Abstract
An important question in developmental biology is how relatively shallow gradients of morphogens can reliably establish a series of distinct transcriptional readouts. Current models emphasize interactions between transcription factors binding in distinct modes to cis-acting sequences of target genes. Another recent idea is that the cis-acting interactions may amplify preexisting biases or prepatterns to establish robust transcriptional responses. In this study, we examine the possible contribution of one such source of prepattern, namely gene length. We developed quantitative imaging tools to measure gene expression levels for several loci at a time on a single-cell basis and applied these quantitative imaging tools to dissect the establishment of a gene expression border separating the mesoderm and neuroectoderm in the early Drosophila embryo. We first characterized the formation of a transient ventral-to-dorsal gradient of the Snail (Sna) repressor and then examined the relationship between this gradient and repression of neural target genes in the mesoderm. We found that neural genes are repressed in a nested pattern within a zone of the mesoderm abutting the neuroectoderm, where Sna levels are graded. While several factors may contribute to the transient graded response to the Sna gradient, our analysis suggests that gene length may play an important, albeit transient, role in establishing these distinct transcriptional responses. One prediction of the gene-length-dependent transcriptional patterning model is that the co-regulated genes knirps (a short gene) and knirps-related (a long gene) should be transiently expressed in domains of differing widths, which we confirmed experimentally. These findings suggest that gene length may contribute to establishing graded responses to morphogen gradients by providing transient prepatterns that are subsequently amplified and stabilized by traditional cis-regulatory interactions.
Keywords: Morphogen gradient, transcriptional delay, computational modeling, prepattern, multiplex in situ hybridization, Drosophila
Introduction
Pattern formation along the dorsal-ventral axis of the Drosophila embryo is initiated by a nuclear concentration gradient of the NFκ-B-related transcription factor Dorsal (Roth et al., 1989; Rushlow et al., 1989; Steward, 1989), wherein high levels of Dorsal in ventral regions specify expression of the key mesodermal determinants Twist (Twi) and Snail (Sna) (Ip et al., 1992b; Jiang et al., 1991; Kosman et al., 1991; Leptin, 1991; Rao et al., 1991; Ray et al., 1991; Thisse et al., 1991), while lower levels in lateral regions activate expression of neuroectodermal genes (Francois et al., 1994; Ip et al., 1992a; Jazwinska et al., 1999a; Jazwinska et al., 1999b; Kosman et al., 1991; Leptin, 1991; McDonald et al., 1998; Mellerick and Nirenberg, 1995; Rao et al., 1991; Ray et al., 1991). Expression of neuroectodermal genes is excluded from the mesoderm by the Sna repressor, which binds to cis-regulatory enhancer sequences of these direct target genes (Francois et al., 1994; Ip et al., 1992a; Kosman et al., 1991; Leptin, 1991; Mellerick and Nirenberg, 1995; Rao et al., 1991). Sna expression is restricted to the most ventral nuclei of the embryo because the Dorsal binding sites in its cis-regulatory enhancer are low-affinity sites that can only be fully occupied by the high nuclear concentrations of Dorsal present ventrally (Ip et al., 1992b; Jiang et al., 1991; Kosman et al., 1991; Leptin, 1991; Rao et al., 1991; Ray et al., 1991). Full ventral activation of Sna also depends on the Twi transcription factor (Ip et al., 1992b; Thisse et al., 1988), which, like Sna, is activated by Dorsal. In contrast to the mesodermal targets of Dorsal, low levels of Dorsal can activate neuroectodermal genes because their cis-regulatory elements contain high-affinity Dorsal binding sites (Ip et al., 1992a; Mellerick and Nirenberg, 1995; Rusch and Levine, 1996) and optimal spacing between Dorsal and Twist binding sites (Crocker et al., 2008; Szymanski and Levine, 1995). These neuroectodermal genes are therefore expressed more laterally than Sna, but are repressed in ventral-most nuclei by Sna, which acts directly by binding to sites in neuroectodermal enhancers. Sna expression sharpens during early blastoderm stages culminating in the formation of an on/off border with the neuroectoderm. Expression of Sna in this sharply defined domain results in full repression of all neuroectodermal genes in the presumptive mesoderm.
Whereas the genetic mechanisms leading to establishment of mesodermal versus neuroectodermal domains have been well characterized, less is known regarding the temporal sequence by which the relevant gene expression patterns resolve into their final sharp domains. To analyze this highly dynamic process with greater precision, we developed quantitative imaging and data analysis tools to measure the expression levels of several genes at a time using multiplex in situ hybridization on a nucleus-by-nucleus basis (Kosman et al., 2004). Analysis of such multiplex data reveals a transient intermediate stage in the formation of the Mesoderm/Neuroectoderm Border (MNB) in which expression of neuroectodermal genes is differentially repressed by Sna in a nested pattern within the mesoderm. While several factors are likely to contribute to this graded response, quantitative modeling of this process suggests that one important input is the delay in transcriptional repression associated with the differing transcription unit lengths of neural genes. We confirm an expectation of this transcriptional-delay model, namely that the gap gene knirps (kni), a short gene, and its paralog knirps-related (knrl), a long gene, should transiently be expressed in domains of differing width. We propose that gene length may provide a transient prepattern bias that is then amplified and stabilized by well-characterized interactions between transcriptional regulators and cis-regulatory modules (CRMs).
Materials and Methods
In situ hybridization and immunofluorescence
A detailed description of fluorescent methods used to detect multiple mRNA transcripts is given in (Kosman et al., 2004). Briefly, Bluescript (sna, brk, vnd, rho) or pNB40 (sog) vectors harboring full-length cDNAs containing the entire coding sequence (1.7kb sna; 4.7kb sog; 3kb brk; 3kb vnd transcript that corresponds to the shorter transcription unit expressed early in embryogenesis; 2.5kb rho) were linearized using HindIII (sna, sog, rho) or Xba (brk, vnd) and transcribed with RNA polymerase T3 (sna, rho) or T7 (sog, brk, vnd) to generate RNA antisense probes labeled with digoxigenin (DIG), biotin (BIO), dinitrophenol (DNP) and fluorescein (FITC) haptens. Primary antibodies used in this study were: sheep anti-DIG (Roche, diluted at 1:1,000), mouse anti-BIO (Roche, diluted at 1:1,000), rabbit anti-DNP (Invitrogen, diluted at 1:2,000), guinea-pig-anti-FITC (diluted at 1:1,000), and guinea-pig-anti-Sna (kindly provided by Michael Levine, diluted at 1:300). Alexa-fluor-coupled secondary antibodies were obtained from Invitrogen and diluted at 1:500 (donkey anti-sheep, donkey anti-mouse, donkey anti-rabbit, donkey anti-guinea pig). To avoid an observed unspecific cross-reaction between the guinea pig anti-Sna and mouse anti-BIO, incubation and secondary detection of mouse anti-BIO was carried out before incubation with guinea pig-anti-Sna.
Drosophila stocks
Oregon-R strain was used as wild type. Df(2L)Sco[rv25]/CyO (Bloomington Stock Center) contains a deficiency for the sna locus and was used to generate sna−/+ embryos. A single sna NTD per nucleus distinguishes sna−/+ embryos from wild-type and sna−/− embryos.
Image Acquisition
All fluorescently stained embryos were counterstained with the fluorescent nuclear marker DAPI, flat-mounted, and imaged as optical sections on a Leica SP2 or a Zeiss LSM700 scanning confocal microscope with a 63X objective lens and digital zoom of either 1x or 2.5x. The gain and offset of the photomultiplier tubes were set so that all the pixels of interest fell within the 8-bit dynamic range.
Data Analysis and Modeling
The Supporting Text outlines the computational methods used to analyze the fluorescence images, together with a model of the dynamic sna cytoplasmic mRNA profile and a model relating the calculated sna profile to the neuroectodermal NTD profiles.
Results and Discussion
Transient formation of a sna gradient in the dorsal mesoderm
It has previously been noted that sna expression is initiated in the 10–12 most ventral mesodermal cells and then expands to occupy the full mesoderm (18 cells wide) (Fowlkes et al., 2008; Ip et al., 1992b). During this period of early sna expression, neural genes are transiently expressed in the mesoderm before sufficient levels of Sna repressor accumulate to shut them off. Once sna expression occupies the full expanse of the mesoderm, its dorsal border abutting the neuroectoderm becomes very sharp (O'Neill and Bier, 1994), and shortly thereafter leads to the induction of a single row of single minded (sim) expressing mesectodermal cells along the ventral border of the neuroectoderm (Bardin and Schweisguth, 2006; Chitnis, 2006; Cowden and Levine, 2002; Morel et al., 2003; Morel and Schweisguth, 2000; Schweisguth, 2004). By this stage neuroectodermal gene expression in the mesoderm is fully repressed.
To examine the refinement of the MNB with greater precision, we coupled multiplex FISH (Kosman et al., 2004) with image segmentation techniques developed in this work (see Supporting Text) to analyze the simultaneous expression of several gene markers within the mesoderm on a nucleus-by-nucleus basis at various times during nuclear cycle 14 of the blastoderm embryo. This strategy allowed us to create detailed snap-shots of Sna and neuroectodermal gene expression in the mesodermal domain. We used DAPI staining to create 3D segmentations of nuclei thereby resolving cytoplasmic and nuclear signals. Once the nuclei were segmented, we extracted robust signals from sites of nascent transcription located within nuclei, also referred to as nuclear transcription dots or NTDs, by thresholding the nuclear mRNA fluorescence intensity. NTDs arise from newly synthesized mRNAs clustered at the site of genes encoding those nascent transcripts (Wilkie et al., 1999), and are ideally suited to uncover rapid changes in gene expression patterns since they serve as a direct read-out of transcriptional regulation,
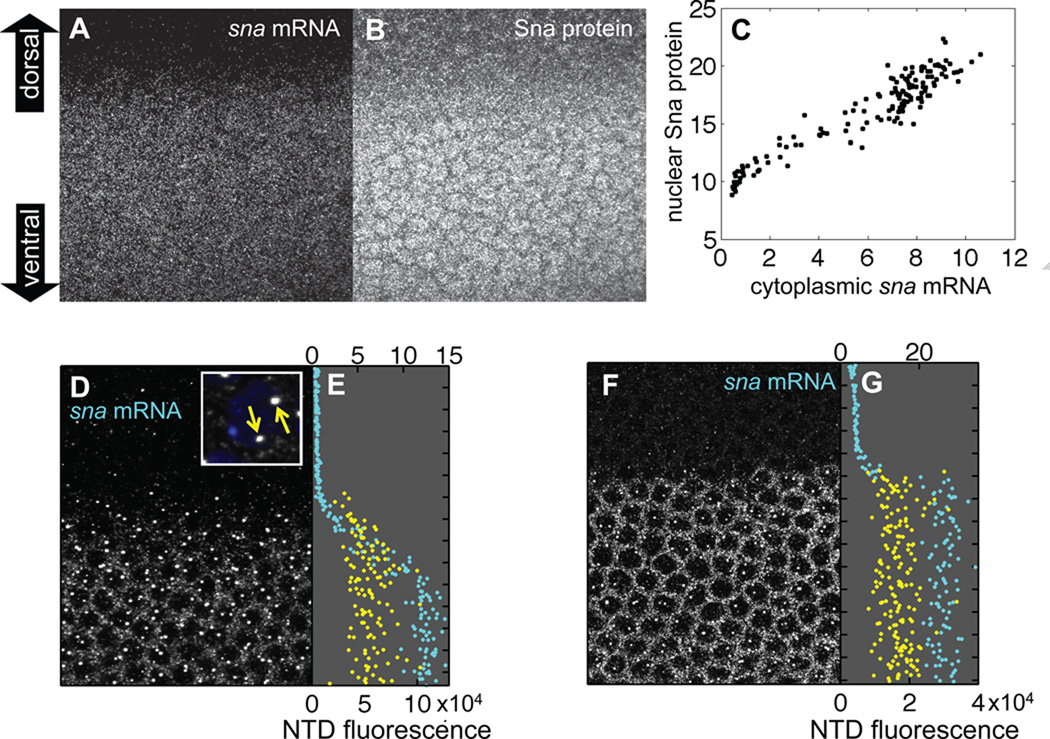
Because the Sna repressor is known to bind directly to the cis-regulatory enhancer sequences of neuroectodermal genes (Gray et al., 1994; Ip et al., 1992a; Jiang and Levine, 1993; Zeitlinger et al., 2007b), we first quantified the establishment of the sna expression profile within the mesodermal domain. Co-staining early-cycle 14 blastoderm embryos with an antisense mRNA probe to detect sna transcripts and an anti-Sna antibody to detect Sna protein revealed gradients of both sna mRNA (Fig. 1A) and Sna nuclear protein (Fig. 1B), consistent with prior reports of a ventral-to-dorsal gradient of sna cytoplasmic mRNA expression (Fowlkes et al, 2008) (Fig. S3A). Quantification revealed a linear correlation between cytoplasmic sna mRNA and nuclear Sna protein over a broad range of individual nuclei (Fig. 1C), validating the use of sna cytoplasmic mRNA as a surrogate for Sna protein levels in the nucleus. In subsequent experiments we made use of this correlation between transcript and protein levels to estimate Sna input protein levels while simultaneously detecting multiple target gene transcripts by multiplex FISH.
Figure 1. Quantification of a transient sna gradient.
(A) Early nuclear cycle 14 embryo stained for sna mRNA via Fluorescent In Situ Hybridization. The sna mRNA fluorescence shown in the image is a maximum projection of a confocal stack spanning the apical cytoplasm where sna cytoplasmic mRNA is localized. Embryo is oriented with ventral side downwards. Anterior is to the left and posterior is to the right. (B) Same embryo co-stained for Sna protein. The image is a maximum projection of a confocal stack spanning the apical-basal extent of the nuclei. The fluorescence has been amplified by a factor of 4 to better visualize the nuclear localization of Sna protein, but the original unamplified data was used in our analysis. (C) Sna protein in individual nuclei is linearly correlated with sna cytoplasmic mRNA. The signals represent voxel intensities averaged over nuclear segmentations and cytoplasmic Voronoi cellular reconstructions. Data was taken from the individual embryo shown in panels A, B. (D) Another early cycle 14 embryo stained for sna mRNA representing a confocal stack spanning the nuclei. Cytoplasmic mRNA stains in a dorsal-ventral gradient (raw data amplified by factor 2.5 to make gradient more apparent in this panel but unamplified data used in subsequent analysis). Two strong foci of sna mRNA fluorescence intensity (referred to as NTDs in the main text) are visible in most nuclei in the presumptive mesoderm (lower half of panel). Inset shows a high-magnification image of two NTDs (yellow arrows) in a single nucleus (indicated by DAPI staining in blue). (E) sna NTD dorsal-ventral profile, corresponding to the single embryo shown in (D), is plotted in yellow with NTD intensities corresponding to the bottom axis. Dorsal-ventral position of the center of mass of each nucleus is plotted in cyan against the mean fluorescence intensity per voxel of sna mRNA in the associated cytoplasm (quantification of fluorescence intensity is indicated on the top axis). (F, G) Same as (D, E) except that data was acquired from a single embryo in the late cellularization stage of nuclear cycle 14. Again sna mRNA signal is amplified (by a factor of 2) in panel F but not in panel G. Notice the much sharper profile of sna mRNA intensity compared to that in panels D, E.
We next analyzed sna cytoplasmic mRNA levels as a function of dorsal-ventral position of individual nuclei (Fig. 1D). A nucleus-by-nucleus quantification revealed a sna concentration gradient with highest levels surrounding the 12 most-ventral nuclei of the mesoderm, and progressively lower levels more dorsally in the border zone, comprising approximately 3 mesodermal nuclei on each side of the MNB (cyan profile in Fig. 1E, corresponding to the field of view shown in panel D). It is noteworthy that in contrast to the graded profile of cytoplasmic sna mRNA (and presumably also Sna protein since these two variables are linearly correlated), the distribution of sna NTDs exhibited a much sharper border along the dorsal-ventral axis (yellow profile in Fig. 1E). An informative detail of this analysis (see supporting text) is that the graded sna cytoplasmic mRNA profile (cyan) trails the frontier of nascent sna expression (yellow) defined by the NTDs (Fig. 1E). By the end of cellularization stage of cycle 14, the sna cytoplasmic mRNA gradient sharpens to form a one-nucleus-sharp profile (Fig 1F with quantification indicated by cyan profile in G).
An expansion-accumulation model accounts for the transient sna gradient
We analyzed the graded profile of sna cytoplasmic mRNA near the MNB in early cycle 14 embryos and found it unlikely to be caused by diffusion (Supporting Text). This result prompted us to search for an alternative explanation for the observed sharpening of the sna gradient. The previously noted dorsally expanding domain of sna expression (Fowlkes et al., 2008) (Ip et al., 1992b) suggested a potential mechanism for generating a transient sna gradient abutting the MNB, in which an on-off profile of sna transcriptional activity encompassing the 10 ventral-most nuclei at t=0 min (onset of cycle 14), expands dorsally at a steady rate to encompass the 18 ventral-most nuclei at t=20 min; see Fig. S3B. Using this advancing front of sna transcriptional activity as input to a mathematical model that invokes only synthesis and turnover of sna mRNA (Eq. [S2] in Supporting Text), we obtained a dynamic sna profile which is graded at times shortly after the onset of expansion (Fig. S3E; t=20 min). This profile is similar to that observed in early cycle 14 (Fig. 1E) and results from the differential accumulation of sna mRNA at different locations as the front expands. The profile then gradually sharpens as the border of sna transcription advances dorsally to its final location at the MNB. Finally, attenuation of sna levels (due to turnover of sna cytoplasmic mRNA) generates an abrupt expression border (Fig. S3E; t ≈ 50 min), similar to what is experimentally observed in late cycle 14 (Fig. 1G); see detailed description in Supporting Text.
Neural genes are expressed in a stratified pattern in the mesoderm of early embryos
As mentioned above, many neuroectodermal genes are transiently expressed in the mesoderm prior to sufficient levels of Sna accumulating to repress them ventrally. To test whether the degree of neural gene expression was related to the dynamic pattern of Sna expression, we examined the relative pattern of neural gene expression during the expansion phase of Sna expression.
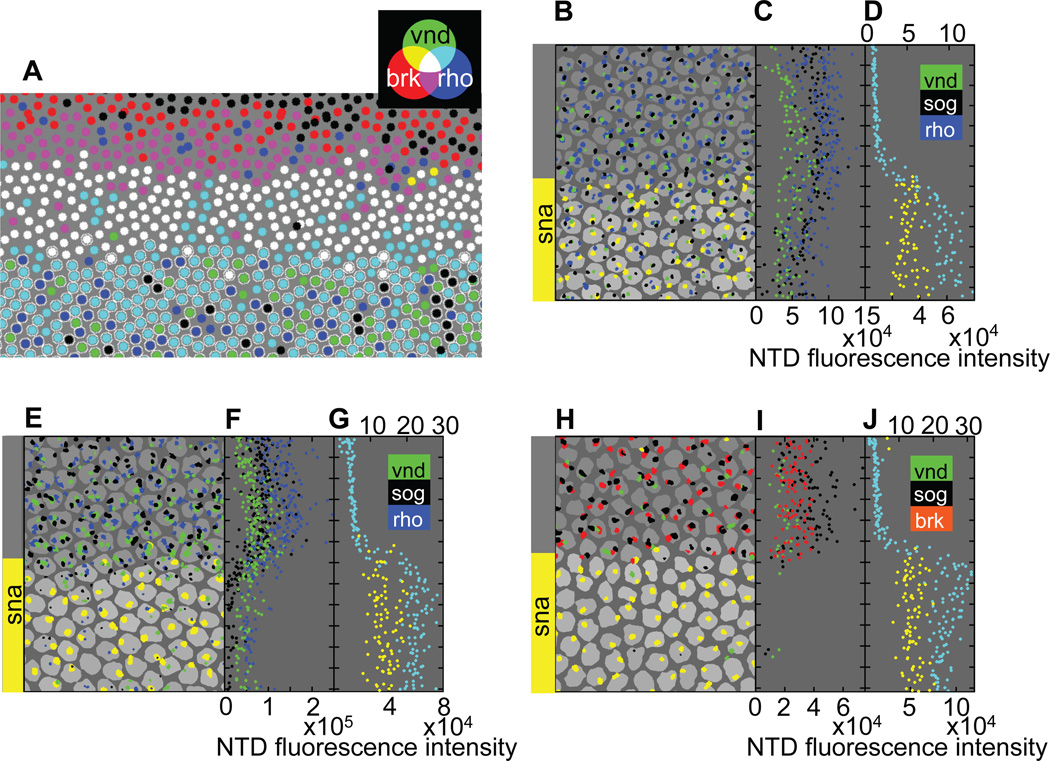
We quantified the extent and overlap of neural transcription patterns at different stages in the mesoderm by creating nuclear masks for fields of nuclei straddling the MNB. In these reconstructed fields of nuclei, each nucleus is represented by a circle whose color indicates the combination of neuroectodermal genes active in that nucleus (Fig. 2A). In early blastoderm-stage embryos (20–25 minutes into cycle 14), these on/off coded gene expression maps accurately reproduce the known relative positions of dorsal borders of neuroectodermal gene expression (Francois et al., 1994; Jazwinska et al., 1999a; Kosman et al., 2004; Mellerick and Nirenberg, 1995). For instance, brinker (brk) expression shown in red extends further dorsally than rhomboid (rho), shown in magenta, followed by ventral nervous system defective (vnd), shown in white. In addition, this analysis revealed a transient nested series of distinct ventral borders for these genes within the presumptive mesoderm. In these embryos, the ventral limits of rho (indicated by cyan) and vnd (indicated by green) extended approximately 1–2 nuclear diameters into the presumptive mesoderm (as defined by the ventral brk border). vnd has previously been shown to be expressed approximately one nuclear diameter more ventrally than rho (Zinzen et al., 2006), and our data is consistent with this assessment. Finally, expression of the short gastrulation (sog) gene is transcribed yet further into the mesoderm (shown in black in the later panels of Fig. 2; see below).
Figure 2. Neuroectodermal genes are differentially repressed in the mesoderm.
(A) Reconstruction of a low-magnification in situ hybridization confocal stack taken from an individual embryo, imaged at early-mid cycle 14, showing expression of three neuroectodermal genes (brk, vnd, and rho) and the mesodermal transcriptional repressor sna. The circles represent nuclei and the colors indicate the combination of Nuclear Transcription Dots (NTDs) detected for the various neuroectodermal genes. Black indicates that no neuroectodermal NTDs were detected. A circle circumscribed by a white outline indicates a nucleus in which a sna NTD was detected. (B) Reconstruction of the individual embryo shown in Fig. 1D with the same dorsal-ventral and anterior-posterior orientation. The area of each NTD is proportional to the projected area of the 3D NTD from which it is reconstructed, but the projected areas are magnified by a fixed factor to aid visualization. Also shown is a single optical section through the set of 3D nuclear segmentations, where the shading of the nuclear masks is proportional to the mean voxel intensity of sna cytoplasmic mRNA in the corresponding Voronoi cells surrounding each nucleus (see Supporting Text). Notice the jagged but sharp sna NTD border. (C) The dorsal-ventral position of each NTD shown in (B) is plotted against the integrated fluorescence intensity of the NTD. Tick marks represent average nuclear spacing. Raw spatial profiles are uncorrected for background, variations in number of fluorophores associated with a target mRNA, or fluorophore efficiency. (D) sna NTD and cytoplasmic mRNA dorsal-ventral profile are reproduced from Fig. 1E for direct comparison with neuroectodermal gene expression. (E) Same format as panel B, but for an individual embryo in late cellularization stage of cycle 14 stained for brk instead of rho. Notice that an on/off border separates the sna and neuroectodermal gene expression domains, which are virtually mutually exclusive at this stage and that the sna expression border defined by sna NTDs is straight in contrast to the jagged, but equally sharp, NTD border present in earlier embryos (e.g., compare the meandering yellow border of reconstructed sna NTDs in this panel to the straight border evident in panel B). (F, G) Same format as panels C and D.
At the low magnification used for imaging the embryo in Fig. 2A, signals from NTDs are difficult to quantify since they consist of only a small number of voxels. To study the pattern of NTDs more quantitatively, we imaged expression of sna and various combinations of neuroectodermal genes at higher magnification in another embryo and reconstructed a field of nuclei at a higher voxel density (Fig. 2B; same embryo as in Fig. 1D and E). In the coded gene expression map representing this high-magnification reconstruction, the degree of white-to-gray in a nuclear segmentation represents the level of Sna mRNA staining with white representing the highest levels. As in the case of the lower-magnification field (Fig. 2A), overlapping patterns of neuroectodermal gene expression were clearly resolved within the presumptive mesoderm (Fig. 2B). By comparing gene expression maps obtained with various combinations of the four probes at this early stage, we confirmed our conclusions based on lower-magnification reconstructions that sog exhibits the broadest degree of mesodermal expression of the neuroectodermal genes analyzed (Fig. 2B), followed by vnd (Fig 2A, B) and rho (Fig. 2A), and that brk is strictly excluded from the mesoderm at all stages analyzed (Fig. 2A, B).
It is also possible to extract relative intensities of the spectral outputs of NTDs when imaged at high magnification by integrating voxel fluorescence intensity over each NTD (Supporting Text). We plotted the integrated spectral outputs (total intensity) from each NTD as a function of dorsal-ventral position in the embryo (Fig. 2C). In the case of sog, which is expressed furthest into the mesoderm, we found that there is a graded ventral decline in the total intensity of NTDs (Fig. 2C; see also Fig. 2B). The nested expression patterns of neuroectodermal genes within the dorsal region of the mesoderm and the graded size of NTDs in this same zone suggest that the expression of these genes is regulated in response to a gradient.
Gene-length correlates with the observed patterns of nested neural gene expression in the mesoderm
The dorsal-most portion of the mesoderm over which sna levels are graded coincides with the zone of differential neuroectodermal gene expression (compare Fig. 2D with B or C), suggesting that Sna may differentially repress neuroectodermal genes within this region. Previous detailed studies have established that neural gene repression in the mesoderm is accomplished by direct binding of Sna to enhancers of these genes (Gray et al., 1994; Ip et al., 1992a; Jiang and Levine, 1993; Zeitlinger et al., 2007b), and differential DNA occupancies of Sna protein on these CRMs has been determined using whole-genome tiling arrays (MacArthur et al., 2009). One obvious possibility is that the CRMs of the various neural genes have differing affinities or numbers of Sna binding sites and that genes transiently expressed more ventrally into the mesoderm (e.g., sog) have fewer and/or weaker Sna sites than those whose expression is strictly limited to the neuroectoderm (e.g., brk). However, the predicted order of nested gene expression based on relative DNA occupancy of Sna protein differs from that observed. The relative CRM occupancy order is sog, rho, brk, vnd, in which sog/rho exhibit weak Sna affinity whereas brk/vnd exhibit high Sna affinity (Fig. S6A), whereas the observed expression order is sog, vnd, rho, brk‥ Thus, differing sensitivities of neural CRMs to Sna do not seem to play the primary role in this patterning process, although they are likely to contribute to it (see analysis of sna+/− heterozygote data below).
Since differential cis-mediated regulation of neural target genes by Sna seemed unable to explain the nested pattern of neuroectodermal gene expression in the mesoderm, we considered alternative models. One correlation we observed is that the order of neural gene expression in the mesoderm corresponds with that of transcription unit length. We reasoned that if Sna acted by repressing initiation of gene expression, then longer genes would take more time to be repressed than shorter ones since it would take more time for previously loaded RNA Polymerase (RNAP) molecules to traverse longer genes. For example, a long transcription unit such as sog (~22 kb in length) requires ≈ 20 minutes to be fully transcribed, whereas a short gene such as brk (~3.5 kb) would take only about 3.5 minutes to be transcribed assuming that RNAP progresses at its typical rate of ≈1 kb per minute (O'Brien and Lis, 1993; O'Farrell, 1992). Thus, completion of transcription and hence onset of repression for such a pair of long versus short genes could amount to a differential delay of ≈ 15 minutes, which is a significant fraction of the cellularization stage of nuclear cycle 14. To examine this idea more fully, we developed a “delayed-repression model” based on the transient Sna gradient acting on target genes of differing gene lengths. As described below, this simple model can indeed account for much of the nested pattern of nascent neuroectodermal gene expression observed in vivo in wild-type embryos (Fig. 2C).
A simple gene-length model predicts the observed expression data in wild-type embryos
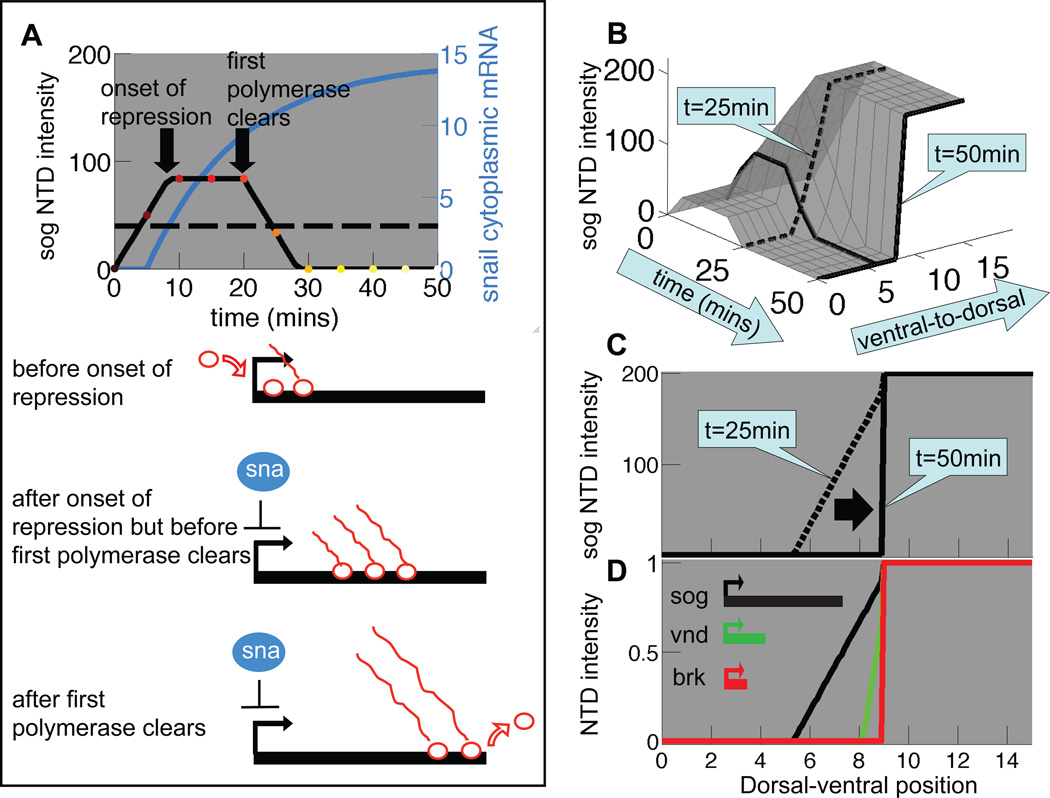
We developed a model based upon both the pattern and levels of neural gene expression obtained experimentally. The integrated fluorescence intensity levels of an NTD is expected to be proportional to the number of nascent transcripts on the gene when plotted in individual cells against their relative positions to the MNB. For example, to determine the expected space- and time-dependent intensity profiles of sog NTDs, we developed a mathematical model (Eq. [S3] in Supporting Text) that incorporates the kinetics of forming the Sna gradient with the delayed repression effect described above. Fig. 3A depicts a typical sna cytoplasmic mRNA time course (blue) as obtained from the expansion-accumulation model, and a threshold concentration (horizontal dashed line) corresponding to a hypothetical Sna level (Eq. [S5]) that completely blocks transcriptional initiation. The black solid curve in Fig. 3A is the resulting time course of the sog NTD intensity (Eq. [S3]; see discussion in the Supporting Text.) Aligning the time courses of sog NTD intensity for all nuclei along the dorsal-ventral axis yields the surface depicted in Fig. 3B. In Fig. 3C, we extracted two time slices from this calculated surface, yielding a transient gradient of sog NTD intensity (t=25 min) that sharpens to form an on-off profile (t=50 min.). These calculated profiles can account for the experimentally observed sog patterns at early and late cellularization stage of cycle 14 (the black scatter plots in Fig. 2C and 2F respectively).
Figure 3. Differences in neuroectodermal gene length yield a stratified pattern of neuroectodermal nascent gene expression in a delayed-repression model.
(A) Time course of sog NTD intensity (black line) in a mesodermal nucleus as calculated in the delayed-repression model (Eq. S3), using as input the sna cytoplasmic mRNA levels (blue line) calculated in the expansion-accumulation model (Eq. S2). The horizontal dashed line indicates the threshold concentration for sna repression (K = 3; see Eq. S5). The delay in repression arising from the length of the sog gene is assumed to be τ = 20 min. (Eq. S4). (B) sog NTD time courses for various nuclei can be combined to construct a surface describing the spatiotemporal dependence of sog NTD intensity. The black line corresponds to the temporal profile analyzed in (A). Spatial profiles at early (t=25 min.) and late (t=50 min.) cellular blastoderm are indicated. (C) Slices taken from the surface in (B) at early (t=25 min.) and late (t=50 min.) stages reveal a transient spatial gradient of sog NTD intensity. (D) The delayed-repression model (Eq. S3) predicts that transient nested gradients of sog, vnd and brk NTD intensity, similar to those observed experimentally (Fig. 2C), occur in early cycle 14 (t=25 min.) due solely to differences in neuroectodermal gene length. Transcriptional delays are assumed to be τ = 20 min. (sog), 6 min. (vnd) and 2 min. (brk), corresponding respectively to gene lengths of 22 kb (sog), 7 kb (vnd), and 3 kb (brk). These estimated gene lengths are based on RNA-seq data available through the modEncode Drosophila genome browser located at http://modencode.oicr.on.ca/fgb2/gbrowse/fly/ and on ChIP-chip polymerase-II binding data in early embryos ((Zeitlinger et al., 2007a); see also Fig. S2 of Ref (Boettiger and Levine, 2009)). Note that vnd has an alternate remote upstream promoter but the modEncode RNA-seq data indicate that this is not used until later in embryogenesis. The transcription initiation rate was chosen to be αon = 10 per min (same for all neuroectodermal genes), though its actual value does not affect our conclusions (αon is defined in Eq. S5).
The respective transcription-unit lengths of the sog, vnd and brk genes during cycle 14 are: sog ≈ 22 kb, vnd ≈ 7 kb, and brk ≈ 3.5 kb. The delayed-repression model predicts the transient formation of a gradient of neuroectodermal nascent gene expression, which has a width (the dorsal-ventral distance over which neuroectodermal gene expression changes) that varies in proportion to gene length (explained in Supporting Text) as depicted in Fig. 3D. These predicted early gene expression profiles are similar to those observed experimentally (Fig. 2C).
Thus, the simple model presented here, based on a ventral-to-dorsal expansion of sna expression and the delayed repression of neuroectodermal genes of differing lengths, accounts for not only the observed gradient in sna mRNA concentration near the MNB, but also much of the nested pattern of neuroectodermal nascent gene expression within the mesoderm of wild-type embryos. We emphasize that according to this highly simplified model, the cis-regulatory regions of brk, vnd and sog need not be differentially sensitive to Sna repression to generate the distinct spatial expression profiles shown in Fig. 3D. Of course, different sensitivities to Sna repression for each of the neuroectodermal enhancers are also likely to contribute to generating such patterns (as detailed below).
Widespread ectopic neural gene expression in the mesoderm of sna+/− embryos
In addition to examining the pattern of neural gene expression in the mesoderm of wild-type embryos, we also examined their expression in sna−/+ heterozygous embryos. This genetic background allowed us to test the cis-regulatory and gene-length models by comparing observed expression patterns with those predicted by a 50% reduction of Sna levels (see Supporting Text). As expected, there is a graded profile of sna (Fig. 4D) the same width (~4 nuclei), but with half the maximal level measured in wild-type embryos (Fig. 1E and 2D)
Figure 4. Widespread ectopic neuroectodermal gene expression in sna−/+ heterozygous embryos illustrates the haploinsufficiency of sna.
(A) Reconstruction of an individual sna−/+ embryo in early-mid cellularization stage of cycle 14 (same format as Fig. 2A). There is widespread mis-expression of vnd and rho in the mesoderm; in contrast brk is completely excluded from the mesoderm as in wild-type embryos. (B–D) Reconstructed image of a single early cycle 14 embryo, similar to Fig. 2B–D. Note that mesodermal nuclei contain only one NTD of sna each, confirming that the embryo is a sna−/+ heterozygote. (E–G) Mid-cellularization-stage cycle 14 embryo. (H–J) Late cellularization-stage cycle-14 embryo.
In sna−/+ embryos of early-mid cellularization stage of cycle 14, on/off plots of NTD expression for brk, vnd and rho revealed that vnd (green) and rho (blue) become distributed broadly and chaotically throughout the mesoderm, whereas expression of brk (red) retains a sharp ventral border (boundary between cyan and white in Fig. 4A). Quantitative analysis of NTD intensities confirmed the conclusions from the on/off analysis (Fig. 4B–D). In contrast to the nested gradients of sog and vnd NTD intensity previously observed in wild-type embryos (Fig. 2C), the mesodermal intensities of NTDs for these genes in early sna−/+ heterozygous embryos are nearly equal to their respective values in the neuroectoderm (Fig. 4C), indicating a nearly complete de-repression of these genes ventrally. Ventral de-repression of neuroectodermal genes is still apparent in the mid-stage embryo shown in Fig. 4E & F, although a fraction of nuclei have lost sog and vnd NTDs by this stage. Despite the prolonged persistence of high-level neuroectodermal gene expression in the mesoderm of sna+/− embryos relative to wild-type embryos, by late cellularization stage the typical one-nucleus sharp sna gradient is eventually established (Fig. 4H–J).
A role for cis-acting inputs in Sna-mediated repression of neural genes
The observed relative expression patterns of neuroectodermal genes in sna−/+ heterozygotes would not be predicted by models based only on CRM sensitivity as the response of the vnd CRM would need to be weak like that of sog/rho (all of which are ventrally de-repressed) whereas Sna ChIP data (Fig. S6A) indicate that the vnd CRM is actually more fully occupied than that of brk (which is never ventrally de-repressed). Widespread and prolonged mis-expression of sog (a long gene) in the mesoderm of sna−/+ embryos during early-mid cycle 14 (Fig. 4B–G) is predicted, however, by the delayed-repression model developed above (see Fig. S5B, C and Supporting Text). In addition, this simple model correctly predicts (Fig. S5C) the observed repression of sog from the mesoderm by the end of cellular blastoderm stage (Fig. 4H–J), giving way to a common sharp border of nascent expression. Another expectation of the model is that expression of the short gene brk would remain restricted in sna−/+ heterozygotes as it is in wild-type embryos.
Although the simple gene length model captures many features of the data presented thus far, one observation that is not accounted for is that the expression patterns of rho/vnd in sna−/+ embryos expand chaotically within the mesoderm, similar to that of sog (Fig. 4). The gene-length model predicts that rho/vnd should behave more like brk (which is always ventrally repressed), however, since all three genes are relatively short. The discrepancy can be resolved by incorporating differential CRM sensitivity to Sna repression into the gene-length model. We therefore scaled the threshold sna mRNA concentration for the onset of neuroectodermal repression (K in Eq. S5) with the inverse of the Sna sensitivity score (Fig. S6B) in a revised model, which alters its predictions (compare Fig. S6C and D) and more accurately models the observed data (see Supplementary Text for additional details).
A natural test of the gene-length hypothesis
As described above, the modified gene-length model accounts for key features of the observed transient patterns of neural gene expression within the mesoderm. A natural test of this model is fortuitously provided by a pair of genes, knirps (kni, a short gene - 3kb) and knirps-related (knrl, a long gene - 23kb), whose expression along the anterior-posterior axis is thought to be controlled by a common CRM (Rothe et al., 1989; Rothe et al., 1992). This identified CRM is located just upstream of the kni gene and drives reporter gene expression in a pattern coinciding with that of the endogenous central stripe of both kni and knrl at blastoderm stage (Rothe et al., 1992). Analysis of this phase of kni and knrl expression has implicated two repressor gradients, Hunchback and Giant, that respectively contribute to setting the anterior and posterior limits of kni/knrl expression (Capovilla et al., 1992; Eldon and Pirrotta, 1991; Hewitt et al., 1999; Hulskamp et al., 1994; Hulskamp et al., 1990; Rothe et al., 1994; Zinzen and Papatsenko, 2007).
On the lateral surface of the embryo, the kni stripe undergoes substantial shifts along the anterior-posterior axis during mid to late blastoderm stages of cycle 14 (Jaeger et al., 2004). In particular, the kni posterior border moves towards the anterior during the time interval defined by 0–50% membrane invagination (Fig. S4 of Ref. (Fowlkes et al., 2008)), presumably as a result of transcriptional repression by the Giant protein. The gene-length model predicts that the observed anteriorly-advancing front of kni transcriptional repression should leave behind a wake of NTDs of the longer knrl gene, mainly in nuclei located posterior of the kni stripe.
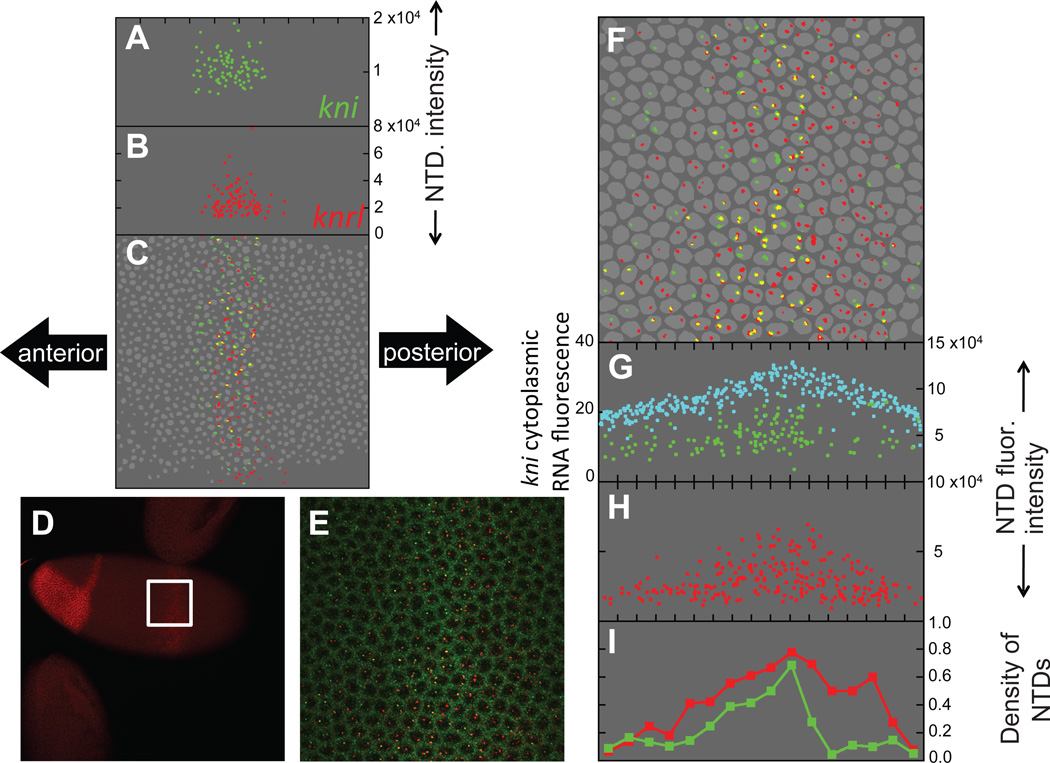
We tested the prediction that the knrl expression domain is broader than that of kni by comparing the expression domains of these sibling genes along the lateral surface of early (Fig. 5A–C) and mid-late (Fig. 5D–I) cellularization-stage embryos. Reconstruction of the kni and knrl NTDs (Fig. 5C and F) and quantitations of their anterior-posterior profiles (Fig. 5A–B and 5G–I) indicated that nascent knrl transcripts (red) coincided with kni NTDs (green) at early stages (Fig. 5A–C), but were more broadly distributed than those of kni at later stages (Fig. 5F–H). The expanded expression of knrl relative to kni was most notable along the posterior border of these gene expression domains (Fig. 5I). The difference between knrl and kni expression observed in this embryo was also observed in other embryos (Figs. S8, 9). We conclude that the gene-length hypothesis is likely to account for the observed differences in kni versus knrl expression.
Figure 5. The dynamic expression patterns of kni and knrl constitute a natural test of the gene-length hypothesis.
(A–C) Reconstruction (similar to Figs. 2 and 4) of an early-blastoderm-stage embryo with the field of view spanning the central kni/knrl stripe. Anterior is to the left, posterior to the right, ventral is down and dorsal is up. (D) Mid-blastoderm stage embryo stained for knrl mRNA (red) showing expression in an anterior patch and a central stripe. A higher magnification confocal stack (white square) spanning the central stripe was acquired and analyzed in (E–I). (E) Maximum projection of the central kni/knrl stripe stained for kni mRNA (green) and knrl mRNA (red). Image corresponds to the white square shown in (D). (F–H) Reconstruction of the embryo in (D) and (E). In (G), kni cytoplasmic mRNA fluorescence (cyan) is plotted on the left axis whereas kni NTD fluorescence intensity (green) is plotted on the right axis. (I) Anterior-posterior profile of NTD density. For each gene, we quantitated the spatial density of NTDs along the dorsal-ventral axis by binning the anterior-posterior axis, calculating the number of NTDs in each bin, and then normalizing by twice the number of nuclei in the corresponding bin (the factor of two arises because each gene exists in two copies in each nucleus).
Timing as a prepatterning bias to enhance morphogen patterning
The studies reported here suggest that variations in gene length of target genes may contribute to robust interpretation of crude positional information provided by morphogen gradients. As a generalization of the trend we observed in the mesoderm, we propose that one can define an activation-repression axis for many well characterized morphogen gradients. Signal transduction pathways can activate gene expression either by directly promoting transcription and/or by relieving constitutive repression of target genes (Barolo and Posakony, 2002). In cases where morphogens also act by a double-negative mechanism, this repressor transcriptional delay mechanism could act as a timing mechanism to generate spatial pattern. Direct activation of gene expression can also, in principle, provide temporal/spatial information based on gene length whereby shorter genes will be transcribed and translated sooner than longer genes. Both delayed repression and precocious activation would act in a coupled fashion by favoring short genes to be expressed near the activation (A) pole and at the same time favoring longer genes to be expressed near the repressor (R) pole of a gradient.
We surveyed several well-studied morphogen gradients and their known direct target genes to ask whether there is any correlation between gene length and position on the A/R axis (Fig. 6). This analysis suggests that there is indeed a general trend to obey the A/R rule for target genes of the Dorsal, Sna (this study), and Dpp gradients in the embryo, as well as the Hh and Dpp gradients in the wing imaginal disc. Noteworthy is a recent analysis of Hh signaling in the wing disc that suggests that an early transient pattern of Hh activation is consolidated by stabilizing feedback mechanisms (Nahmad and Stathopoulos, 2009).
Figure 6. Target genes of the Dl, BMP, and Hh morphogens generally follow the A/R rule.
(A) Relative positions of dorsal-most expression borders of dorsal target genes, together with their genomic length (in kb), are shown schematically on the left of the embryo. Relative positions of ventral-most expression borders of sna target genes, together with their genomic length (in kb), are shown on the right of the embryo. The arrows on the morphogen gradients indicate the hypothesized direction of gradient movement in the region of the embryo where the target genes are expressed. (B) Relative border positions of dpp targets genes in the dorsal epidermis (left) and relative dorsal-most borders of dpp targets in the neuroectoderm (right) of the embryo. Also shown are the oppositely directed gradients of dpp and brk. Genomic length in kb indicated in brackets. (C) Relative extents of gene expression of Dpp target genes in the wing imaginal disc with opposing gradients of Dpp and brk (genomic length in kb indicated in brackets). (D) Relative border positions of Hh target genes in the anterior half of the imaginal wing disc along with the reciprocal gradients of Hh and Ci (genomic length in kb indicated in brackets). In all four panels, there is a general trend, represented by target-gene names in bold type, favoring borders of short genes near the activation (A) pole and borders of longer genes near the repressor (R) pole of the corresponding morphogen gradient.
The general idea that timing plays an important role in developmental patterning is not new. In the example of stabilization of Hh response in the wing disc, differential rates of protein degradation was proposed as an important factor in this process (Nahmad and Stathopoulos, 2009). Another example of this patterning principle occurs in somitogenesis, where a moving maturation wave front generates a periodic array of somite boundaries. As the wave moves over the tissue, cells at the frontier of the wave become “locked-in” to different states according to their phase in a gene expression cycle (Dequeant and Pourquie, 2008; Ozbudak and Pourquie, 2008). Indeed, transcriptional delay is partly responsible for the oscillating gene expression underlying the moving maturation front (Takashima et al., 2011). Another example is the temporally ordered sequence of gene activation in distinct domains of the mesoderm and neuroectoderm in the early Drosophila embryo. In this case, more ventrally expressed genes are activated earlier (e.g., sna -> vnd -> ind -> msh). Interestingly, their order of activation corresponds to the sequence of cross-repressive interactions observed among transcription factors encoded by these genes (i.e., Sna represses all neuroectodermal genes, Vnd represses expression of ind and msh, and Ind represses expression of msh), a chain of interactions that is referred to as “ventral dominance” (Cowden and Levine, 2003). With regard to the neuroectoderm, prior expression of Vnd should reinforce its ability to effectively repress ind expression in the most ventral cells of the neuroectoderm, and similarly for Ind to gain dominance over msh in more lateral cells. Patterning of the vertebrate spinal cord has also been proposed to rely on similar timing mechanisms (Kutejova et al., 2009; Lek et al., 2010; Liem et al., 1997).
As mentioned above, there appears to be a general gene-length versus A/R axis trend extending to a variety of patterning systems, including those acting in the wing disc over much longer developmental periods (days) than establishment of the embryonic dorsal-ventral axis (≈1 hour). Thus, it may be that even though typical gene-length variation (1 kb – 100 kb) can only act over a period of an hour or so, it may nonetheless seed a refinement process in which robustness is gained from the early prepattern. For example, the gene-length mechanism may contribute to reliably setting closely spaced borders in cases where noise makes it difficult to initially distinguish corresponding thresholds in a gradient, as in situations where closely spaced borders are set by morphogen gradients (Yu and Small, 2008). Yet another possibility is that transient patterns function to bias cell-fate decisions controlled by commonly occurring cross-repressive interactions and feedback loops. In such cases, bi-stability may arise in which pre-steady-state dynamics can affect the outcome of which genes are eventually expressed in different domains.
In all of the cases described above, transient biases in gene expression acting in conjunction with a morphogen gradient can help establish discrete gene expression patterns that are then amplified and stabilized by other mechanisms including autoactivating positive feedback loops and cross-repressive interactions. It will be interesting in future studies to test whether transient spatial patterns generated by transcriptional/translational delay mechanisms such as those described here may also contribute to patterning effected by other morphogen gradients.
Is the early Sna gradient and graded repression of neural genes biologically relevant?
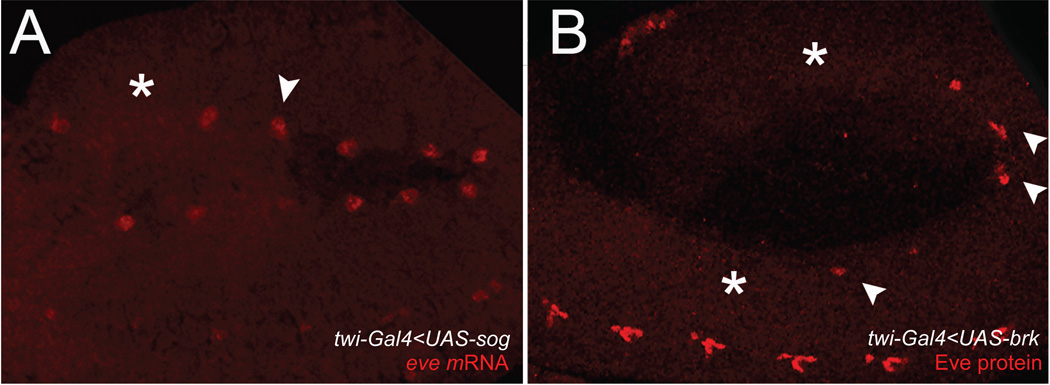
A fair question to ask regarding the transient formation of a Sna gradient described here is whether it plays any biological role or is just an epiphenomenon associated with the mechanics of Sna activation by the Dorsal gradient. Thus, we tested whether the relative sensitivity of the two genes on the extreme end of the spectrum, sog versus brk, might be biologically relevant. These two genes make for an informative comparison since they both act to inhibit the BMP signaling pathway. BMP signaling plays an essential role in the formation of somatic, visceral, and cardiac components during early gastrulation and blocking BMP signaling during this phase leads to severe defects in mesodermal patterning. Sog is an extracellular inhibitor, which prevents BMP ligands from binding the receptor, whereas Brk acts within the nucleus as a transcriptional repressor. Because Sog protein can readily diffuse, the protein diffusing from the neuroectoderm accumulates in a graded pattern within the mesoderm (Srinivasan et al., 2002), even after its expression is extinguished in the mesoderm by Sna. However, Sog would not be expected to affect BMP signaling during gastrulation, since it acts by inhibiting signaling by the heterodimeric Dpp:Scw ligand, and Scw is only produced during blastoderm stages. In contrast, Brk acts cell-autonomously and its expression is absent from the mesoderm at both the mRNA and protein levels. Hence the ectopic presence of Brk in the mesoderm should produce defects similar to those resulting from global loss of BMP signaling. Thus, we reasoned that Sog misexpression in the mesoderm of gastrulating embryos should have little effect on patterning there in contrast to Brk misexpression, which should lead to gross defects. We tested these predictions by expressing sog versus brk in the mesoderm using a twist-GAL4 driver (Fig. 7). As expected, sog misexpression had little effect on specification of eve-positive cells of the visceral mesoderm, while comparable expression of brk severely reduced their numbers (Fig. 7). Thus, evolutionary selection on transient sog mRNA expression in the mesoderm might have been more relaxed than for brk. Alternatively, there may have been positive selection for Sog gene length relevant to its known patterning effects in the dorsal epidermis or to yet undiscovered functions it may play in mesodermal patterning.
Figure 7. Mis-expression of Sog versus Brk in the developing mesoderm has divergent effects on developing visceral cells.
(A) in situ hybridization for eve, which labels precursor cells of the visceral mesoderm, in a twi-Gal4<UAS-sog E11 embryo. The majority of the visceral mesoderm precursor cells are present (arrowhead), with only a few exceptions (asterisk). (B) anti-Eve protein staining in a twi-Gal4<UAS-brk E11 embryo. Note that most eve+ cells are missing (asterisks). In both cases, expression of eve in the ventral nerve cord is normal. Embryos are oriented with anterior to the left.
Additional circumstantial evidence indicates that the Sna gradient might also be relevant to mesoderm invagination. For instance, expression patterns of genes involved in mesoderm invagination, such as folded gastrulation, are excluded from the region occupied by the graded portion of the Sna profile, whereas cells within the graded region undergo distinct morphological changes. Those changes in cell shape create an inflection in apical-basal versus basal-apical constriction, creating the Omega shape of invaginating epithelium (Martin et al., 2010). Whether additional components of this morphogenic system are expressed in patterns controlled directly or indirectly by the Sna gradient remains to be determined as this process is further dissected.
Possible evolutionary considerations
Non-coding DNA such as intergenic or intronic sequences tend to evolve more rapidly than coding sequences (Petrov and Hartl, 1998). Thus, altering gene length by lengthening or shortening intronic sequences should occur often during evolution (Petrov, 2002). If the type of timing bias we report is broadly relevant to other morphogen patterning systems, one could imagine that gene-length would be a readily evolvable means for creating a prepattern. Thus, "one-step" insertions or deletions that change gene length may result in dramatic and immediate changes. Gene-length mechanisms may also fine-tune the enhancer sensitivities of target genes to morphogen gradients during evolution. An interesting evolutionary question to consider as additional genomes are sequenced in various organisms will be whether there are correlations in gene length along A-R axes of various morphogen gradients in other species, as there tend to be in Drosophila.
Research Highlights.
A transient Sna gradient forms in the dorsal portion of the mesoderm in Drosophila embryos
Neural genes are transiently expressed in the mesoderm of Drosophila embryos.
A transcriptional-delay model based on gene length accounts for many aspects of the data.
The gene-length model correctly predicts relative expression patterns of kni, a short gene and knrl, a long gene.
Supplementary Material
Acknowledgements
We thank William Beaver, Ella Tour, Yoav Freund, and Michael Levine for valuable discussions and comments. Michael Levine provided the anti-Sna antibody, and fly stocks were obtained from the Bloomington Stock Center. P.M. thanks Arthur Lander for critical comments and suggestions, and the Center for Complex Biological Systems (UCI) where this work was completed. C.M.M. thanks Cheryl Hsia and William Beaver for advice with collection of confocal images. D.L.M. and M.B. were supported by start-up funds provided by CWRU to C.M.M. This work was supported by grants from E.B. (NSF IBN 0120728 and IOS-0920785, NIH R01 NS29870) and W.M. (NIH grant R37HD28315). P.M. and T.H. acknowledge the support of the NSF through the Center for Theoretical Biological Physics (Grant PHY-0822283).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
P.M., C.M.M., D.K., W.M., E.B., T.H. designed research; P.M., C.M.M., D.K., D.L.M, M.B., A.H., W.M., E.B., T.H. performed the research; P.M. contributed new analytic tools; P.M., C.M.M., D.K., W.M., E.B., T.H. analyzed the data; P.M., C.M.M., W.M., E.B., T.H. wrote the paper.
References
- Bardin AJ, Schweisguth F. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev Cell. 2006;10:245–255. doi: 10.1016/j.devcel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. doi: 10.1101/gad.976502. [DOI] [PubMed] [Google Scholar]
- Boettiger AN, Levine M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science. 2009;325:471–473. doi: 10.1126/science.1173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M, Eldon E, Pirrotta V. The giant gene of Drosophila encodes a B-ZIP DNA-binding protein that regulates the expression of other segmentation gap genes. Development. 1992;114:99–112. doi: 10.1242/dev.114.1.99. [DOI] [PubMed] [Google Scholar]
- Chitnis AB. Keeping single minded expression on the straight and narrow. Mol Cell. 2006;21:450–452. doi: 10.1016/j.molcel.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Cowden J, Levine M. The Snail repressor positions Notch signaling in the Drosophila embryo. Development. 2002;129:1785–1793. doi: 10.1242/dev.129.7.1785. [DOI] [PubMed] [Google Scholar]
- Cowden J, Levine M. Ventral dominance governs sequential patterns of gene expression across the dorsal-ventral axis of the neuroectoderm in the Drosophila embryo. Dev Biol. 2003;262:335–349. doi: 10.1016/s0012-1606(03)00395-6. [DOI] [PubMed] [Google Scholar]
- Crocker J, Tamori Y, Erives A. Evolution acts on enhancer organization to fine-tune gradient threshold readouts. PLoS Biol. 2008;6:e263. doi: 10.1371/journal.pbio.0060263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequeant ML, Pourquie O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- Eldon ED, Pirrotta V. Interactions of the Drosophila gap gene giant with maternal and zygotic pattern-forming genes. Development. 1991;111:367–378. doi: 10.1242/dev.111.2.367. [DOI] [PubMed] [Google Scholar]
- Fowlkes CC, Hendriks CL, Keranen SV, Weber GH, Rubel O, Huang MY, Chatoor S, DePace AH, Simirenko L, Henriquez C, Beaton A, Weiszmann R, Celniker S, Hamann B, Knowles DW, Biggin MD, Eisen MB, Malik J. A quantitative spatiotemporal atlas of gene expression in the Drosophila blastoderm. Cell. 2008;133:364–374. doi: 10.1016/j.cell.2008.01.053. [DOI] [PubMed] [Google Scholar]
- Francois V, Solloway M, O'Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Gray S, Szymanski P, Levine M. Short-range repression permits multiple enhancers to function autonomously within a complex promoter. Genes Dev. 1994;8:1829–1838. doi: 10.1101/gad.8.15.1829. [DOI] [PubMed] [Google Scholar]
- Hewitt GF, Strunk BS, Margulies C, Priputin T, Wang XD, Amey R, Pabst BA, Kosman D, Reinitz J, Arnosti DN. Transcriptional repression by the Drosophila giant protein: cis element positioning provides an alternative means of interpreting an effector gradient. Development. 1999;126:1201–1210. doi: 10.1242/dev.126.6.1201. [DOI] [PubMed] [Google Scholar]
- Hulskamp M, Lukowitz W, Beermann A, Glaser G, Tautz D. Differential regulation of target genes by different alleles of the segmentation gene hunchback in Drosophila. Genetics. 1994;138:125–134. doi: 10.1093/genetics/138.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulskamp M, Pfeifle C, Tautz D. A morphogenetic gradient of hunchback protein organizes the expression of the gap genes Kruppel and knirps in the early Drosophila embryo. Nature. 1990;346:577–580. doi: 10.1038/346577a0. [DOI] [PubMed] [Google Scholar]
- Ip YT, Park RE, Kosman D, Bier E, Levine M. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 1992a;6:1728–1739. doi: 10.1101/gad.6.9.1728. [DOI] [PubMed] [Google Scholar]
- Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992b;6:1518–1530. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Surkova S, Blagov M, Janssens H, Kosman D, Kozlov KN, Manu, Myasnikova E, Vanario-Alonso CE, Samsonova M, Sharp DH, Reinitz J. Dynamic control of positional information in the early Drosophila embryo. Nature. 2004;430:368–371. doi: 10.1038/nature02678. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Kirov N, Wieschaus E, Roth S, Rushlow C. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell. 1999a;96:563–573. doi: 10.1016/s0092-8674(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Rushlow C, Roth S. The role of brinker in mediating the graded response to Dpp in early Drosophila embryos. Development. 1999b;126:3323–3334. doi: 10.1242/dev.126.15.3323. [DOI] [PubMed] [Google Scholar]
- Jiang J, Kosman D, Ip YT, Levine M. The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 1991;5:1881–1891. doi: 10.1101/gad.5.10.1881. [DOI] [PubMed] [Google Scholar]
- Jiang J, Levine M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell. 1993;72:741–752. doi: 10.1016/0092-8674(93)90402-c. [DOI] [PubMed] [Google Scholar]
- Kosman D, Ip YT, Levine M, Arora K. Establishment of the mesoderm-neuroectoderm boundary in the Drosophila embryo. Science. 1991;254:118–122. doi: 10.1126/science.1925551. [DOI] [PubMed] [Google Scholar]
- Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- Kutejova E, Briscoe J, Kicheva A. Temporal dynamics of patterning by morphogen gradients. Curr Opin Genet Dev. 2009;19:315–322. doi: 10.1016/j.gde.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lek M, Dias JM, Marklund U, Uhde CW, Kurdija S, Lei Q, Sussel L, Rubenstein JL, Matise MP, Arnold HH, Jessell TM, Ericson J. A homeodomain feedback circuit underlies step-function interpretation of a Shh morphogen gradient during ventral neural patterning. Development. 2010;137:4051–4060. doi: 10.1242/dev.054288. [DOI] [PubMed] [Google Scholar]
- Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr., Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- MacArthur S, Li XY, Li J, Brown JB, Chu HC, Zeng L, Grondona BP, Hechmer A, Simirenko L, Keranen SV, Knowles DW, Stapleton M, Bickel P, Biggin MD, Eisen MB. Developmental roles of 21 Drosophila transcription factors are determined by quantitative differences in binding to an overlapping set of thousands of genomic regions. Genome Biol. 2009;10:R80. doi: 10.1186/gb-2009-10-7-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J Cell Biol. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Holbrook S, Isshiki T, Weiss J, Doe CQ, Mellerick DM. Dorsoventral patterning in the Drosophila central nervous system: the vnd homeobox gene specifies ventral column identity. Genes Dev. 1998;12:3603–3612. doi: 10.1101/gad.12.22.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellerick DM, Nirenberg M. Dorsal-ventral patterning genes restrict NK-2 homeobox gene expression to the ventral half of the central nervous system of Drosophila embryos. Dev Biol. 1995;171:306–316. doi: 10.1006/dbio.1995.1283. [DOI] [PubMed] [Google Scholar]
- Morel V, Le Borgne R, Schweisguth F. Snail is required for Delta endocytosis and Notch-dependent activation of single-minded expression. Dev Genes Evol. 2003;213:65–72. doi: 10.1007/s00427-003-0296-x. [DOI] [PubMed] [Google Scholar]
- Morel V, Schweisguth F. Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- Nahmad M, Stathopoulos A. Dynamic interpretation of hedgehog signaling in the Drosophila wing disc. PLoS Biol. 2009;7:e1000202. doi: 10.1371/journal.pbio.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T, Lis JT. Rapid changes in Drosophila transcription after an instantaneous heat shock. Mol Cell Biol. 1993;13:3456–3463. doi: 10.1128/mcb.13.6.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH. Developmental biology. Big genes and little genes and deadlines for transcription. Nature. 1992;359:366–367. doi: 10.1038/359366a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JW, Bier E. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques. 1994;17:870, 874–875. [PubMed] [Google Scholar]
- Ozbudak EM, Pourquie O. The vertebrate segmentation clock: the tip of the iceberg. Curr Opin Genet Dev. 2008;18:317–323. doi: 10.1016/j.gde.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Petrov DA. Mutational equilibrium model of genome size evolution. Theor Popul Biol. 2002;61:531–544. doi: 10.1006/tpbi.2002.1605. [DOI] [PubMed] [Google Scholar]
- Petrov DA, Hartl DL. High rate of DNA loss in the Drosophila melanogaster and Drosophila virilis species groups. Mol Biol Evol. 1998;15:293–302. doi: 10.1093/oxfordjournals.molbev.a025926. [DOI] [PubMed] [Google Scholar]
- Rao Y, Vaessin H, Jan LY, Jan YN. Neuroectoderm in Drosophila embryos is dependent on the mesoderm for positioning but not for formation. Genes Dev. 1991;5:1577–1588. doi: 10.1101/gad.5.9.1577. [DOI] [PubMed] [Google Scholar]
- Ray RP, Arora K, Nusslein-Volhard C, Gelbart WM. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development. 1991;113:35–54. doi: 10.1242/dev.113.1.35. [DOI] [PubMed] [Google Scholar]
- Roth S, Stein D, Nusslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Rothe M, Nauber U, Jackle H. Three hormone receptor-like Drosophila genes encode an identical DNA-binding finger. EMBO J. 1989;8:3087–3094. doi: 10.1002/j.1460-2075.1989.tb08460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Pehl M, Taubert H, Jackle H. Loss of gene function through rapid mitotic cycles in the Drosophila embryo. Nature. 1992;359:156–159. doi: 10.1038/359156a0. [DOI] [PubMed] [Google Scholar]
- Rothe M, Wimmer EA, Pankratz MJ, Gonzalez-Gaitan M, Jackle H. Identical transacting factor requirement for knirps and knirps-related Gene expression in the anterior but not in the posterior region of the Drosophila embryo. Mech Dev. 1994;46:169–181. doi: 10.1016/0925-4773(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Rusch J, Levine M. Threshold responses to the dorsal regulatory gradient and the subdivision of primary tissue territories in the Drosophila embryo. Curr Opin Genet Dev. 1996;6:416–423. doi: 10.1016/s0959-437x(96)80062-1. [DOI] [PubMed] [Google Scholar]
- Rushlow CA, Han K, Manley JL, Levine M. The graded distribution of the Dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989;59:1165–1177. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Regulation of notch signaling activity. Curr Biol. 2004;14:R129–R138. [PubMed] [Google Scholar]
- Srinivasan S, Rashka KE, Bier E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev Cell. 2002;2:91–101. doi: 10.1016/s1534-5807(01)00097-1. [DOI] [PubMed] [Google Scholar]
- Steward R. Relocalization of the Dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989;59:1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- Szymanski P, Levine M. Multiple modes of dorsal-bHLH transcriptional synergy in the Drosophila embryo. EMBO J. 1995;14:2229–2238. doi: 10.1002/j.1460-2075.1995.tb07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Ohtsuka T, Gonzalez A, Miyachi H, Kageyama R. Intronic delay is essential for oscillatory expression in the segmentation clock. Proc Natl Acad Sci U S A. 2011;108:3300–3305. doi: 10.1073/pnas.1014418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Stoetzel C, Gorostiza-Thisse C, Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988;7:2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Perrin-Schmitt F, Stoetzel C, Thisse B. Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell. 1991;65:1191–1201. doi: 10.1016/0092-8674(91)90014-p. [DOI] [PubMed] [Google Scholar]
- Wilkie GS, Shermoen AW, O'Farrell PH, Davis I. Transcribed genes are localized according to chromosomal position within polarized Drosophila embryonic nuclei. Curr Biol. 1999;9:1263–1266. doi: 10.1016/s0960-9822(99)80509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Small S. Precise registration of gene expression boundaries by a repressive morphogen in Drosophila. Curr Biol. 2008;18:868–876. doi: 10.1016/j.cub.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, Levine M, Young RA. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet. 2007a;39:1512–1516. doi: 10.1038/ng.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J, Zinzen RP, Stark A, Kellis M, Zhang H, Young RA, Levine M. Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 2007b;21:385–390. doi: 10.1101/gad.1509607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzen RP, Papatsenko D. Enhancer responses to similarly distributed antagonistic gradients in development. PLoS Comput Biol. 2007;3:e84. doi: 10.1371/journal.pcbi.0030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzen RP, Senger K, Levine M, Papatsenko D. Computational models for neurogenic gene expression in the Drosophila embryo. Curr Biol. 2006;16:1358–1358. doi: 10.1016/j.cub.2006.05.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.