n=5). (p<0.005, T-test) Bars represent mean ± SD. (D) Quantification of K1 and K10 mRNA levels in tissue. (p<0.005, T-test) Bars represent mean ± SD. See also Figure S4.
ACTL6a represses differentiation in part through KLF4
To search for downstream targets through which ACTL6A represses differentiation, we performed Gene Set Enrichment Analysis (GSEA) using the ACTL6a gene set. A number of previously characterized transcriptional activators of epidermal differentiation were included among ACTL6a target genes, including KLF4, GRHL3, PRDM1, and HOPX, all of which were significantly upregulated by ACTL6A knockdown. When comparing the genes regulated by these transcription factors, we found that 227 of the 467 genes within the ACTL6A-regulated gene set (48.6%) are also regulated by KLF4 with a significant p-value of 3.5E-69. In contrast, only 8% of ACTL6a gene set is regulated by GRHL3, PRDM1, and HOPX combined (Fig. 5A-C). This suggests that a significant portions of the genes regulated by ACTL6a are also KLF4 targets.
Figure 5. ACTL6a suppresses differentiation partially by repressing KLF4.
(A) Gene set enrichment analysis (GSEA) comparing the ACTL6a controlled gene set (fold change > 2) with the gene sets controlled by the known epidermal differentiation-mediating transcription factors whose expression levels changed with ACTL6a loss, including KLF4, GRHL3, PRDM1, and HOPX. Shared genes are indicated in dark blue. (B) Bar graph comparing the percentage of shared gene numbers with ACTL6a data set. KLF4 controls 48.6% of ACTL6a gene set. (C) Bar graph comparing the p-Value (−log corrected p-value) in GSEA analysis. (D) Staining of KLF4 in vivo in adult mice epidermis comparing KO vs littermate HET controls on Day 16 after initial tamoxifen treatment. KLF4 is induced on the basal layer in KO mouse tissue. (E) Staining of the KLF4 target, CDSN, comparing KO mouse tissue VS HET controls. (F) Relative mRNA expression level of KLF4 and ACTL6a in double RNAi experiment using ACTL6a shRNA and KLF4 siRNA. (p<0.0001, ANOVA) Bars represent mean ± SD. (G) Relative mRNA expression of differentiation associated genes with ACTL6a and KLF4 double RNAi. (p<0.0001, ANOVA) Bars represent mean ± SD. (H) K1 staining (green) of human epidermal tissue sections from tissues with ACTL6a KLF4 double RNAi as compared to controls. Note rescue of ACTL6a tissue collapse by KLF4 depletion. (I) K10 staining (red) of human epidermal tissue sections from tissues with ACTL6a KLF4 double RNAi as compared to controls. See also Figure S5.
KLF4, the transcription factor Kruppel-like factor 4, in addition to its well known role in the embryonic stem cell setting, is also highly expressed in differentiated layers of epidermal tissue where it has been characterized by targeted gene disruption as a critical and non-redundant activator of differentiation (Jaubert et al., 2003; Segre et al., 1999). Loss of ACTL6a in KO mouse epidermal tissue causes derepression of KLF4 and dysregulation of KLF4 targets such as CDSN (Fig 5 D,E; Fig. S2F; Fig S5D). To validate the genetic interaction between ACTL6A and KLF4, we performed double RNAi studies to investigate the impact of KLF4 depletion in the context of ACTL6a loss. KLF4 depletion effectively suppressed the induction of differentiation genes caused by ACTL6a loss, indicating that KLF4 is required for the de-repression of differentiation observed with ACTL6a loss (Fig. 5F-I, Fig. S5B,C). Therefore, both bioinformatic and genetic studies identify KLF4 as a downstream target through which ACTL6a exerts a portion of its repression actions on differentiation.
ACTL6a regulates epidermal homeostasis through the SWI/SNF complex
Recent studies suggest that ACTL6a can associate with different epigenetic regulators, including the Tip60 HAT complexes, KAT2a HAT complexes, and the SWI/SNF chromatin remodeling complex (Park et al., 2002; Tea and Luo, 2011; Zhao et al., 1998). To examine which complex might be relevant to ACTL6a function in this setting, we performed loss-of-function analysis for key functional components of each of these complexes. Depletion of either KAT2a or Tip60 failed to significantly alter differentiation gene expression (Fig. S6I,J and data not shown). In contrast, depletion of the largest component of the SWI/SNF complex, BAF250a/ARID1A, but not BAF250b/ARID1B, produced impacts similar to ACTL6a loss, decreased clonogenic growth and premature induction of differentiation (Fig. S6A-H). ACTL6a shares a very similar distribution pattern in the FPLC fractions of undifferentiated human keratinocyte cellular extracts with the Brg1/Brm catalytic subunits of the SWI/SNF complex (Fig. S7A). In addition, overexpression of ACTL6a mutant M1 with K226/E227A mutations that impairs binding with Brg1 (Nishimoto et al., 2012) also impaired suppression of differentiation as compared to wild-type ACTL6a (Fig. S4B-D). Taken together, these results suggest that the function of ACTL6a in repressing progenitor differentiation may involve the SWI/SNF complex.
ACTL6a loss permits SWI/SNF complex binding to differentiation genes
To explore the basis for the functional relationship between ACTL6a and the SWI/SNF complex, we performed ChIP analysis to examine Brg1/Brm binding to differentiation promoters as a function of ACTL6a. Since ACTL6a expression is significantly down regulated during human keratinocyte differentiation, we first compared the localization of SWI/SNF complex in undifferentiated human keratinocytes with calcium-induced differentiated keratinocytes. Compared to undifferentiated cells, differentiated keratinocytes displayed enhanced binding by SWI/SNF, as well as RNA polymerase II, at the promoters of differentiation genes, including KLF4, as well as KRT10, S100A9, SPRR3, and BMP6 (Fig. 6A,B). Moreover, depletion of ACTL6a in undifferentiated progenitor populations enhanced the binding of both Brg1/Brm as well as RNA polymerase II to differentiation gene promoters while failing to alter binding to other gene promoters (Fig. 6C,D; Fig. S7C,D). This suggests that ACTL6a loss permits SWI/SNF to bind and activate differentiation genes, a model that would predict that loss of Brg1/Brm SWI/SNF complex catalytic subunits would impair differentiation.
Figure 6. ACTL6a loss facilitates SWI/SNF complex targeting to differentiation genes.
(A) ChIP analysis of the Brg1/Brm SWI/SNF complex components at differentiation gene promoters, comparing undifferentiated and differentiated human keratinocytes. (P<0.0001, ANOVA) Bars represent mean ± SD. (B) ChIP analysis of RNA polymerase II at differentiation gene promoters, comparing undifferentiated and differentiated human keratinocytes. (P<0.0001, ANOVA) Bars represent mean ± SD. (C and D) ChIP analysis of the SWI/SNF complex and RNA polymerase II enrichment at promoter regions of representing differentiation-associated genes using undifferentiated human keratinocytes treated with shRNA against ACTL6a or control shRNA. (P<0.05, ANOVA) Bars represent mean ± SD. (E) Brg1/Brm double depletion using two independent sets of siRNAs inhibits differentiation in regenerating organotypic human epidermal tissue. [K1=green, K10=orange, dotted line=basement membrane] (F) Quantification of the knock down efficiency of siRNAs targeting Brg1 or Brm. Bars represent mean ± SD. (G) Loss of Brg1/Brm also inhibits the expression of KLF4 in differentiated human keratinocytes. Bars represent mean ± SD. See also Figure S6 and S7.
Consistent with this model, previously reported Brg1/Brm double knockout mice displayed impaired epidermal differentiation gene induction and deficient tissue barrier formation (Indra et al., 2005). In agreement with these prior data, simultaneous loss of both Brm and Brg1 by two independent sets of RNAi sequences suppressed expression of differentiation genes in organotypic human epidermal tissue (Fig. 6E,F). This suppression of differentiation mediated by Brg1/Brm loss is also accompanied by suppression of the transcription factor KLF4 (Fig. 6G), indicating that intact SWI/SNF complex function is required for full induction of KLF4 and epidermal differentiation. Taken together, our data suggest a model in which ACTL6a helps to maintain the undifferentiated progenitor state by inhibiting SWI/SNF complex binding to and activation of KLF4 and other differentiation gene promoters (Fig. 7).
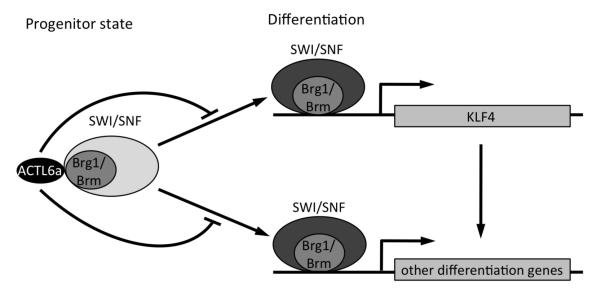
Figure 7. Hypothetical model of ACTL6a action in regulating epidermal tissue differentiation.
In undifferentiated conditions, ACTL6a prevents Brg1/Brm-containing SWI/SNF complex binding to and activation of KLF4 and other differentiation gene promoters. During differentiation, ACTL6a down-regulation facilitates SWI/SNF binding to and activation of KLF4 and other differentiation genes. In addition, the composition of the SWI/SNF also changes during differentiation (reflected by different shade of gray).
Discussion
Here we present data indicating that ACTL6a is required to repress epidermal progenitor differentiation, in part via suppression of KLF4, and that ACTL6a prevents SWI/SNF complex binding to the promoters of KLF4 and other differentiation genes. The necessity of SWI/SNF complex function in induction of epidermal differentiation was confirmed by double depletion of the Brg1/Brm catalytic subunits, a loss of function experiment that confirmed that full KLF4 induction also requires SWI/SNF action. Taken together, these data support a hypothetical model in which ACTL6a sustains the epidermal progenitor phenotype, at least in part, by preventing the SWI/SNF complex from binding to and activating the expression of differentiation genes.
Although recent studies demonstrate that ACTL6a can associate with multiple epigenetic regulatory complexes, our data indicate that the SWI/SNF complex may be most relevant to ACTL6a action in this setting. Previous studies in various systems suggest that, in addition to the SWI/SNF chromatin remodeling complex, ACTL6a can associate with HAT complexes, including those containing Tip60 and KAT2a (Lee et al., 2003; Tea and Luo, 2011). The majority of the previous functional analysis on ACTL6a’s role during development has been in the nervous system and only recently in the hemopoietic system (Krasteva et al., 2012; Lessard et al., 2007; Yoo et al., 2009). For Drosophila olfactory projection neuron dendrite targeting, the ACTL6a homolog Bap55 functions through Tip60 histone acetyl-transferase (Tea and Luo, 2011). However in vertebrates, the switch of ACTL6a by its homolog ACTL6b in the SWI/SNF complex is critical for the differentiation process of neuron stem cells (Lessard et al., 2007). The present studies in the mammalian epidermal setting indicate that the Tip60 histone acetyltransferase is not required for ACTL6a effects, whereas multiple subunits associated with the SWI/SNF complex, including BAF250a and Brg1/Brm are actively involved in repressing differentiation in the progenitor state and in activating normal differentiation gene expression.
The presence of ACTL6a in progenitor cell-containing populations is associated with suppression in the targeting of the SWI/SNF complex to differentiation genes and ACTL6a depletion relieves this suppression in these undifferentiated cells. Previous studies in lymphocytes indicated that >80% of total SWI/SNF complexes in resting lymphocytes does not associate tightly with chromatin, while a rapid and tight association of the complex can be induced by cell stimulation (Zhao et al., 1998). A similar mechanism may operate during epidermal differentiation in that ACTL6a loss may stimulate the relocation of a portion of SWI/SNF complexes to chromatin at differentiation gene promoters. This would also be in agreement with recent studies in T helper cells, in which Brg1/Brm binding to differentiation genes was associated with gene induction (De et al., 2011).
The targeting of the SWI/SNF complex during differentiation may result from a variety of inputs. The combination of different regulatory subunits and the resulting overall conformation are hypothesized to directly affect the targeting and activity of the SWI/SNF complex (Wu et al., 2009). Therefore the physical association between ACTL6a and the SWI/SNF complex is likely to form a specific conformation that may have less affinity to differentiation genes. On the other hand, multiple transcription factors and histone modifications can also play an important role in recruiting the SWI/SNF complex (Debril et al., 2004; Kowenz-Leutz and Leutz, 1999; Salma et al., 2004). Therefore, it is formally possible that the recruitment of the SWI/SNF complex to differentiation genes upon ACTL6a loss is indirectly mediated by the transcription factors de-repressed by ACTL6a loss, such as KLF4. It is noteworthy, however, that KLF4 depletion did not totally account for the full spectrum of differentiation gene de-repression caused by ACTL6a loss. This partial rescue of the ACTL6a loss defect could be due to a number of factors, including action by transcription factors other than KLF4, such as GRHL3, PRDM1, HOPX and others.
KLF4 is an essential transcription regulator directly dictating multiple biological processes including cell proliferation, differentiation, tumorigenesis and pluripotency (Rowland and Peeper, 2006; Vangapandu and Ai, 2009). However, very little is known about how the expression levels of KLF4 are precisely controlled to enable its multiple roles in different settings. During epidermal differentiation, KLF4 expression is increased substantially and both KLF4 loss of function and ectopic expression significantly disturb epidermal growth and differentiation (Jaubert et al., 2003; Segre et al., 1999). Our findings have identified ACTL6a as a negative regulator of KLF4 in the undifferentiated progenitor state, and the down regulation of ACTL6a during epidermal tissue differentiation thus appears to enable the upregulation of KLF4 to help drive differentiation.
In addition to ACTL6a, we have observed that several other actin-like proteins also change their expression during differentiation, including ACTR8, which is functionally related to the Ino80 chromatin remodeling complex. It is interesting to note that the expression of ACTL6b is below the level of detection by multiple methods in our lab, including quantitative RT-PCR and RNA seq. ACTL6b was previously shown to replace ACTL6a to associate with the SWI/SNF complex and dictate the neuronal differentiation processes (Lessard et al., 2007). The lack of detectable epidermal ACTL6b expression indicates that the regulation of differentiation at the level of nuclear actin-like proteins is significantly different in epidermis versus the neuronal tissue. Consistent with our findings in the epidermal tissue, ACTL6a is essential for the progenitor function in the hemopoietic system where little ACTL6b is detected (Krasteva et al., 2012; Kuroda et al., 2002; Olave et al., 2002). Future studies on the functions of other actin-like proteins in multiple tissues will further enhance our knowledge of developmental regulation by these regulators and the epigenetic regulatory complexes with which they associate.
Experimental Procedures
Cells and Organotypic Culture
Primary human keratinocytes were isolated from fresh surgically discarded newborn foreskin, and cultured in complete Keratinocyte-SFM (Life Technologies #17005-142) and Medium 154 (Life Technologies #M-154-500). Organotypic regeneration of human epidermal tissue were performed as previously described (Truong et al., 2006). Biologic replicates were performed in all cases using primary cells from at least 3 independent unrelated donors.
Gene Transfer and Knockdown
Gene transfer by viral transduction was performed as described (Sen et al., 2010). shRNAs targeting ACTL6a were ordered from Openbiosystems. For siRNA knockdown, 1×106 cells were electroporated with 1 nmol siRNA using Amaxa Human Keratinocyte Nucleofector Kit (Lonza VPD-1002). For Brg1/Brm knockdown, either the ON-TARGETplus SMARTpool (Dharmacon) were used, or single ON-TARGETplus siRNA were used, including Brg1 Si A (J-010431-12, Dharmacon), Brg1 Si B (J-010431-09, Dharmacon), Brm Si A (J-017253-07, Dharmacon), and Brm Si B (J-017253-05, Dharmacon). For KLF4 siRNA, the ON-TARGETplus siRNA (D-005089-19, Dharmacon) were used.
Knockout Mice
ACTL6a targeted mice were generated as separately described (J.Tang, in preparation). Mouse toes or 3mm of tail tissue were cut with clean surgical scissors and heated in 75uL of Reagent A (25mM NaOH, 2mM EDTA) at 95°C for 1 hour. After cooling down to room temperature, tissue was mashed with pipette tip to aid the release of genomic DNA. 75uL of Reagent B (40mM of Tris-HCl, pH7.5) were then added to neutralize. 1uL of each tissue genomic DNA extraction was used in a 20uL PCR reaction for genotyping. Tamoxifen (T5648-1G, Sigma) were dissolved in 100% ethanol at the concentration of 0.5mg per 100uL. Mice were aged for least 40 days and their back hair was shaved prior to tamoxifen treatment. Each mouse receives 0.5 mg tamoxifen applied directly on their back skin each time for every two days, 3 times total.
Protein Expression and Tissue Analysis
For immunoblot analysis, 20-50 μg of cell lysates were loaded per lane for SDS-PAGE and transferred to PVDF membranes. For immunofluorescence staining, tissue sections (7 μm thick) were fixed using either 50% acetone and 50% methanol, or 4% formaldehyde. Primary antibodies were incubated at 4°C overnight and secondary antibodies were incubated at room temperature for 1 hour. The affinity purified antiserum against ACTL6a and the J1 antiserum against Brg1/Brm were raised by the Crabtree Lab. Other antibodies used in this study include: anti-BAF53a (Novus Biologicals), anti-Krt1 (Covance), anti-Krt10 (Neomarkers), anti-Ki67 (Neomarkers), anti-Loricrin (Covance), ms-anti-CollagenVII (Millipore), pAb-anti-CollagenVII (Calbiochem), goat-anti-CDSN (Santa Cruz), and rat-anti-Nidogen (Santa Cruz).
qRT-PCR Expression Analysis
For qRT-PCR, total RNA was extracted using the RNeasy Plus (Qiagen) and subsequently subjected to reverse transcription using SuperScript VILO cDNA synthesis kit (Invitrogen). qRT-PCR analysis was performed using the Mx3000P instrument with the SYBR Green Master Mix (Fermentas). Samples were run in duplicate and normalized to levels of GAPDH mRNA or 18S ribosomal RNA for each reaction. Primer sequences are listed in Supplemental Table S1.
mRNA Expression Profiling and Analysis
Amplification and labeling of cDNA probes and hybridization to the HG-U133 plus 2.0 microarray chip (Affymetrix) were performed by the Stanford PAN Facility. Data analysis was performed using R. Each data set for an experiment was filtered for probes that had an expression value ≥100 in at least 1 of the samples along with a p-value ≤ 0.05 based on SAM analysis. Pair-wise comparisons between the RNAi-treated samples and the control samples were performed to find probes that showed ≥ 2-fold expression change. Additional gene sets were acquired from GEO (GRHL3: GSE7381 (Yu et al., 2006); KLF4: GSE32685 (Sen et al., 2012)) or taken from supplementary tables for HOPX (Yang et al., 2010) and PRDM1 (Magnusdottir et al., 2007). Significant genes were identified as having greater than 2 fold change with p-value<0.05. Gene Set Enrichment Analysis (GSEA) was conducted using Genomica software with a p-value cutoff of 0.05.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed essentially as previously described (Euskirchen et al., 2011) with minor modifications. Human keratinocytes was cross-linked either with 1% formaldehyde alone, or with dual cross-linking of both 2mM DSG and 1% formaldehyde. The chromatin was sonicated to achieve fragments with an average length between 200-500 bp. The sonicated chromatin was immunoprecipitated overnight at 4°C with J1 antibody (Khavari et al., 1993), Poll II, or same amount of IgG control. Following reverse-cross-linking, the samples were treated with RNase and Protease K, and the DNA was purified using the Qiagen PCR Purification Kit. 2 μL ChIP product was used for each qPCR reaction.
Colony Formation Assay
Mouse fibroblast 3T3 cells were treated with 15 μg/mL mitomycin C (Sigma) in DMEM for 2 hours, then trypsinized and plated at 8×105 cells per well in a 6-well plate. The media was changed to KGM 24 hours after plating. 300 keratinocytes were seeded onto the feeder layer 24 hours after the media change. Media was changed every two days for 14 days. At the end of 14 days, the cells were washed with PBS to remove the 3T3 cells, then fixed in 1:1 acetone/methanol for 5 minutes. The plate was allowed to air dry for 3-5 minutes and then colonies were stained with crystal violet.
Skin Barrier Analysis
The skin barrier analysis was performed essentially as previously published (Scholl et al., 2007). In brief, live newborn mice or E17.5 embryos (dissected and washed in PBS) were incubated overnight at 37°C in 5-bromo-4-chloro-3-indlyl-β-D-galactopyranoside (X-gal) reaction mix (100 mM NaH2PO4, 1.3 mM MgCl2, 3 mM K3Fe[CN]6, 3 mM K4Fe[CN]6, and 1 mg/ml X-gal [pH 4.5]). Images were taken next morning after incubation.
Supplementary Material
Highlights.
ACTL6a is down regulated during epidermal differentiation.
ACTL6a loss in epidermis causes progenitor loss and terminal differentiation.
ACTL6a is required to repress KLF4 and other epidermal differentiation genes.
ACTL6a prevents binding of the SWI/SNF complex to differentiation genes.
Acknowledgments
We thank H Chang and A Oro for presubmission review, B Zarnegar for the FPLC fractions of undifferentiated human keratinocytes, D Johnston for technical support, and L Boxer for suggestions and reagents. We thank L Morcom and P Bernstein for administrative assistance. Profiling data has been deposited with GEO Accession Code GSE36222.This work is supported by the U.S. Department of Veterans Affairs Office of Research and Development, by NIAMS NIH R01 AR45192 (P.A.K), and by an F32 Award (AR061230) to X. B.
References
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- De S, Wurster AL, Precht P, Wood WH, 3rd, Becker KG, Pazin MJ. Dynamic BRG1 recruitment during T helper differentiation and activation reveals distal regulatory elements. Mol Cell Biol. 2011;31:1512–1527. doi: 10.1128/MCB.00920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debril MB, Gelman L, Fayard E, Annicotte JS, Rocchi S, Auwerx J. Transcription factors and nuclear receptors interact with the SWI/SNF complex through the BAF60c subunit. J Biol Chem. 2004;279:16677–16686. doi: 10.1074/jbc.M312288200. [DOI] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell I, Oda H, Blanco S, Nascimento E, Humphreys P, Frye M. The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin. EMBO J. 2011;31:616–629. doi: 10.1038/emboj.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euskirchen GM, Auerbach RK, Davidov E, Gianoulis TA, Zhong G, Rozowsky J, Bhardwaj N, Gerstein MB, Snyder M. Diverse roles and interactions of the SWI/SNF chromatin remodeling complex revealed using global approaches. PLoS Genet. 2011;7:e1002008. doi: 10.1371/journal.pgen.1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I. Actin and myosin as transcription factors. Curr Opin Genet Dev. 2006;16:191–196. doi: 10.1016/j.gde.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Indra AK, Dupe V, Bornert JM, Messaddeq N, Yaniv M, Mark M, Chambon P, Metzger D. Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation. Development. 2005;132:4533–4544. doi: 10.1242/dev.02019. [DOI] [PubMed] [Google Scholar]
- Jaubert J, Cheng J, Segre JA. Ectopic expression of Kruppel like factor 4 (Klf4) accelerates formation of the epidermal permeability barrier. Development. 2003;130:2767–2777. doi: 10.1242/dev.00477. [DOI] [PubMed] [Google Scholar]
- Khavari PA, Peterson CL, Tamkun JW, Mendel DB, Crabtree GR. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature. 1993;366:170–174. doi: 10.1038/366170a0. [DOI] [PubMed] [Google Scholar]
- Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, Nam JS, Kim H, Chung H, Lee HW, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21:7787–7795. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowenz-Leutz E, Leutz A. A C/EBP beta isoform recruits the SWI/SNF complex to activate myeloid genes. Mol Cell. 1999;4:735–743. doi: 10.1016/s1097-2765(00)80384-6. [DOI] [PubMed] [Google Scholar]
- Krasteva V, Buscarlet M, Diaz-Tellez A, Bernard MA, Crabtree GR, Lessard JA. The BAF53a subunit of SWI/SNF-like BAF complexes is essential for hemopoietic stem cell function. Blood. 2012 doi: 10.1182/blood-2012-04-427047. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Oma Y, Nishimori K, Ohta T, Harata M. Brain-specific expression of the nuclear actin-related protein ArpNalpha and its involvement in mammalian SWI/SNF chromatin remodeling complex. Biochem Biophys Res Commun. 2002;299:328–334. doi: 10.1016/s0006-291x(02)02637-2. [DOI] [PubMed] [Google Scholar]
- LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, Morrisey EE, Millar SE. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell. 2010;19:807–818. doi: 10.1016/j.devcel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chang SH, Shim JH, Lee JY, Yoshida M, Kwon H. Cytoplasmic localization and nucleo-cytoplasmic shuttling of BAF53, a component of chromatin-modifying complexes. Mol Cells. 2003;16:78–83. [PubMed] [Google Scholar]
- Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis NM, Morey L, Mejetta S, Pascual G, Janich P, Kuebler B, Cozutto L, Roma G, Nascimento E, Frye M, et al. Regulation of human epidermal stem cell proliferation and senescence requires polycomb-dependent and -independent functions of Cbx4. Cell Stem Cell. 2011;9:233–246. doi: 10.1016/j.stem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Magnusdottir E, Kalachikov S, Mizukoshi K, Savitsky D, Ishida-Yamamoto A, Panteleyev AA, Calame K. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc Natl Acad Sci U S A. 2007;104:14988–14993. doi: 10.1073/pnas.0707323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejetta S, Morey L, Pascual G, Kuebler B, Mysliwiec MR, Lee Y, Shiekhattar R, Di Croce L, Benitah SA. Jarid2 regulates mouse epidermal stem cell activation and differentiation. EMBO J. 2011;30:3635–3646. doi: 10.1038/emboj.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino G, Annicchiarico-Petruzzelli M, Piredda L, Candi E, Gentile V, Davies PJ, Piacentini M. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol. 1994;14:6584–6596. doi: 10.1128/mcb.14.10.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento EM, Cox CL, MacArthur S, Hussain S, Trotter M, Blanco S, Suraj M, Nichols J, Kubler B, Benitah SA, et al. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat Cell Biol. 2011;13:1395–1405. doi: 10.1038/ncb2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Watanabe M, Watanabe S, Sugimoto N, Yugawa T, Ikura T, Koiwai O, Kiyono T, Fujita M. Heterocomplex Formation by Arp4 and beta-Actin Involved in Integrity of the Brg1 Chromatin Remodeling Complex. J Cell Sci. 2012 doi: 10.1242/jcs.104349. [DOI] [PubMed] [Google Scholar]
- Olave I, Wang W, Xue Y, Kuo A, Crabtree GR. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes Dev. 2002;16:25092517. doi: 10.1101/gad.992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wood MA, Cole MD. BAF53 forms distinct nuclear complexes and functions as a critical c-Myc-interacting nuclear cofactor for oncogenic transformation. Mol Cell Biol. 2002;22:1307–1316. doi: 10.1128/mcb.22.5.1307-1316.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha) EMBO J. 1998;17:6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh N, Zhang X, Namour F, Fejer G, Wen YD, Yao YL, Gyory I, Wright K, Seto E. Targeted recruitment of a histone H4-specific methyltransferase by the transcription factor YY1. Genes Dev. 2003;17:1019–1029. doi: 10.1101/gad.1068003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- Salma N, Xiao H, Mueller E, Imbalzano AN. Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the peroxisome proliferator-activated receptor gamma nuclear hormone receptor. Mol Cell Biol. 2004;24:4651–4663. doi: 10.1128/MCB.24.11.4651-4663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Barragan DI, Harada K, Bissonauth V, Charron J, Khavari PA. Mek1/2 MAPK kinases are essential for Mammalian development, homeostasis, and Raf-induced hyperplasia. Dev Cell. 2007;12:615–629. doi: 10.1016/j.devcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Sen GL, Boxer LD, Webster DE, Bussat RT, Qu K, Zarnegar BJ, Johnson D, Siprashvili Z, Khavari PA. ZNF750 Is a p63 Target Gene that Induces KLF4 to Drive Terminal Epidermal Differentiation. Dev Cell. 2012 doi: 10.1016/j.devcel.2011.12.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tea JS, Luo L. The chromatin remodeling factor Bap55 functions through the TIP60 complex to regulate olfactory projection neuron dendrite targeting. Neural Dev. 2011;6:5. doi: 10.1186/1749-8104-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangapandu H, Ai W. Kruppel like factor 4 (KLF4): a transcription factor with diverse context-dependent functions. Gene Ther Mol Biol. 2009;13:194–204. [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa N, Percipalle P. Nuclear functions of actin. Cold Spring Harb Perspect Biol. 2010;2:a000620. doi: 10.1101/cshperspect.a000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugde S, Ferrai C, Miluzio A, Hauben E, Marchisio PC, Crippa MP, Bussi M, Biffo S. Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol Cell. 2006;23:749–755. doi: 10.1016/j.molcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Sim SM, Kim HY, Park GT. Expression of the homeobox gene, HOPX, is modulated by cell differentiation in human keratinocytes and is involved in the expression of differentiation markers. Eur J Cell Biol. 2010;89:537–546. doi: 10.1016/j.ejcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Ye J, Zhao J, Hoffmann-Rohrer U, Grummt I. Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes Dev. 2008;22:322–330. doi: 10.1101/gad.455908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–646. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Lin KK, Bhandari A, Spencer JA, Xu X, Wang N, Lu Z, Gill GN, Roop DR, Wertz P, et al. The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev Biol. 2006;299:122–136. doi: 10.1016/j.ydbio.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, Crabtree GR. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell. 1998;95:625–636. doi: 10.1016/s0092-8674(00)81633-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.