Abstract
Objectives
To investigate grape seed extract proanthocyanidins’ (PA) capability in improving dentin collagen’s sustainability in an enzymatic environment, given that the size and shape of the collagen samples, and the manner to apply PA are both clinically relevant.
Methods
Human dentin was sectioned into 6-μm-thick films. After demineralization in 35 wt% phosphoric acid for 15 s, the films were subject to 30 s of treatment at PA concentrations of 0% (control), 0.5%, 1%, 2%, 3.75%, 7.5% and 15% (w/w), respectively. The films were then digested in 0.1 wt% collagenase for 1 h and 24 h. The amount of degraded collagen in the liquid digests was determined by MALDI-TOF mass spectroscopy. The trend of PA’s incorporation into dentin collagen was analyzed by ATR-FTIR.
Results
The control exhibited complete digestion in 1 h. In contrast, collagen treated with 0.5% and 1% PA afforded 13.84 ± 4.69% and an undetectable level of degradation, respectively in the first 1 h of digestion, and additional 17.48 ± 4.38% and 4.50 ± 1.68%, respectively in the following 23 h. Collagen treated with ≥2 wt% PA was not significantly digested regardless of digestion time. FTIR spectroscopy revealed that PA incorporation was saturated at ≥2 wt% PA.
Conclusion
Thirty seconds of PA treatment at 2 wt% and above could provide optimal protection for dentin collagen against collagenase digestion.
Clinical significance
This study demonstrated PA’s extraordinary efficiency in stabilizing demineralized dentin collagen when it is applied in a clinical relevant manner, and identified the optimal conditions for its utilization.
Keywords: Dentin collagen, Proanthocyanidins, Collagenase digestion, MALDI-TOF, ATR-FTIR
1. INTRODUCTION
Contemporary composite restorations rely on dental adhesives to promote proper bonding between the filling material and underlying tooth structure. Although current adhesives generally deliver a satisfactory result in immediate bonding strength, the bonding’s long-term stability is still subpar compared to traditional amalgam restorations.1 In particular, the durability of adhesive-dentin bonding was found to be inferior to that of adhesive-enamel bonding.2 The vulnerability of dentin bonding results from the instability of the very structure that provides for the adhesives’ bonding force: the adhesive/dentin hybrid layer.3 The creation of this hybrid layer requires the demineralization of dentin collagen matrix to enable the infiltration of resin monomers. However, because of such factors as imperfect resin infiltration and incomplete monomer conversion, the demineralized collagen fibrils cannot be reverted to the fully-encapsulated state by resin later upon light-curing.4, 5 As a result, collagen fibrils in the hybrid layer are partially exposed and susceptible to hydrolytic and enzymatic challenges. Moreover, the commonly-used self-etching adhesives were found to activate the collagenolytic and gelatinolytic activities of host-derived matrix metalloproteinases (MMPs),6 which further increases the risk of collagen degradation in the hybrid layer.
In light of this, it should come as no surprise that researchers have put a lot of efforts into finding the best method to enhance collagen’s longevity in an enzymatic environment. Generally speaking, the investigated approaches address the issue either from the perspective of collagen itself, or from the perspective of the environment. The former approach seeks to introduce auxiliary cross-links to the collagen matrix and improve its biological, thermal and mechanical tenacity as a result. In this regard, a number of synthetic or naturally-occurring cross-linking agents such as glutaraldehyde, genipin and grape seed extract proanthocyanidins (PA) have been found to be quite efficient.7–10 The latter approach, on the other hand, attempts to lower the hostility of the environment and thus enhance collagen’s sustainability, through the inhibition of the collagen-degrading enzymes. For example, chlorhexidine, an MMP inhibitor could deter the degradation of hybrid layer in vivo if applied to tooth cavity after acid-etching.11, 12 In another instance, quaternary ammonium compounds were found to inhibit MMPs as effectively as chlorhexidine while having the potential to be incorporated in the monomeric structure of adhesive resins.13 It is especially noteworthy that some small polyphenolic compounds such as catechin, epicatechin gallate and epigallocatechin gallate have also exhibited various degrees of collagenase-inhibiting capabilities.14, 15 Considering these molecules are the building blocks of PA, PA therefore could potentially increase collagen’s lifetime against enzymatic erosion via both approaches synergistically.
The direct inclusion of PA in current adhesive systems, however, suffers from a hampered degree of polymerization of the resin16–18 due to the radical-scavenging ability of PA.19 Moreover, if the water-soluble PA was incorporated directly, its eventual release could pose additional threat to the integrity of the entire adhesive layer. Consequently, the alternative use of PA, i.e. as a priming agent (especially, water rinse after priming) seems to be a more favorable approach. In a respective, qualitative study that was done earlier we have revealed that PA is strikingly effective in improving the acid-etched dentin’s stability against collagenase degradation following only tens of seconds of treatment (unpublished data). In more detail, the demineralized collagen, when pre-treated with PA for 15 – 30 s, could withstand 24 h of collagenase digestion without any perceivable changes in collagen’s fibrillar structure and characteristic banding pattern. In contrast, the untreated control and glutaraldehyde-treated counterpart were completely consumed after 1 h and 16 h of digestion, respectively. It was reasoned that the extraordinary efficiency of PA resulted from the non-covalent nature of its interaction with collagen, and the diminished influence of the rate-limiting factor of diffusion due to the minute thickness of the demineralized collagen layer.
With these previous findings in mind, it becomes a natural topic of interest to quantitatively determine PA’s effect in stabilizing dentin collagen in a clinically relevant manner. In the present study, we used a mass spectroscopic method to evaluate ultra-thin (6 μm) dentin films’ digestion behavior after they were acid-etched and then subject to 30 s of treatment with grape seed extract PA at various concentrations. The conditions of enzymatic challenge including collagenase concentration (0.1 wt%) and digestion time (1 h and 24 h) were selected based on pilot studies so that the dentin films, either untreated or treated at comparable conditions with glutaraldehyde, showed complete degradation. In addition, the FTIR spectroscopy was employed to analyze the amount of incorporated PA with respective to the concentration of treatment solutions. The null hypothesis is that the fraction of dentin collagen susceptible to collagenase digestion is not affected by the concentration of the PA solution that was used to treat it.
2. MATERIALS AND METHODS
2.1 Reagents
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), including collagenase type I (Clostridiopeptidase A from Clostridium histolyticum, 125 U/mg). This also holds true for the matrix materials for matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, including 2,5-dihydroxybenzoic acid (DHB) and trifluoroacetic acid (TFA), which were of the highest purity grade. The powdered grape seed extract (containing > 90% PA) was generously donated by MegaNatural (Madera, CA, USA).
2.2 Dentin film preparation
Non-carious human molars were collected after obtaining the patients’ informed consent under a protocol approved by the University of Missouri-Kansas City Adult Health Sciences Institutional Review Board. Extracted teeth were stored at 4 °C in 0.96% (w/v) phosphate buffered saline (PBS) containing 0.002% sodium azide. A water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL) was used to remove the occusal one-third to one-half of the crown, followed by four additional cuts in the occusal-apical direction to remove all side walls of the enamel. The resultant dentin block was sectioned in the mesial-distal direction, with a tungsten carbide knife mounted on an SM2500S microtome (Leica, Deerfield, IL), into dentin films 6 μm thick. The final size of each film was approximately 5 mm × 5 mm.
2.3 Proanthocyanidins (PA) and collagenase solution preparation
PA solutions at selected concentrations (0.5%, 1%, 2%, 3.75%, 7.5% and 15% w/w) were prepared by adding the powdered grape seed extract to deionized water. For the collagenase solution, TESCA buffer was first made by the addition of N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (11.5 g), sodium azide (50 mg) and calcium chloride dihydrate (53 mg) into distilled water to a total volume of 1000 mL, followed by pH adjustment to 7.4. Then collagenase (1 g) was dissolved in the TESCA buffer to a final concentration of 0.1% (w/v).
2.4 Demineralization and PA treatment
Each dentin film was demineralized with 35 wt% phosphoric acid for 15 s, rinsed in deionized water for 10 s, and then spread on a plastic cover slip (Fisher Scientific, Pittsburgh, PA, USA). With the excessive water blotted way, the demineralized film (while still moist) was immediately immersed in a small drop (approx. 0.2 ml) of selected PA solution or deionized water (control). After treatment for 30 s, the excessive PA solution was thoroughly rinsed off with deionized water, and the film was air dried prior to the following collagenase digestion and MALDI-TOF experiments.
2.5 Collagenase digestion and MALDI-TOF analysis
Each PA-treated film was first subject to 1 h of degradation in 30 μl of collagenase solution at 37 °C. The resultant digest was saved, and the remnant film was removed from the digest, rinsed with copious amount of deionized water, and blot dried. A fresh 30 μl of collagenase solution was then added to digest the remnant film for an additional period of 23 h, after which the digest was collected again. The amount of degraded collagen in the two digests was determined by a method as described by Nimptsch et al.,20 and all experiments were performed in pentaplicate following a procedure customized as follows.
First, to obtain the standard curve, six demineralized but PA-free films were digested for 1 h in the same way as their PA-treated counterparts, except that the volume of collagenase solution was 30 μl, 40 μl, 60 μl, 120 μl, 240 μl and 1920 μl, respectively. Meanwhile, a collagenase blank (with no dentin film in it) also went through the digestion process as if there were collagen film in it. Since all the films were completely digested in 1 h, this resulted in standard digests with degraded collagen at 100%, 75%, 50%, 25%, 12.5%, 1.56% and 0% per 30 μl of collagenase, respectively. For each standard digest, 8 μl of the liquid was transferred to an Eppendorf vial, and 4 μl of Arg-Gly-Asp tripeptide solution (0.25 mg/ml) was added to the vial and thoroughly mixed with the digest liquid. Positive ion detection matrix was prepared by dissolving DHB in acetonitrile/water (60/40, v/v) containing 0.2% TFA to a final concentration of 20 mg/ml. After introducing the DHB matrix into the digest/tripeptide mixture at a 2:1 volume ratio, 1 μl of the resultant solution was spotted on the target for subsequent mass spectroscopic (MS) analysis using a Voyager DE Pro MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA, USA). For each MS spectrum, the peak intensities at mass-to-charge (m/z) ratios of 329.2 and 351.2 were summed (IGPR), representing the Gly-Pro-Arg tripeptide from degraded collagen. The same was done for the peaks at 347.2 and 369.2 (IRGD), representing the Arg-Gly-Asp tripeptide internal standard. The intensity ratios (IGPR/IRGD) of the standard digests were plotted against their corresponding percentage of degraded collagen, and the standard curve was then obtained by the least-square curve fitting.
Next, the amount of degraded collagen in the sample digests collected from the PA-treated dentin films was calculated, based on the standard curve, with their respective IGPR/IRGD ratios acquired in the same way as the standard digests.
2.6 FTIR spectroscopy
FTIR spectra of dentin films were collected before demineralization, before PA treatment, and after PA treatment, at a resolution of 4 cm−1 with a Fourier transformed infrared (FTIR) spectrometer equipped with an attenuated total reflectance (ATR) attachment (Spectrum One, Perkin-Elmer, Waltham, MA, USA). The ATR crystal was diamond with a transmission range between 650 and 4000 cm−1, and a gauge force of 75 was applied to ensure a good contact between the films and the ATR top-plate. The band intensities at 1235 cm−1 (amide III) and 1450 cm−1 (CH2 scissoring) were determined following a two-point baseline correction and band area integration, and the band ratio (A1450/A1235) was calculated.
2.7 Statistical analysis
Data were expressed as means ± standard deviation. Comparisons of IGPR/IRGD and A1450/A1235 ratios between groups were analyzed by one-way ANOVA and Turkey’s post hoc test with 95% confidence level.
3 RESULTS
3.1 MALDI-TOF analysis
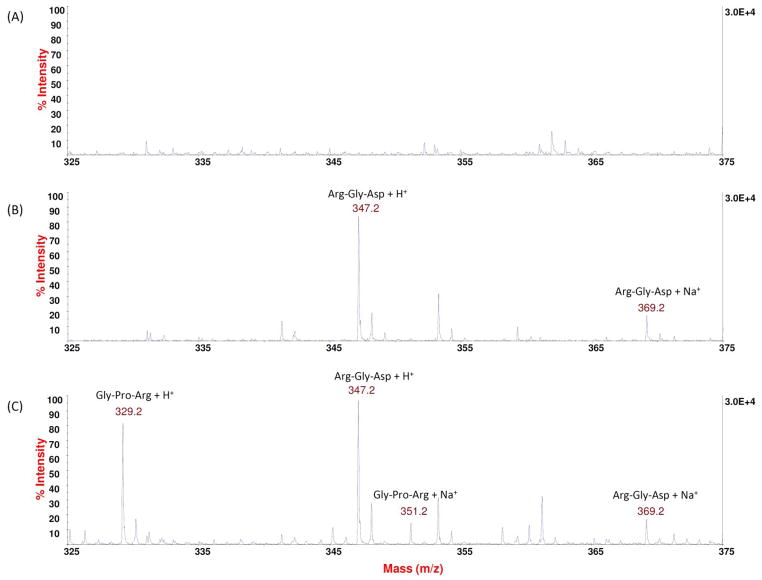
The MS spectrum of collagenase solution (Figure 1A) had no prominent peaks in the m/z range of 325 – 375. In contrast, the spectrum of the standard blank (Figure 1B), which consisted of collagenase solution and the added Arg-Gly-Asp internal standard, displayed two pronounced peaks at m/z ratios of 347.2 and 369.2, corresponding to the H+ and Na+ adduct of the internal standard, respectively. Further, when digested collagen was present, as seen in the representative spectrum of standard digest (Figure 1C), the traces at m/z ratios of 329.2 and 351.2 were evident, which were attributed to the Gly-Pro-Arg tripeptide segment that was resulted from the enzymatic breakdown of collagen molecules.
Figure 1.
Representative MS spectra (m/z range: 325 – 375) of (A) collagenase solution only; (B) standard blank which contains collagenase and the Arg-Gly-Asp tripeptide internal standard; and (C) standard digest which contains collagenase, the tripeptide internal standard, and degraded collagen.
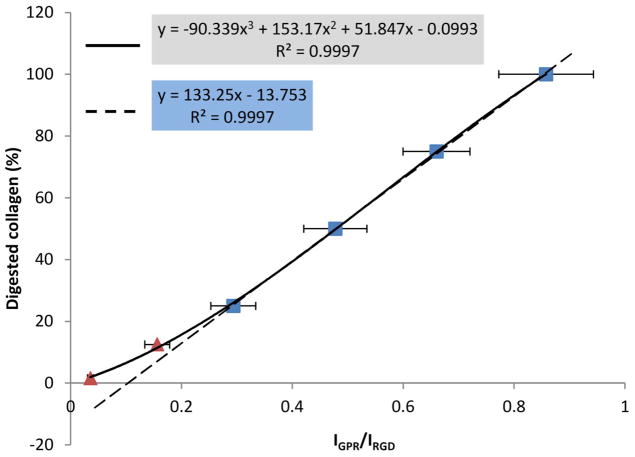
The IGPR/IRGD ratios were significantly different among the standard digests (Table 1) with one exception. Namely, the IGPR/IRGD ratio of the blank was statistically identical to that of the digest with 1.56% of film consumed (p = 1). This finding suggests that the low limit of detectable digested collagen was approximately 1.56%, or 1/64 pertaining to the experimental conditions of the present study. Accordingly, the blank digest was not included in the standard curve fitting. An almost perfectly linear standard curve could be obtained when plotting the higher amount of digested collagen (25%, 50%, 75% and 100%) against their respective IGPR/IRGD ratios (R2 = 0.9997, dashed line in Figure 2), but the line deviated from the IGPR/IRGD ratios of the digest containing less degraded collagen (12.5% and 1.56%). As a result, a third-order polynomial curve was used to fit all data points (R2 = 0.9997, solid line in Figure 2).
Table 1.
The IGPR/IRGD ratios of the standard digests and blank (n=5).
| Digested (%)
|
IGPR/IRGD
|
|---|---|
| 0 | 0.0389 ± 0.0116a |
| 1.56 | 0.0358 ± 0.0052a |
| 12.5 | 0.1562 ± 0.0225b |
| 25 | 0.2935 ± 0.0405c |
| 50 | 0.4776 ± 0.0570d |
| 75 | 0.6601 ± 0.0603e |
| 100 | 0.8578 ± 0.0853f |
Values labeled with the same letters are statistically equivalent.
Figure 2.
Standard curves and their corresponding standard equations (n=5). Dashed line: linear fitting for the standard digests containing more than 25% of digested collagen. Solid line: third-order polynomial fitting for all data points.
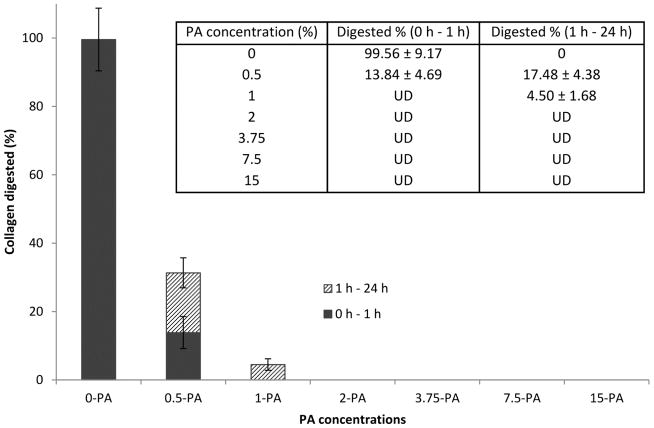
The amount of degraded collagen in the sample digests was calculated by plugging their respective IGPR/IRGD ratio in the standard equation. If a sample digest’s IGPR/IRGD ratio was not significantly higher than that of the low-limit standard digest mentioned above, its corresponding amount of dissolved collagen was marked as “UD”, or undetectable. As seen in Figure 3, the untreated control was completely consumed after 1 h of digestion. In contrast, by treating the dentin film with PA solution at the lowest concentration studied (0.5 wt%) for 30 s, the fraction of decomposed collagen fell drastically to around 13.84% after 1 h of digestion, followed by an additional 17.48% if the digestion was extended to 24 h. When the PA concentration was increased further to 1 wt%, the degree of degradation fell to an undetectable level in 1 h, and to approximately 4.5% in the next 23 h. Finally, by treating the dentin film with PA solution concentrated at 2 wt% and above for 30 s, the collagenase challenge did not afford any detectable level of decomposed collagen regardless of digestion time.
Figure 3.
Degree of degradation of the dentin films in the initial 1 h and the following 23 h of digestion (n=5).
3.2 FTIR analysis
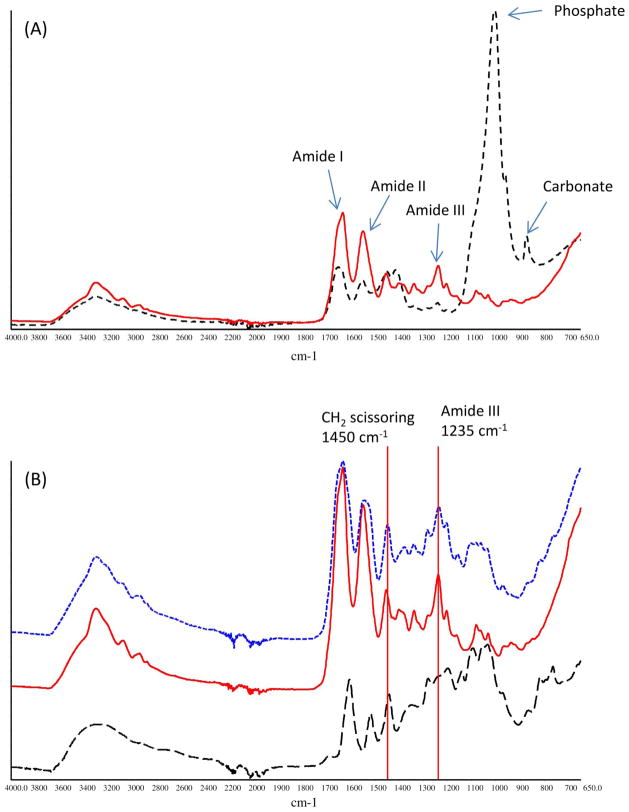
Representative FTIR spectrum of un-demineralized dentin film was shown in Figure 4A (black dashed line). Bands characteristic of collagen matrix as well as hydroxyapatite mineral were identified and assigned as have been well documented in the literature,21–23 including C=O stretching at ~1633 cm−1 for the amide I, out-of-phase combination of N-H bending and C-N stretching at ~1544 cm−1 for the amide II, in-phase combination of N-H bending and C-N stretching at ~1235 cm−1 for the amide III, υ3 vibration at ~1005 cm−1 for phosphate ion PO43−, and υ2 vibration at ~872 cm-1 for carbonate ion CO32−. After the dentin films were treated with phosphoric acid for 15 s, the bands due to the collagen content remained intact, whereas those due to the mineral content including PO43− at ~1005 cm−1 and CO32− at ~872 cm−1 vanished entirely (Figure 4A, red solid line), indicating the complete demineralization of dentin films. Following PA treatment, collagen’s amide III band at ~1235 cm−1 (Figure 4B, blue dotted line) was hardly augmented by the signals of PA (Figure 4B, black dashed line) because pure PA’s band intensity at the nearby wave numbers is almost non-existent. In contrast, collagen’s band at ~1450 cm−1, which is assigned to the scissoring mode of CH2 groups, was greatly enhanced by the CH2 groups of PA. As a result, the band ratio (A1450/A1235) was found to be positively correlated to the concentration of PA that was used to treat the collagen (Figure 5). More specifically, the ratio significantly increased from about unity (when no PA treatment was applied) to ~2.5 (when PA concentration was 2 wt%), and then did not significantly change as more concentrated PA was used.
Figure 4.
ATR-FTIR spectra of (A) un-demineralized dentin (black dashed line) and demineralized dentin (red solid line); (B) PA (black dashed line), demineralized dentin before PA treatment (red solid line) and after treatment (blue dotted line).
Figure 5.
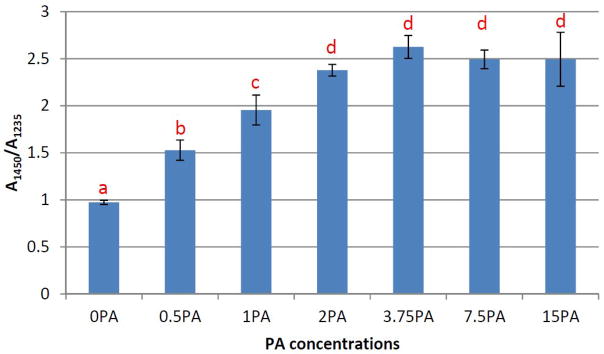
The band ratio between ~1450 cm−1 and ~1235 cm−1 for demineralized dentin films treated with PA at various concentrations (n=5). Bars labeled with the same letters are statistically equivalent.
4 DISCUSSION
The null hypothesis that the fraction of dentin collagen susceptible to collagenase digestion is not affected by the concentration of the PA solution has to be rejected. To the best of the author’s knowledge, the present study is the first quantitative investigation of PA’s efficacy in enhancing collagen’s enzymatic stability that meets the following two criteria: (1) the treatment time is as short as 30 s, and (2) the interference due to PA’s incomplete diffusion into the sample is suppressed to a large extent. These criteria ensure the result from the study has practical meaning, as the clinical setting is exactly a situation where the treatment time has to be short, and PA’s diffusion into the demineralized collagen layer plays a minimal role due to the minute thickness of the layer.
When the treatment time is so short, the form/shape factor of the samples must be selected with caution. A sample with too small of a surface area with respective to its volume would exaggerate the effect of PA’s diffusion into the sample, and thus skew the experimental result. Thus, the option to process dentin into slabs is ruled out. Another common choice, i.e. to grind dentin into fine powder would require the specimen to undergo extremely low temperature and then massive mechanical stress (and local heat along with it), which at least brings uncertain consequences to the structure of collagen. In comparison, dentin films with thickness at the micron scale can be easily obtained at room temperature with relatively low stress, and more importantly, they can best mimic the dimension and morphology of the demineralized collagen layer in a clinical situation. Therefore, we chose to section dentin into films 6 μm thick.
When it comes to the analysis of collagen’s resistance toward enzymatic digestion, two methods are the most frequently cited in the literature: the gravimetric method and the fluorometric method, or hydroxyproline (HYP) assay. The gravimetric method determines the ratio of a sample’s residual weight with respect to its original weight, but the mass of a dentin film is too little to afford an accurate measurement. The HYP assay, on the other hand, gauges the amount of degraded collagen in the digest liquid, by monitoring the absorbance of a red chromophore at ~550 nm which is produced by a series of transformation of the hydroxyproline content of the digested collagen.24, 25 However, when grape seed extract PA is present, the validity of the HYP assay also becomes questionable. As PA is a red substance as well, its presence could severely interfere with the absorbance of the red chromophore, distorting the assay result.
Finally, we resorted to a novel mass spectroscopic method as proposed by Nimptsch et al.20 This technique quantifies the amount of degraded collagen in the sample digest, by monitoring the strength of the MS signals attributed to the tripeptide product of the bacterialcollagenolytic digestion of collagen (IGPR), and comparing it to the strength of signals due to an added tripeptide internal standard (IRGD) at a pre-selected concentration. As seen in Figure 1, the peaks of the Gly-Pro-Arg tripeptide from collagen’s degradation, including the H+-adduct at m/z ratio of 329.2 and Na+-adduct at m/z ratio of 351.2, do not overlap with the peaks of the Arg-Gly-Asp internal standard, including the H+-adduct at m/z ratio of 347.2 and Na+-adduct at 369.2. In addition, neither set of peaks could be identified in the spectrum of collagenase solution. Therefore, it is appropriate to evaluate the amount of digested collagen using the signals of the Gly-Pro-Arg tripeptide.
The rigorousness of the MS method can be seen from the exceptionally high correlation coefficient (R2 = 0.9997) when a linear regression is used to fit the standard digests containing > 25% of degraded collagen (Figure 2). When the amount of degraded collagen is lower, however, the experimental IGPR/IRGD ratios become deviated from the values predicted by the linear fit, suggesting the noises from the DHB matrix and collagenase solution start to interfere with the signals of the Gly-Pro-Arg tripeptide. The low-limit of detectable digested collagen is found to be around 1.56%, in which case the IGPR/IRGD ratio of the standard digest becomes statistically equivalent to the blank (Table 1). It is noteworthy that this low limit is pertaining to the experimental conditions of a particular study, and therefore may be different from one occasion to another. Measures such as reducing the volume of digestion media, increasing the film size or the number of films in the digestion media can effectively enhance the signal-to-noise ratio of the tripeptide of interest, and thus increase the sensitivity of the technique. In the end, a third-order polynomial standard equation is derived to incorporate all data points with a detectable level of digested collagen (Figure 2). Using the polynomial standard equation, the amount of PA-treated collagen that is consumed in the initial 1 h of digestion and the following 23 h of digestion is shown in Figure 3. Obviously, the treatment with PA significantly and substantially improved collagen’s sustainability under collagenolytic stress. An increased concentration of PA leads to a decreased degree of collagen degradation, and this stabilizing effect levels off when the concentration of PA reaches 2 wt%, at which point nearly 100% of the collagen is preserved regardless of digestion time. These results suggest that the optimal concentration of PA, when it is used as a priming agent, should be 2 wt% and above.
Additionally, the extent of PA’s incorporation in the demineralized collagen is analyzed by ATR-FTIR. The two bands of interest, ~1235 cm−1 (amide III) and ~1450 cm−1 (CH2 scissoring) (Figure 4B) were actually often cited in the literature to study collagen’s triple helical structure. More specifically, a band ratio (A1450/A1235) of unity indicates an intact triple helical structure, whereas a ratio around 2 suggests the triple helix is destroyed like gelatin 26. In our case, there is no reason to believe the collagen-stabilizing PA disrupts collagen’s triple helical structure. Therefore, the increase in the A1450/A1235 band ratio that is observed in PA-treated samples (Figure 5) must be due to the fact that PA significantly contributes to the band at ~1450 cm−1 but not amide III (Figure 4B). Consequently, the A1450/A1235 ratio can reflect the amount of PA incorporated in the demineralized dentin films. As seen in Figure 5, the trend of the degree of PA incorporation features a sigmoid pattern, with the turning point at 2 wt%. This result matches very well with the digestion study discussed earlier, and further verifies that a concentration of 2 wt% and above would be appropriate when using PA as a priming agent.
The results of the present work suggest that a saturated incorporation of PA into ultra-thin demineralized dentin collagen films can be achieved in 30 s, on the condition that the treatment solution is at a concentration of 2 wt% or above. Such a quick saturation was never reported before. For example, Ku et al. found that the equilibrium adsorption of PA onto bovine achilles tendon collagen was not reached until after 285 min when the PA concentration was 0.01 –0.04%.27 In another instance, Castellan et al. showed that the mechanical strength of demineralized dentin slabs was reinforced by 10 min of treatment with 6.5% PA, and could be further reinforced if the treatment time was elongated to 60 min,8 indicating the absence of a saturated PA incorporation within 10 min. Similarly, Liu et al. revealed that demineralized dentin slabs continuously gained weight when being immersed in 3.75% PA for 10 s – 720 min.10 According to our study presented here, the apparent inability to reach a state of PA saturation in a shorter time, as shown in these studies, is due to the slow diffusion rate of PA into the samples instead of a slow reaction rate between collagen and PA. Indeed, these previously-published reports either used collagen particles with 20 – 40 mesh size (400 – 841 μm),27 or processed dentin into slabs with thickness in the millimeter range,8, 10 amplifying the influence of diffusion.
5 CONCLUSION
This study quantified PA’s extraordinary efficiency in stabilizing collagen against enzymatic digestion when the restriction of diffusion was lifted. It was established that the treatment of 2 wt% or more concentrated PA for 30 s was enough to render the demineralized dentin collagen protected from degradation. In our future plans, we would like to apply PA in a primer style at the optimal conditions discovered in this report, and we are interested to see if a more collagenolytically defiant collagen layer translates to a more stable dentin bonding in the long term by examining the bonding strength over time.
Acknowledgments
This investigation was supported in part by USPHS Research Grants R15-DE021023 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: Methods and results. Journal of Dental Research. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 2.Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RHM, et al. Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Operative Dentistry. 2008;33:79–88. doi: 10.2341/07-42. [DOI] [PubMed] [Google Scholar]
- 3.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. Journal of Biomedical Materials Research. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. Journal of Biomedical Materials Research. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. Journal of Dental Research. 2003;82:141–5. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 6.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. European Journal of Oral Sciences. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Bedran-Russo AKB, Pereira PNR, Duarte WR, Drummond JL, Yamaychi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2007;80:268–72. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 8.Castellan CS, Pereira PN, Grande RHM, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dental Materials. 2010;26:968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Wang Y. Cross-linked demineralized dentin maintains its mechanical stability when challenged by bacterial collagenase. Journal of Biomedical Materials Research -Part B Applied Biomaterials. 2011;96 B:242–8. doi: 10.1002/jbm.b.31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dental Materials. 2013 doi: 10.1016/j.dental.2013.01.013. http://dx.doi.org/10.1016/j.dental.2013.01.013. [DOI] [PMC free article] [PubMed]
- 11.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 12.Carrilho MRO, Geraldeli S, Tay F, De Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 13.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. Journal of Dental Research. 2011;90:535–40. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochimica et Biophysica Acta - Protein Structure and Molecular Enzymology. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 15.Madhan B, Krishnamoorthy G, Rao JR, Nair BU. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. International Journal of Biological Macromolecules. 2007;41:16–22. doi: 10.1016/j.ijbiomac.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, et al. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. Journal of Dentistry. 2010;38:908–15. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hechler B, Yao X, Wang Y. Proanthocyanidins alter adhesive/dentin bonding strengths when included in a bonding system. American Journal of Dentistry. 2012;25:276–80. [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Wang Y. Effect of proanthocyanidins and photo-initiators on photo-polymerization of a dental adhesive. Journal of Dentistry. 2012;41:71–9. doi: 10.1016/j.jdent.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewandte Chemie - International Edition. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 20.Nimptsch A, Schibur S, Ihling C, Sinz A, Riemer T, Huster D, et al. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell and Tissue Research. 2011;343:605–17. doi: 10.1007/s00441-010-1113-2. [DOI] [PubMed] [Google Scholar]
- 21.Barth A, Zscherp C. What vibrations tell us about proteins. Quarterly Reviews of Biophysics. 2002;35:369–430. doi: 10.1017/s0033583502003815. [DOI] [PubMed] [Google Scholar]
- 22.Arai Y, Sparks DL. ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. Journal of Colloid and Interface Science. 2001;241:317–26. [Google Scholar]
- 23.Antonakos A, Liarokapis E, Leventouri T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials. 2007;28:3043–54. doi: 10.1016/j.biomaterials.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Analytical Biochemistry. 1981;112:70–5. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clinical Biochemistry. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 26.Sylvester MF, Yannas IV, Salzman EW, Forbes MJ. Collagen banded fibril structure and the collagen-platelet reaction. Thrombosis Research. 1989;55:135–48. doi: 10.1016/0049-3848(89)90463-5. [DOI] [PubMed] [Google Scholar]
- 27.Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007;67:1618–27. doi: 10.1016/j.chemosphere.2006.11.037. [DOI] [PubMed] [Google Scholar]