Abstract
Objective
We demonstrated that inflammatory cells and intima-media thickening (IMT) are increased in carotids exposed to low blood flow in the SJL/J (SJL) strain compared to other mouse strains. We hypothesized that the extent of inflammation associated with IMT is a genetically regulated trait.
Approach and Results
We performed a whole genome approach to measure leukocyte infiltration in the carotid intima as a quantitative trait in a genetic cross between C3HeB/FeJ (C3H/F) and SJL mice. Immunostaining for CD45+ (a pan-specific leukocyte marker) was performed on carotids from C3H/F, SJL, F1 and N2 progeny to measure leukocyte infiltration. We identified a nearly significant QTL for CD45+ on chromosome (chr) 11 (17cM, LOD=2.3; significance was considered at threshold P=0.05). Interval mapping showed that the CD45+ locus on chr11 accounted for 8% of the variation in the C3H/FxSJL backcross. Importantly, the CD45+ locus co-localized with the intima-modifier 2 (Im2) locus, which controls 17% of intima variation. We created two Im2 congenic lines of mice (C3H/F.SJL.11.1 and C3H/F.SJL.11.2) to better understand the regulation of IMT by the chr11 locus. The C3H/F.SJL.11.1 congenic mouse showed ~30% of the SJL trait confirming that CD45+ cell infiltration contributed to the intima trait.
Conclusions
We discovered a novel locus on chromosome 11 that controls leukocyte infiltration in the carotid. Importantly this locus overlaps with our previously published Im2 locus on chr11. Our study reveals a potential mechanistic relationship between leukocyte infiltration and IMT in response to decreased blood flow.
Keywords: genetics, SJL/J, C3HeB/FeJ, leukocytes, congenic
Introduction
In response to variations in hemodynamic parameters the vessel wall remodels to compensate for changes in blood flow1–4. An important clinical measurement of vascular remodeling is carotid intima-media thickening (IMT), which is associated with increased cardiovascular risks such as stroke, myocardial infarction and peripheral vascular disease5. Carotid IMT is a complex trait determined by genetic factors. Individuals with functional variations in genes associated with matrix deposition, inflammation and lipid metabolism have a greater risk for carotid IMT6. Genetic linkage analyses have provided insight into genes that regulate IMT7–9. In the Framingham Heart Study a quantitative trait locus (QTL) was identified for internal carotid IMT on human chromosome (chr) 12 (LOD = 3.4)9. More recently, Wang et al reported QTLs for IMT in a study of Dominican families on chr’s 7 and 14, which houses a primary gene candidate, proline-rich membrane anchor 1 (PRiMA1)8. Genetic studies of IMT in animals have also been reported10, 11. Our group published a cross of C3HeB/FeJ (C3H/F) and SJL/J (SJL) mice that identified three intima modifier (Im) QTLs in response to decreased blood flow on chr’s 2, 11 and 18 termed Im1, Im2 and Im3, respectively11.
There are reported differences in immune response to various inflammatory stimuli between C3H/F and SJL mice12–15. We have shown that in response to low blood flow SJL mice develop significantly greater IMT accompanied by increased leukocyte infiltration in comparison to C3H/HeJ (C3H/H), C3H/F and FVB/NJ mice12. This suggests there is genetic regulation of IMT that is partially driven by inflammation. In the current study we utilized our previously characterized C3H/FxSJL cross to better understand the genetic regulation of inflammatory cell infiltration during vascular remodeling. Our goal was to identify a genomic locus which houses candidate genes that affect the inflammatory response during carotid IMT in response to low flow.
Results
Quantitative CD45+ and α1-actin+ Immunostaining in C3H/FxSJL N2 Progeny
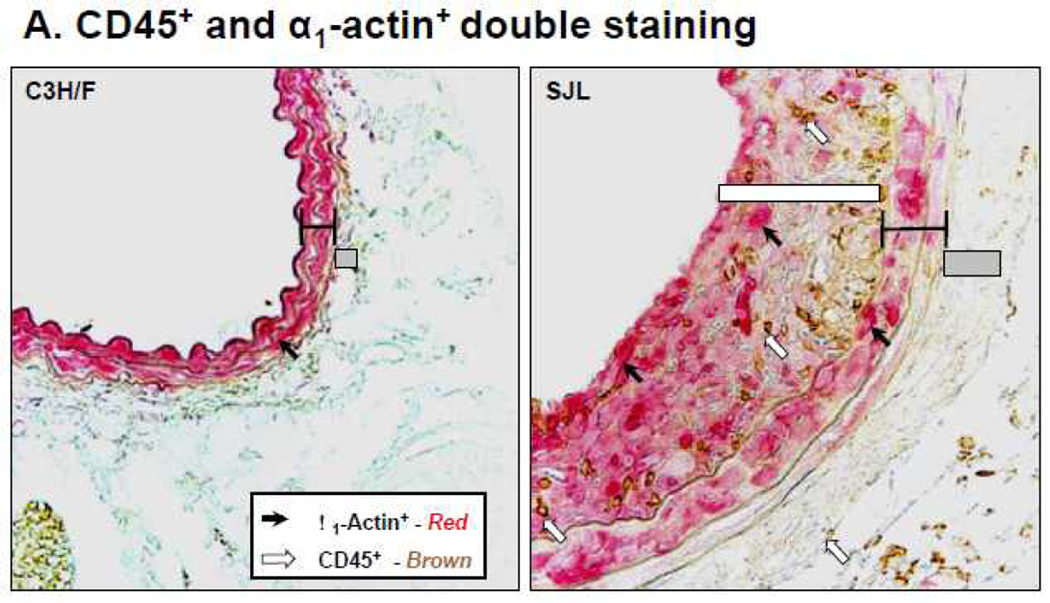
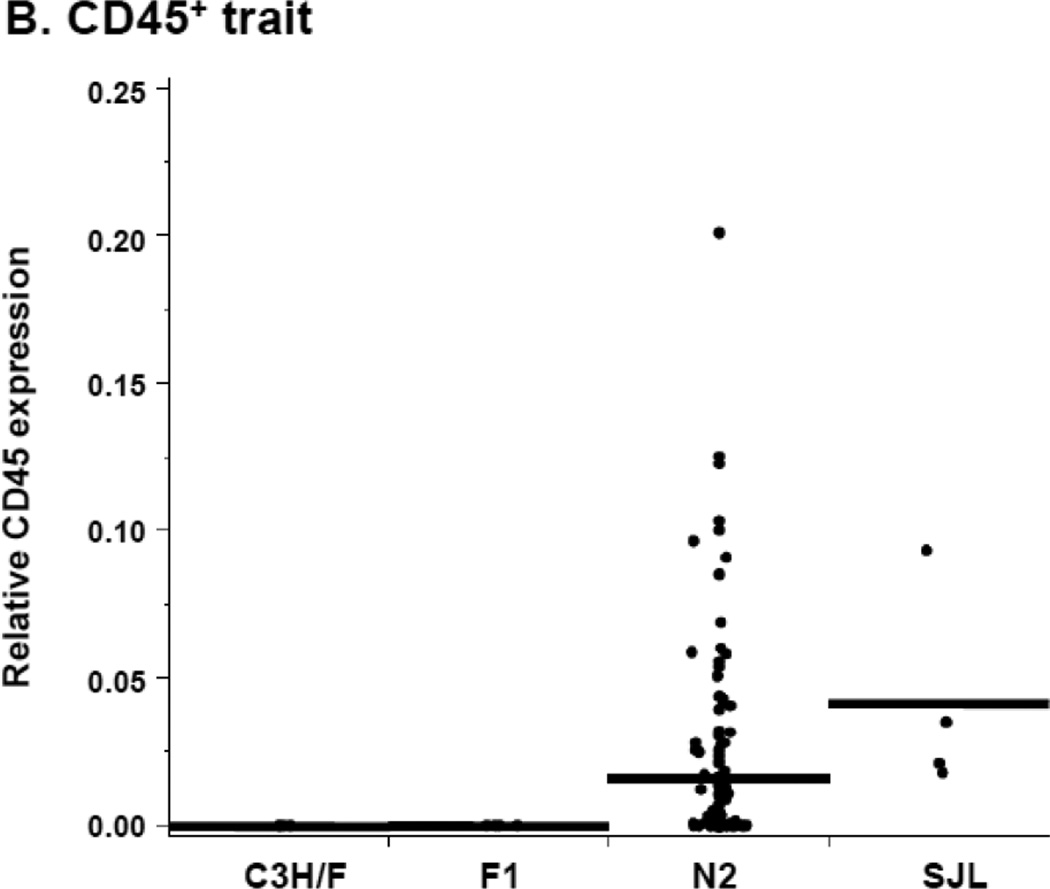
We reported that in response to decreased blood flow there was an increase in CD45+ cells in the intima of SJL relative to C3H/F12. In agreement with these previous findings, immunohistochemistry on carotid sections from the C3H/FxSJL cross demonstrated that infiltration of CD45+ cells in SJL was significantly greater compared to C3H/F (Fig. 1A–B). In the N2 population there was an intermediate phenotype for CD45+ cell infiltration (Fig. 1B). The CD45+ distribution in the parentals and N2 strikingly resembled the distribution of IMT11. When normalized to smooth muscle α1-actin+, we found that the ratio of CD45+ expression to α1-actin+mirrored the relative staining of CD45+ alone (compare Fig. 1B with Supplemental Fig. SI). Therefore, α1-actin+ expression did not significantly affect the CD45+ trait. These results indicate that leukocyte infiltration contributes to IMT in SJL mice and the N2 progeny from the C3H/FxSJL cross.
Figure 1. Relative CD45+ and α1-actin+ immunostaining in the C3H/FxSJL backcross.
A. Representative CD45+ and α1-actin+staining in C3H/F and SJL mice. B. Relative CD45+ expression in the vascular wall of C3H/F, F1 and N2 progeny and SJL mice 14 days after injury. CD45+ expression in the N2 progeny exhibited an intermediate phenotype.
Quantitative Trait Linkage (QTL) Analysis of CD45+ Expression
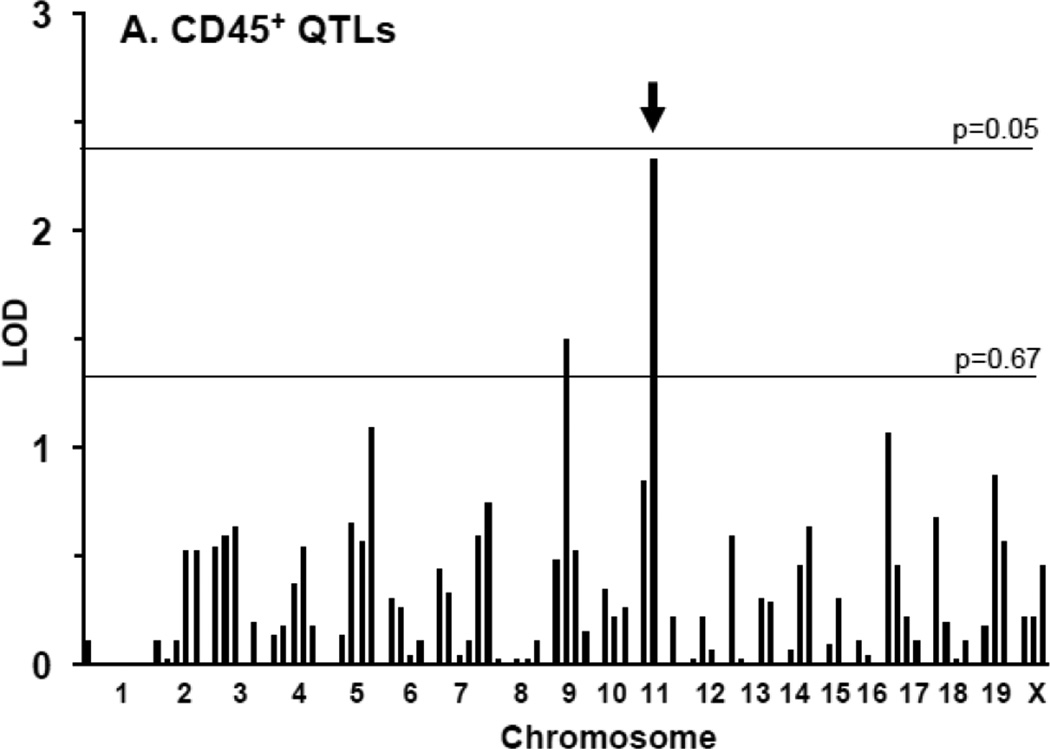
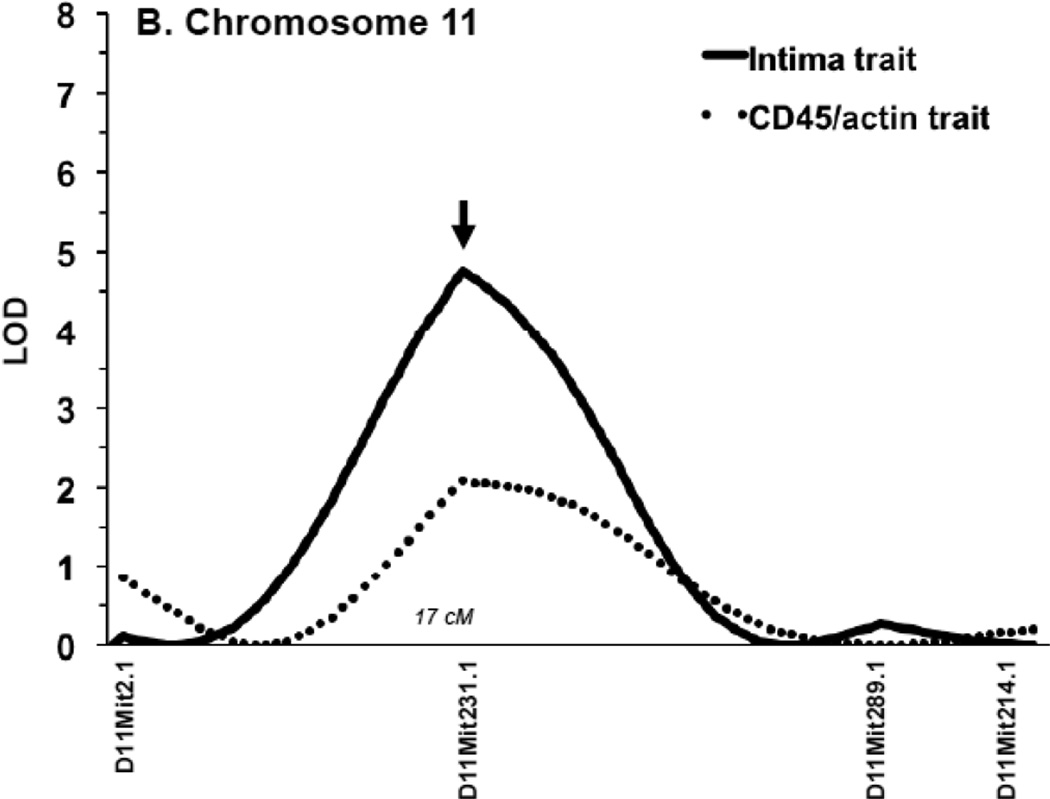
We performed QTL analysis for the CD45+ staining in the N2 progeny. We found a nearly significant locus on chr11 (LOD = 2.3; significance was considered at threshold P=0.05) and a second suggestive one on chr9 (LOD = 1.5; suggestive was considered at threshold P=0.67) (Table 1; Fig. 2A). Interval mapping showed a peak of the locus on chr11 at marker D11Mit231.1 (Fig. 2B). Importantly, the CD45+ locus and its peak directly coincide with our previously reported Im2 peak on chr11 for IMT11 (Fig. 2B). This finding suggests that inflammation (defined by CD45+) contributes to IMT associated with the Im2 locus. To determine whether there was an association between myointimal cells and inflammation we performed a QTL analysis for the CD45+/α1-actin+ratio. The ratio resembled that of the CD45+ trait alone (Table 1; Supplemental Fig. SII) indicating that α1-actin+does not affect the inflammatory trait. In summary, the CD45+ trait maps to the same intima locus on chr11 suggesting that leukocyte infiltration and the intima trait are both regulated by the Im2 locus.
Table 1.
QTLs for CD45+ and CD45+/α1-actin+ identified in C3H/F and SJL backcross
| Trait | Chromosome Marker (cM) | LOD | CI (cM) | Variance (%) |
|---|---|---|---|---|
| CD45+ | D9Mit336.1 (36) | 1.5 | 78 | 5 |
| D11Mit231.1 (17) | 2.3 | 52 | 8 | |
| LOD, | Logarithm of | |||
| CD45+/α1-actin+ | D9Mit336.1 (36) | 1.4 | 83 | 5 |
| D11Mit231.1 (17) | 2.3 | 51 | 8 | |
| odds. | ||||
Suggestive QTL LOD≥1.3, significant QTL LOD≥2.4. CI, confidence interval. Variance (%), a percentage of the total phenotypic variance detected in the N2 progeny.
Figure 2. Quantitative trait linkage analysis for CD45+ expression.
A. All N2 progeny were used for QTL analysis of CD45+ expression. A. A nearly significant QTL was found on chr11 (LOD 2.4) (arrow). A nearly suggestive QTL was identified on chr9 (LOD 1.3). B. The QTL on chr11 for CD45+ expression (dotted line) mapped to the same locus as the previously identified intima QTL (Im2) (black line). The peak of both QTLs was at marker D11Mit231.1.
IMT in Im2 Congenic Mice
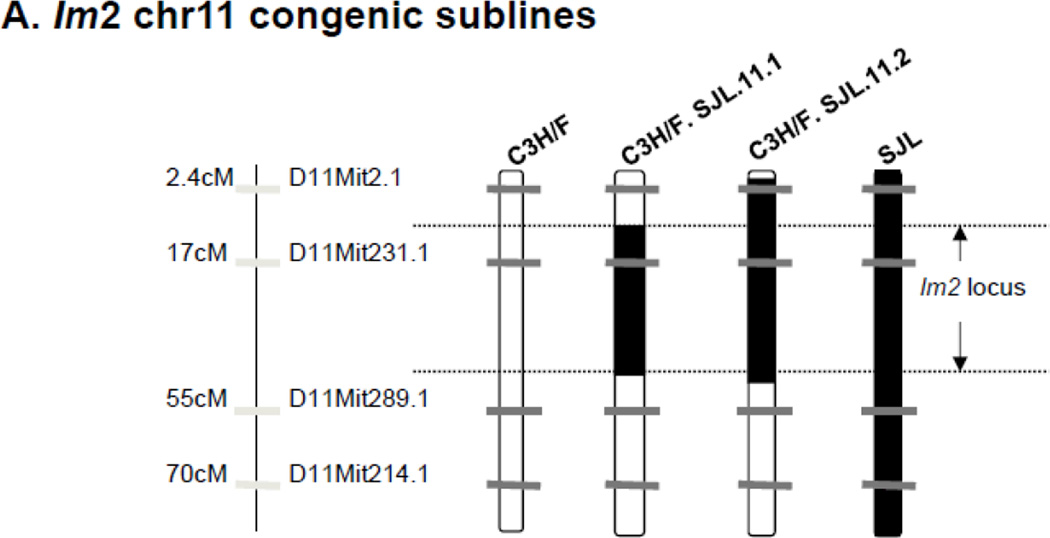
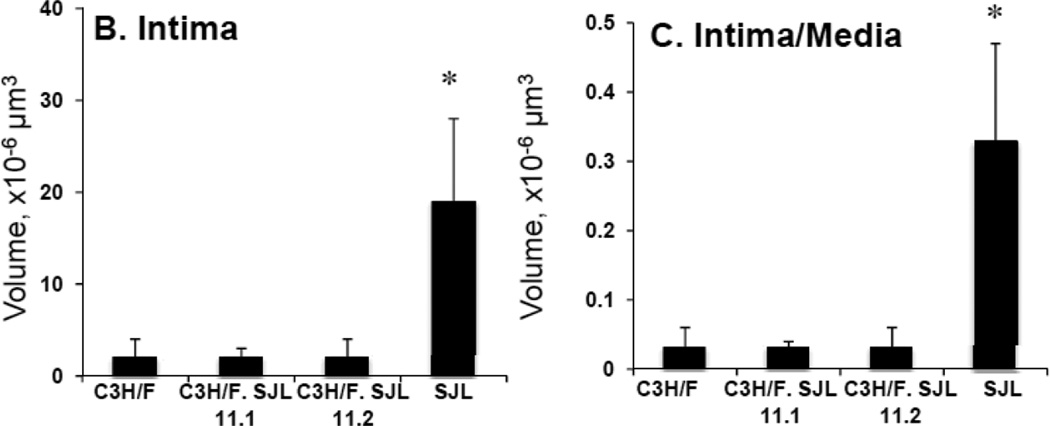
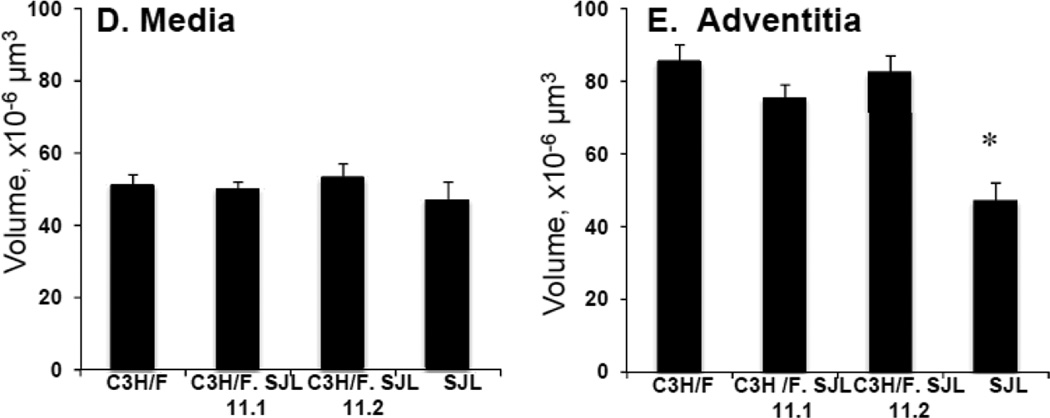
Since there was clear overlap of the CD45+ locus with the Im2 locus we created congenic mice to study the regulation of IMT by the locus. We introgressed the Im2 chr11 locus from SJL onto the C3H/F background using marker-assisted genotyping and created two congenic lines: C3H/F.SJL.11.1 and C3H/F.SJL.11.2 (Fig. 3A). Both lines contain the entire Im2 locus but C3H/F.SJL.11.2 contains additional SJL genomic material proximal to the peak locus marker (D11Mit231.1) (Fig. 3A). We performed partial carotid ligation to create a low flow environment on these congenic mice, and quantified vessel wall compartment volumes to determine the contribution of the Im2 and CD45+ locus to IMT (Fig. 3B–E; Supplemental Fig. S3A–C). Consistent with our previous findings11, 16 there was a significant increase in intima volume and intima/media ratio in SJL compared to all strains (Fig. 3B–C). The intima phenotype of the congenics was the same compared to the C3H/F background (Fig. 3B). The same observation was found for media or adventitia volume in congenics compared to C3H/F (Fig. 3D–E). We observed that SJL had significant decreases in lumen (Supplemental Fig.IIIA) and adventitia volume (Fig. 3E) compared to C3H/F and congenic lines. There were no significant differences intima+media or external elastic lamina (EEL) between the parental and congenic mice (Supplemental Fig. SIIIB-C).
Figure 3. Im2 congenic mice and morphometric analysis.
A. Diagram of congenic mice that were generated for the Im2 locus on chr11. Using marker-assisted genotyping, SJL genomic material was introgressed onto the C3H/F background. Two congenic lines were created, C3H/F.SJL.11.1 and C3H/F.SJL.11.2. B–E. Intima, intima/media, media and adventitia were quantified in parental and congenic mice 14 days following partial carotid ligation. SJL had significantly greater intima (A) and intima/media (B) volumes and no change in media (C) and significantly less adventitia (D) compared to C3H/F and both congenic lines. C3H/F.SJL.11.1 nor C3H/F.SJL.11.2 generated a measurable intima volume over the length of the left carotid artery. (n≥5; p<0.05 was considered significant*).
CD45+ Cell Infiltration in Im2 Congenic Mice
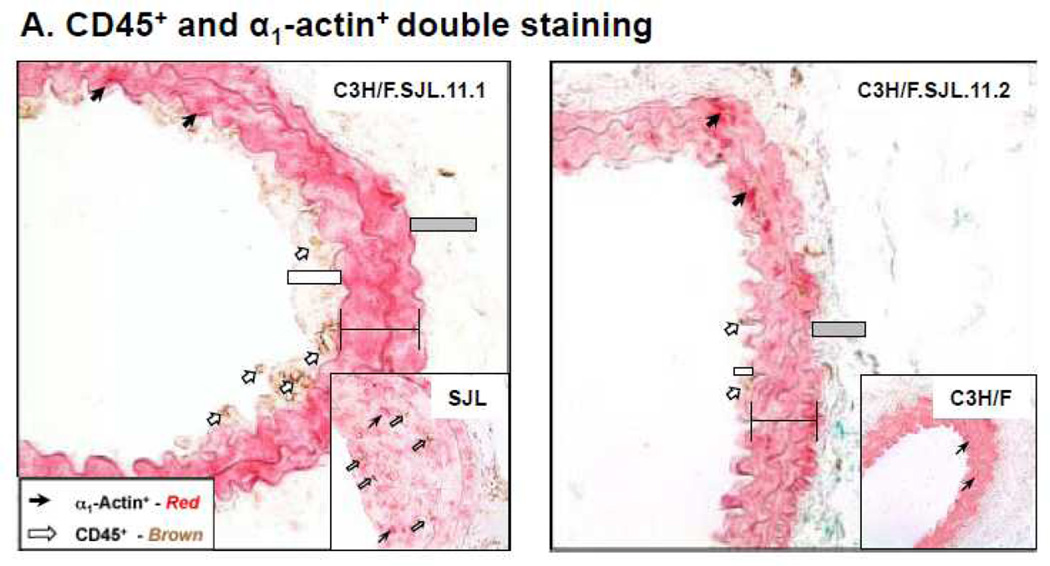
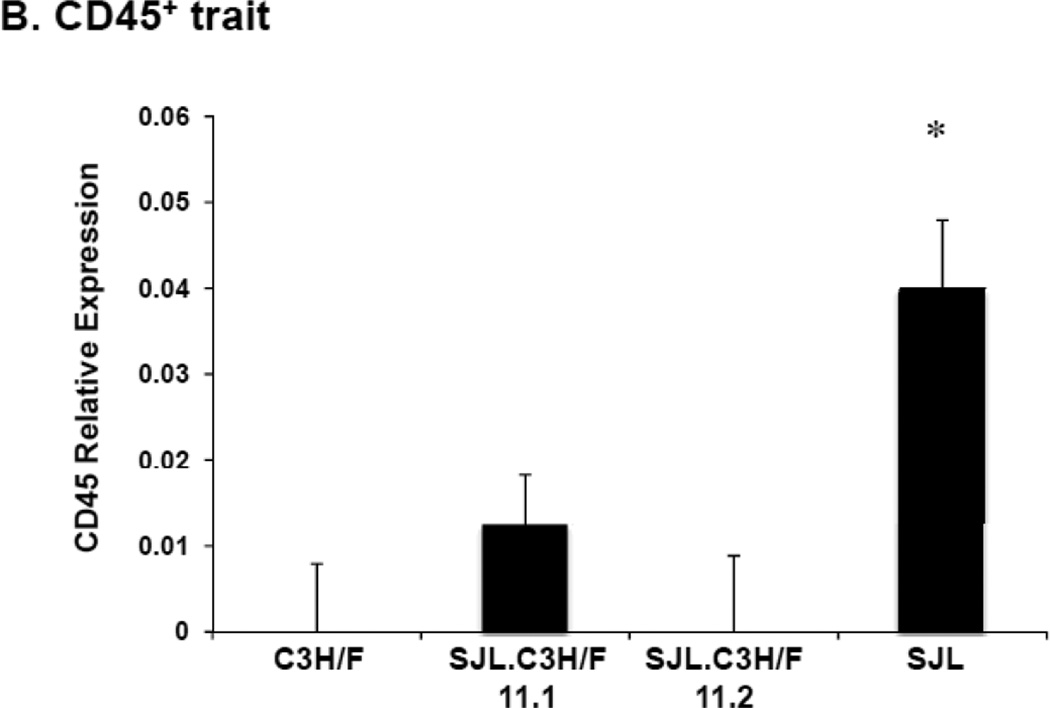
We did not observe an increase in intima volume along the length of the carotids in the congenic mice. However, there were areas along the vessel wall that remodeled with slight increases in IMT. Analysis of these sections demonstrated that CD45+ cells infiltrated the wall in the C3H/F.SJL.11.1 mice but not the C3H/F.SJL.11.2 mice. The amount of CD45+ cell infiltration in the C3H/F.SJL.11.1 mice was approximately 30% of that measured in sections that displayed intima in SJL mice (Fig. 4A–B). CD45+ cell infiltration occurred more frequently in the intima of C3H/F.SJL.11.1 compared to the C3H/F.SJL.11.2 mice (Fig. 4A–B). The CD45+/α1-actin+ ratio for both congenic lines resembled the CD45+ staining alone (Supplemental Fig. SIV).
Figure 4. Relative CD45+ and α1-actin+ immunostaining in congenic mice.
A. Representative CD45+ and α1-actin+ staining in C3H/F.SJL.11.1 and C3H/F.SJL.11.2 mice. Insets are representative images from parental C3H/F and SJL mice. B. Sections from C3H/F.SJL.11.1 and C3H/F.SJL.11.2 where intima area was observed were stained for CD45+ 14 days after injury. Relative CD45+ expression was measurable in C3H/F.SJL.11.1 but not in C3H/F.SJL.11.2 sections.
Discussion
The main finding of this study is the identification of a novel locus on chromosome 11 for CD45+ cell infiltration induced by low blood flow. This locus maps to the same genomic location as our previously published intima-modifier, Im2, locus in the C3H/FxSJL backcross. In the present study we generated congenic mice that express the Im2 locus from the SJL mouse on the C3H/F background. The congenic mice revealed two important findings regarding the association between inflammation and IMT in response to low blood flow: 1) Intima formation in the congenic mice was comparable to C3H/F mice, suggesting that the C3H/F genome functions to protect the vascular wall from intima formation, and 2) Since CD45+ staining was observed in areas of the congenic mice where the vessel wall had remodeled we conclude that leukocyte infiltration only partially explains the intima trait. Thus, the Im2 and CD45+ locus play a role in recruiting or retaining inflammatory cells during vascular remodeling.
Carotid IMT in response to injury or altered blood flow is a complex multifactorial trait. The contribution of specific cell types to IMT remains to be defined. It is well accepted that vascular smooth muscle cell migration and proliferation are the major sources for IMT in response to multiple stimuli, including low blood flow17, 18. However, circulating inflammatory cells and bone marrow-derived progenitor cells have also been shown to play a role in IMT19, 20. Other factors that need to be considered when determining mediators of IMT include the disease model, time course of cell infiltration, and strain background (hence genetic factors).We have shown that the C3H/F and SJL mouse strains that have significant differences in vascular remodeling in response to altered blood flow11, 16. Our interest was to determine if there was genetic regulation of inflammation associated with IMT. Our current study presents evidence that leukocyte infiltration occurs in IMT and that it is a genetically regulated trait.
We utilized our previously published C3H/FxSJL cross11 to measure leukocyte infiltration and map loci that explain this trait. We found overlap on chr11 for the CD45+ locus with our reported Im2 locus. We created two congenic mouse lines that carry SJL genomic information on the C3H/F background to further study the Im2 locus. The intima phenotype in both congenic lines was comparable to the parental C3H/F mice. This indicates that the C3H/F genome is dominant in regulating IMT. However, the genomic locus on chr11 from SJL mice was able to promote a measurable amount of leukocyte infiltration as indicated by the increased CD45+ cell infiltration in the C3H/F.SJL.11.1 congenic line. These findings support a genetic regulation of leukocyte infiltration in carotid IMT. Our data are consistent with a recent study by Orozco et al, which demonstrated using system genetics that inflammatory traits are highly influenced by genetic determinants21. The use of our congenic mice also emphasizes the importance of genetic regulation of complex traits, such as IMT. Even though theIm2 congenic lines did not display a large intima phenotype, the C3H/F.SJL.11.1 did exhibit increased inflammation. Similar to a genome wide association study (GWAS) of inflammation in coronary artery disease where chr’s 6, 9, and 10 were shown to house both protective and promoting inflammatory genes22, our congenic mice presumable express both intima protective and promoting genes. Therefore, we conclude that the C3H/F genome is more dominant in protecting against IMT while the SJL locus promotes inflammatory and intima.
The overlapping Im2 and CD45+ loci suggest that this genomic region houses causal genes that promote IMT. Using the JaxLabphenome and SNP variation database (http://phenome.jax.org/db/q?rtn=docs/genonav) we generated a list of approximately 140 genes with SNPs differing between C3H/F and SJL in the region of the CD45+ locus (17Mb to 63Mb) (Supplementary Table SI; bold font). We observed that two genes on the list were also identified in our previously published microarray data: epidermal growth factor-containing fibulin-like extracellular matrix 1 (Efemp1) and polyribonucleotide nucleotidyltransferase 1 (Pnpt1)15. In an expression QTL (eQTL) analysis of macrophages from C3H/F mice, Pnpt1 expression was significantly increased relative to other strains (http://systems.genetics.ucla.edu/data21). A future direction from this study will be to explore the potential role of macrophage Pnpt1 expression, as well as Efemp1, and its interaction with other inflammatory genes in IMT. IMT is an index of atherosclerosis, which is influenced by inflammation. Recently, a high-resolution GWA analysis of aortic atherosclerosis loci in 22 different mouse strains, including a C3H substrain and SJL, identified genes on chr1 that influence intima and vascular smooth muscle cell function23. Specifically, desmin (Des) was differentially expressed during atherogenesis. It would be interesting to see if Des interacts with the Im2 locus to influence flow induced vascular remodeling.
We did observe an increase in inflammation inthe C3H/F.SJL.11.1 congenic mice despite having a large intima formation. We interpret this to mean that inflammation is not the primary cause of the intima trait and that, by itself, the CD45+ locus within the Im2 locus only partially explains the entire IMT phenotype. In fact, we reported that the Im2 locus only accounts for 17% of the trait11 and it appears that the CD45+ trait contributes to approximately 8% of that. Therefore, we speculate that genes in the Im2 locus are responsible for the immune response while genes in the Im1 locus on chr2 and Im3 locus on chr18 work in trans to promote IMT. Therefore, a future direction of this study would be to determine the relationship of the CD45+ locus with the Im1 and Im3 loci. If the Im2 locus works to promote inflammation during vascular remodeling then it is probable the Im1 and Im3 loci function to promote vascular smooth muscle cell growth and migration as well as endothelial cell signaling. We have phenotypic evidence of IMT in our Im1 mice (data not shown). Therefore, investigating the candidate genes in the Im1 should provide further insight into interaction and regulation of inflammatory and growth genes across these loci.
Our data strongly indicate that the CD45+ locus houses genes that regulate inflammation in response to decreased blood flow. We recognize, however, that there is a limitation to our study. As discussed above, we are not certain if the Im2 locus controls an early inflammatory response or contributes to a sustained immune response. We have only measured CD45+ staining two weeks post ligation. Thus, it is possible that CD45+cell infiltration varied and possibly peaked at an earlier time point such one week following low blood, as was reported in FVB/NJ mice for CD45+ cell count in the vascular wall24. Similarly, a study in ApoE knockout mice demonstrated that leukocyte infiltration peaked one week following injury25. Although we did not measure CD45+cell infiltration at earlier time points, we can conclude that after two weeks of decreased blood flow there appears to be a sustained immune response in SJL and C3H/F.SJL.11.1 congenic mice, as we previously observed for SJL mice12. Therefore, our data indicate that inflammation is a part of vascular remodeling and that the CD45+ locus, which overlaps with the Im2 locus, partially explains prolonged inflammation associated with IMT.
In summary, we have identified a novel locus for leukocyte mediated IMT. Genes within this locus likely contribute to the immune response in vascular remodeling and may provide insight into the treatment of inflammation associated with carotid IMT.
Supplementary Material
Significance.
Carotid IMT is a highly predictive risk factor for cardiovascular events such as myocardial infarction and stroke. It is known that carotid IMT development is a multifactorial process and is highly influenced by genetics. Our study identified a novel genomic region using a mouse genetic model. Our findings indicate that inflammatory genes are partially responsible for vascular remodeling associated with carotid IMT. Our research will allow for more focused investigation of candidate genes for treatment of inflammation related to cardiovascular diseases.
Acknowledgments
We thank Amy Mohan and Christine Christie for assistance with congenic mouse generation and breeding. We also thank the University of Rochester Medical Center Genomic Research Center for assistance with mouse genotyping. We appreciate the scientific communication with Dr. Luz Orozco and Dr. Aldons Jake Lusis from the University of California Los Angeles.
Source of Funding
This study was supported by NIH HL-62826 to B.C.B.
Abbreviations
- IMT
Intima-Media Thickening
- chr
Chromosome
- QTL
Quantitative Trait Locus
- C3H/F
C3Heb/FeJ
- SJL
SJL/J
- Im
Intima-modifier
- GWAS
Genome Wide Association Study
- SNP
Single Nucleotide Polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: Hemodynamic and biochemical mechanisms underlying glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 3.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–319. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- 4.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: A model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 5.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 6.Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, Juo SH, Sacco RL. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: The northern manhattan prospective cohort study. Stroke. 2002;33:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacco RL, Blanton SH, Slifer S, Beecham A, Glover K, Gardener H, Wang L, Sabala E, Juo SH, Rundek T. Heritability and linkage analysis for carotid intima-media thickness: The family study of stroke risk and carotid atherosclerosis. Stroke. 2009;40:2307–2312. doi: 10.1161/STROKEAHA.109.554121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Beecham A, Zhuo D, Dong C, Blanton SH, Rundek T, Sacco RL. Fine mapping study reveals novel candidate genes for carotid intima-media thickness in dominican families. Circ Cardiovasc Genet. 2012;223:177–183. doi: 10.1161/CIRCGENETICS.111.961763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox CS, Cupples LA, Chazaro I, Polak JF, Wolf PA, D'Agostino RB, Ordovas JM, O'Donnell CJ. Genomewide linkage analysis for internal carotid artery intimal medial thickness: Evidence for linkage to chromosome 12. Am J Hum Genet. 2004;74:253–261. doi: 10.1086/381559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Z, Pei H, Roberts DJ, Zhang Z, Rowlan JS, Matsumoto AH, Shi W. Quantitative trait locus analysis of neointimal formation in an intercross between c57bl/6 and c3h/hej apolipoprotein e-deficient mice. Circ Cardiovasc Genet. 2009;2:220–228. doi: 10.1161/CIRCGENETICS.108.792499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korshunov VA, Berk BC. Genetic modifier loci linked to intima formation induced by low flow in the mouse carotid. Arterioscler Thromb Vasc Biol. 2009;29:47–53. doi: 10.1161/ATVBAHA.108.178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korshunov VA, Nikonenko TA, Tkachuk VA, Brooks A, Berk BC. Interleukin-18 and macrophage migration inhibitory factor are associated with increased carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2006;26:295–300. doi: 10.1161/01.ATV.0000196544.73761.82. [DOI] [PubMed] [Google Scholar]
- 13.Mozes E, Isac R, Taussig MJ. Antigen-specific t-cell factors in the genetic control of the immune response to poly(tyr,glu)-polydlala--polylys. Evidence for t- and b-cell defects in sjl mice. J Exp Med. 1975;141:703–707. doi: 10.1084/jem.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berk BC, Korshunov VA. Genetic determinants of vascular remodelling. Can J Cardiol. 2006;22(Suppl B):6B–11B. doi: 10.1016/s0828-282x(06)70980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolock EM, Korshunov VA, Glazko G, Qiu X, Gerloff J, Berk BC. Ribosomal protein l17, rpl17, is an inhibitor of vascular smooth muscle growth and carotid intima formation. Circulation. 2012;126:2418–2427. doi: 10.1161/CIRCULATIONAHA.112.125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: The "glagov phenomenon" is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 17.Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk BC. Cyclophilin a mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 20.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 21.Orozco LD, Bennett BJ, Farber CR, et al. Unraveling inflammatory responses using systems genetics and gene-environment interactions in macrophages. Cell. 2012;151:658–670. doi: 10.1016/j.cell.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherson R, Davies RW. Inflammation and coronary artery disease: Insights from genetic studies. Can J Cardiol. 2012;28:662–666. doi: 10.1016/j.cjca.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Bennett BJ, Orozco L, Kostem E, Erbilgin A, Dallinga M, Neuhaus I, Guan B, Wang X, Eskin E, Lusis AJ. High-resolution association mapping of atherosclerosis loci in mice. Arterioscler Thromb Vasc Biol. 2012;32:1790–1798. doi: 10.1161/ATVBAHA.112.253864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korshunov VA, Solomatina MA, Plekhanova OS, Parfyonova YV, Tkachuk VA, Berk BC. Plasminogen activator expression correlates with genetic differences in vascular remodeling. J Vasc Res. 2004;41:481–490. doi: 10.1159/000081804. [DOI] [PubMed] [Google Scholar]
- 25.Alberts-Grill N, Rezvan A, Son DJ, Qiu H, Kim CW, Kemp ML, Weyand CM, Jo H. Dynamic immune cell accumulation during flow-induced atherogenesis in mouse carotid artery: An expanded flow cytometry method. Arterioscler Thromb Vasc Biol. 2012;32:623–632. doi: 10.1161/ATVBAHA.111.242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.