Abstract
We have recently demonstrated that the ventral premammillary nucleus (PMV) plays a key role in the metabolic control of the female reproductive axis. However, whether PMV neurons modulate the reproductive neural circuitry and/or the expression of sexual behaviors has not been determined. Here, we showed that the expression of estrogen and progesterone receptors in the PMV is modulated by changing levels of sex steroids across the estrous cycle. We also showed that sexual behavior, not the high physiologic levels of sex steroids, induces Fos in PMV neurons. Bilateral lesions of the PMV caused no significant changes in proceptive behavior but a high percentage of PMV-lesioned rats failed to exhibit lordosis behavior when exposed to a sexually-experienced male rat (50% vs. 18% in the control group). Notably, lesions of the PMV disrupted the physiologic fluctuations of Kiss1 and GnRH mRNA expression characteristic of the proestrus-to-estrus transition. This neurochemical imbalance may ultimately alter female reproductive behavior. Our findings suggest that the PMV is a component of the neural circuitry that modulates the physiologic fluctuations of key neuroendocrine players (i.e., Kiss1 and GnRH) in the control of the female reproductive physiology.
Keywords: HPG axis, neuroendocrinology, hypothalamus, lordosis
Introduction
Female reproductive physiology is controlled by a myriad of brain areas. These sites ultimately converge to regulate the synthesis and release of GnRH which, in turn, controls the pituitary secretion of LH and FSH (Freeman, 2006). In cycling females, the rise in the circulating levels of estrogens that occurs on the day of proestrus stimulates GnRH release, which is observed as an increase in the frequency of pulses and sustained high levels of GnRH secretion, thereby triggering an LH surge and ovulation (Levine et al., 1982; Levine and Ramirez, 1982; Moenter et al., 1992; Herbison, 2008). Increases in estrogen levels also induce site-specific changes in the expression of estrogen and progesterone receptors (Simerly and Young, 1991; Simerly et al., 1996; Österlund et al., 1998; Yamada et al., 2009). These genomic and neuronal adaptations, in turn, prime the neuroendocrine axis for the night of behavioral estrus (Pfaff et al., 2006).
Recent studies demonstrated that the hypothalamic ventral premammillary nucleus (PMV) plays an instrumental role in the coordinated control of the neuroendocrine reproductive axis (Donato et al., 2009; Donato and Elias, 2011; Donato Jr. et al., 2011b). The PMV innervates GnRH and kisspeptin neurons and projects to areas involved in the genesis and modulation of sexual behaviors (Canteras et al., 1992; Rondini et al., 2004; Leshan et al., 2009; Donato Jr. et al., 2011b). Lesions of the PMV disrupt female cyclicity, generating an initial state of anestrus followed by the reinstatement of cycles with atypical vaginal cytology. At the time of the LH surge, PMV-lesioned rats display low levels of estradiol and LH and decreased activation of GnRH neurons and key brain nuclei, such as the anteroventral periventricular nucleus (AVPV) (Donato et al., 2009).
Therefore, our previous observation that PMV-lesioned rats display decreased estradiol and gonadotropin secretion at the time of the LH surge suggested that their reproductive physiology is compromised. But whether the lack of PMV inputs ultimately leads to disruption of the related neural circuitry and/or behavioral deficits has not been determined. In the present study, we initially investigated whether PMV neurons are responsive to changing levels of sex steroids across the estrous cycle and whether they are a component of the circuitry engaged in sexual behavior. We then assessed whether the disruption of PMV inputs compromises the neural circuitry involved in neuroendocrine regulation and/or the adequate expression of sexual behaviors.
Experimental procedures
Subjects
Adult male and female Sprague-Dawley rats (8 weeks old) were maintained on a 12-h light/dark cycle in a temperature-controlled (22 ± 2°C) environment with free access to water and food. All experiments were performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and Institutional Committee for Research and Animal Care of the University of São Paulo. Females were perfused in specific periods of their estrous cycles or submitted to stereotaxic surgeries and sexual behavior. Males were used as breeders in the behavioral tests.
Experiment 1: Expression of sex steroids receptors in the PMV across the estrous cycle
Increases in estradiol levels during the proestrus day alter the expression of estrogen receptor alpha (ERα) and the progesterone receptor (PR) in several brain sites (Simerly and Young, 1991; Simerly et al., 1996; Österlund et al., 1998; Yamada et al., 2009). These changes prime the neuronal circuitry for neuroendocrine control of ovulation and sexual behavior (Freeman, 2006; Adachi et al., 2007; Herbison, 2008; Oakley et al., 2009). PMV neurons express ERα and PR (Warembourg et al., 1986; Simerly et al., 1990; Intlekofer and Petersen, 2011); however, whether they are responsive to changing levels of sex steroids across the estrous cycle is not known. To address this question, we used brain sections from female rats previously defined as being in diestrus I (second day of predominant leucocytes in the vaginal lavage) and proestrus (nucleated cells in the vaginal lavage), according to vaginal cytology and hormone profile (Donato et al., 2009). Rats were divided into two groups: a) those perfused during diestrus I (low circulating levels of sex steroids, n = 5) and b) those perfused during proestrus (high circulating levels of sex steroids, n = 4). The estrous cycle was assessed daily in the morning by analysis of vaginal cytology as previously described (Donato et al., 2009), and rats were perfused 1 hour before lights off. Series of hypothalamic sections containing PMV and control sites – i.e., the ventrolateral subdivision of ventromedial nucleus of the hypothalamus (VMHvl), the medial tuberal nucleus (MTu) and the posterodorsal subdivision of the medial nucleus of amygdala (MeApd) – were processed for ERα or PR immunoreactivity.
Experiment 2: Requirement of PMV neurons for female sexual behavior
Previous studies have shown that the PMV neurons of ovariectomized estrogen- and progesterone-primed rats express Fos immunoreactivity after copulation (Pfaus et al., 1993; Coolen et al., 1996; Pfaus and Heeb, 1997). However, one of these studies also found that control rats submitted to the hormone replacement regimen showed numbers of Fos immunoreactive neurons in the PMV that were similar to rats that underwent the behavioral test (Coolen et al., 1996). To assess whether the physiologic increase in sex steroids levels induces Fos expression in PMV neurons, cycling females were divided into 3 groups: a) rats perfused during the afternoon of the proestrus day (high sex steroids levels, Control Proestrus, n = 6); b) rats perfused during the night of estrus, 3h after lights off (Control Behavior, n = 5); and c) rats perfused 40 min after sexual behavior 3 h after lights off (Behavior, n = 9). To further investigate the role played by the PMV in the expression of sexual behaviors, we produced bilateral lesions of the PMV in adult female rats. During the night of estrus, rats were housed with a sexually experienced male, and proceptivity and receptivity were assessed.
Experiment 3: Neuroendocrine characterization of PMV-lesioned female rats following sexual behavior
To further investigate the role played by PMV neurons in the neuroendocrine events that occur during behavioral estrus, rats submitted to the sexual behavior paradigm (same rats of the Experiment 2) were screened for changes in hormone levels (estradiol, progesterone, prolactin, testosterone, LH and FSH) and neuropeptide (GnRH and Kiss1) gene expression.
Stereotaxic surgeries
Stereotaxic surgeries were performed on female rats under Equitesin anesthesia (ip, 3 mg/100 g sodium thiopental, 12.7 mg/100 g chloral hydrate). NMDA (0.15 M, Sigma) was injected iontophoretically from a glass micropipette into the PMV bilaterally [coordinates: anteroposterior (from bregma) = −3.9; mediolateral (from midline) = ± 0.7; dorsoventral (from dura-mater) = −8.5] by applying a −8 μA pulsed current at 7 s intervals for 15 min (n = 27). NMDA is effective in inducing excitotoxic neuronal lesions without affecting fibers of passage (Sisk et al., 1988).
Sexual behavior test
The behavioral test was conducted approximately 8 weeks after stereotaxic surgeries in an acoustically isolated room equipped with red lights. The behavioral test was initiated 2–3 hours after the beginning of the dark cycle on the night of proestrus day (night of estrus), when female rats are expected to be sexually receptive (Pfaff et al., 2006). Females were moved to an acrylic box (40 cm height × 40 cm width × 60 cm length) containing bedding and were allowed to adapt to the environment for 30 min. Then, a sexually experienced male was introduced into the cage and sexual behavior was recorded. Males (n = 3) were randomly allocated to the cages with females, and each male was used in more than one trial.
We evaluated the sexual proceptivity and receptivity of each female in the presence of a male. Proceptivity was defined as the occurrence of stereotypic behaviors including darting, hopping and ear wiggling (Pfaff et al., 2006). To assess sexual receptivity, we evaluated the occurrence of lordosis behavior following 10 attempts to mount (McGinnis and Gorski, 1980).
Perfusion and tissue sectioning
Female rats were deeply anesthetized with a cocktail containing ketamine (5 mg/100 g), xylazine (1 mg/100 g), and acepromazine (0.2 mg/100 g). Immediately before the perfusion, a blood sample was collected from the heart to assess their hormonal profile. Rats were perfused with 4% paraformadehyde in borate-buffer (pH 9.5 at 4°C). Brains were dissected, post-fixed for 1 h and cryoprotected overnight at 4°C in diethylpyrocarbonate (DEPC)-treated 0.1 M phosphate-buffer saline pH 7.4 (PBS) containing 20% sucrose. The brains were cut (30-μm sections) in the frontal plane on a freezing microtome. Five series of sections were collected and stored at −20°C in cryoprotectant.
Evaluation of bilateral lesions and definition of experimental groups
The extent of PMV lesions and contamination of adjacent hypothalamic nuclei were assessed by thionin (Nissl) staining (Donato et al., 2009). We also confirmed the extent of lesions by assessing the expression of NADPH diaphorase (NADPHd) activity (Donato et al., 2010a; Donato et al., 2010b) and cocaine and amphetamine regulated transcript (CART) mRNA by in situ hybridization (Douglass et al., 1995; Cavalcante et al., 2006). Both NADPHd and CART mRNA are highly expressed in the PMV and in adjacent nuclei.
We classified rats as “PMV-lesioned” if they showed specific and bilateral lesions of the PMV. As controls, we used NMDA-injected rats that showed an intact PMV and no lesions of adjacent hypothalamic nuclei (PMV-non-lesioned group). Females with small lesions of the PMV or with lesions of adjacent nuclei were excluded from the analysis.
NADPH diaphorase
To label neurons that express NADPHd activity, a series of hypothalamic sections containing the PMV of control and lesioned female rats were rinsed overnight in PBS and incubated in a solution containing 0.02% of βNADPH (Sigma) and 0.02% of nitroblue tetrazolium (Sigma) in 1 M Tris-HCl, pH 7.4 with 0.1% Triton X-100 at 37°C in the dark, as we reported previously (Donato et al., 2010a; Donato et al., 2010b). Following 2 to 5 h, the reaction was terminated with rinses in PBS. Sections were mounted onto gelatin-coated slides, dehydrated in ascending concentrations of ethanol, delipidated in xylene and coversliped with DPX mounting medium.
Immunohistochemistry
Brain sections of rats on diestrus or proestrus were rinsed in PBS and blocked in 3% normal donkey serum for 1 h, followed by incubation overnight in anti-ERα antiserum raised in rabbit (1:100,000, Millipore, C1355) or in anti-PR monoclonal antibody from mouse (1:2,000, Millipore, MAB462). Subsequently, sections were incubated for 90 min in AlexaFluor 488-conjugated IgG donkey anti-rabbit or anti-mouse (1:500, Invitrogen) for ERα or PR, respectively. Sections were rinsed in PBS, mounted onto gelatin-coated slides, dried and coverslipped with Flouromount G (Electron Microscopy Sciences). Hypothalamic sections of PMV-lesioned and PMV non-lesioned rats were also submitted to ERα immunolabeling to detect lesion in the VMHvl. Additionally, hypothalamic sections of rats on proestrus were also evaluated for Fos immunoreactivity. Sections were blocked in 3% normal donkey serum, and incubated overnight at room temperature in anti-Fos polyclonal primary antisera raised in rabbit (Ab5, 1:70,000, Oncogene). Sections were incubated for 1 h in biotin-conjugated IgG donkey anti-rabbit (1:1,000, Jackson Laboratories) and for 1 h in avidin-biotin complex (1:500, Vector Labs). The peroxidase reaction was performed using 0.05% DAB and 0.025% nickel sulfate as chromogens, and 0.03% hydrogen peroxide.
In situ hybridization histochemistry
The brain sections were mounted onto SuperFrost plus slides (Fisher Scientific). Before hybridization, sections were fixed in 4% formaldehyde for 5 min, pretreated with proteinase K (Roche) at 37°C for 30 min, followed by triethanolamine plus acetic anhydride for 10 min. Sections were then dehydrated in ascending concentrations of ethanol, cleared in xylene for 15 min and re-hydrated in descending concentrations of ethanol. The riboprobes (GnRH, Kiss1, CART and Fos) were generated by in vitro transcription with 35S-UTP. The 35S-labeled probes were diluted (106 dpm/mL) in the hybridization solution. The solution consisted of 50% formamide, 10 mM Tris-HCl (Gibco-BRL), 0.01% sheared salmon sperm DNA, 0.01% yeast tRNA, 0.05% total yeast RNA (Sigma), 10 mM dithiothreitol, 10% dextran sulfate, 0.3 M NaCl, 1 mM EDTA (pH 8.0) and 1x Denhardt’s solution (Sigma). The hybridization solution (120 μl) was applied to each slide, and slides were incubated overnight at 57°C. Sections were then treated with 0.002% RNAase A solution and submitted to stringency washes in decreasing concentrations of sodium chloride/sodium citrate buffer (SSC). Sections were dehydrated and enclosed in X-ray film cassettes with BMR-2 film (Kodak) for 2 to 4 days. Slides were dipped in NTB2 autoradiographic emulsion (Kodak), dried overnight and stored at 4°C for 14 to 30 days (according to the signal on the film). Slides were developed with a Dektol developer (Kodak), dehydrated, cleared in xylene, and coverslipped with DPX.
The GnRH cDNA was kindly provided by Dr. Rexford Ahima (University of Pennsylvania, PA, USA), and its specificity has been described previously (Bond et al., 1989). Kiss1 cDNA was generated in our laboratory, the details and specificity of which has been published previously (Donato et al., 2009; Cravo et al., 2011). The CART and Fos cDNA was kindly provided by Dr. P. Couceyro (Finch University of Health Sciences, Chicago Medical School, North Chicago, IL, USA) and Dr. J.K. Elmquist (University of Texas Southwestern Medical Center, Dallas, TX, USA), respectively, and the specificity of these cDNAs has been previously described (Curran et al., 1983; Douglass et al., 1995).
Data analysis and production of photomicrographs
An observer unaware of the experimental groups performed quantifications. Only one representative section from one side of the brain for each nucleus was quantified; therefore, the data were not corrected for double counting. Brain levels were defined according to the Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, 1997).
The ERα or PR immunoreactive cells in the PMV (level 33) and in control sites –i.e., the VMHvl (level 30), MTu (level 31) and MeApd (level 31) – were quantified, and the means (± SEM) were compared between rats in diestrus or proestrus. ImageJ software (http://rsb.info.nih.gov/ij) was used to count the number of ERα or PR immunoreactive cells and each counted cell was marked avoiding double counting in the same section. Only one section from one side of each nucleus was assessed. The hybridization signal was estimated by analysis of the integrated optical density (IOD) using ImageJ software. Darkfield photomicrographs were acquired using the same illumination and exposure time for every section. No image editing was performed before quantification. For quantification of GnRH and Kiss1 mRNA expression, we assessed the IOD of individual cells. We considered only the cells that had IOD values at least 3 times higher than that of the background, which was determined from regions adjacent to the region of interest. We determined the number of cells and the IOD/cell.
Brain sections were analyzed on a Zeiss Axioplan microscope (Carl Zeiss). Photomicrographs were produced by capturing images with a digital camera (Axiocam, Zeiss) mounted directly on the microscope. Adobe Photoshop CS3 image-editing software was used to integrate photomicrographs into plates. Only sharpness, contrast and brightness were adjusted. Illustrator CS3 was used to produce the schematic drawings using thionin staining as template.
Hormone assays
Commercial radioimmunoassay kits were used to measure the levels of estradiol, progesterone (BioChem ImmunoSystems) and testosterone (DSL). The lower detection limits and the intra-assay coefficients of variation were, respectively, 7.5 pg/mL and 2.5% for estradiol, 4.1 ng/mL and 3.7% for progesterone, and 0.08 ng/mL and 8.5% for testosterone. LH, FSH and prolactin (PRL) were determined by radioimmunoassay using kits provided by the National Hormone and Peptide Program (Harbor-UCLA Medical Center, USA) as described elsewhere (Anselmo-Franci et al., 1997). The reference preparations were LH-RP3, FSH-RP2 and PRL-RP2. The lower detection limits were 0.05 ng/mL, 0.2 ng/mL and 0.2ng/mL, and the intra-assay coefficients of variation were 4%, 3% and 3.5%, for LH, FSH and PRL, respectively.
Statistical analysis
The comparisons between diestrus vs. proestrus or PMV-lesioned vs. PMV-non lesioned groups were carried out using unpaired two-tailed Student’s t test. To compare three groups simultaneously we used one-way ANOVA followed by the pairwise Newman-Keuls test. Data on proceptivity and receptivity were described as the percentage of rats in each group, and no statistical comparisons were performed due to the variability of behavioral data (in control and lesioned rats) and lack of statistical power. The data are expressed as the mean ± SEM. Statistical analysis was performed using GraphPad Prism software (San Diego, CA), and α values of less than 0.05 (P < 0.05) were taken as significant in all analyses.
Results
Experiment 1: Expression of sex steroids receptors immunoreactivity in the PMV across the estrous cycle
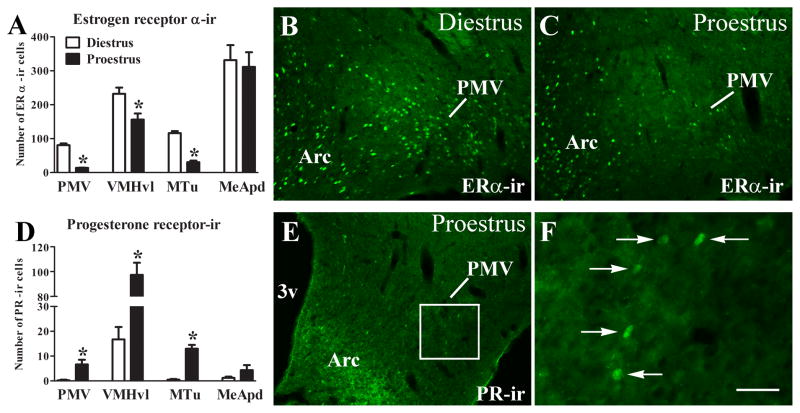
We observed that ERα-immunoreactivity (ERα-ir) in the PMV of rats perfused in the afternoon of the proestrus day (high estrogen levels) was decreased compared with rats perfused during the afternoon of diestrus I (low estrogen levels, Fig. 1A–C). This change follows the pattern observed in the VMHvl and the MTu. By contrast, no difference in the ERα-ir was observed in the MeApd across the estrous cycle (Fig. 1A). We then assessed PR-immunoreactivity (PR-ir) in PMV neurons. Virtually no PR-ir was found in the PMV of females perfused during diestrus (Fig. 1D). However, we observed a small but significant increase in the number of PR-immunoreactive cells in the ventral aspects of the PMV of females perfused during proestrus (Fig. 1D–F). In agreement with earlier studies (MacLusky and McEwen, 1978; Simerly and Young, 1991; Simerly et al., 1996; Österlund et al., 1998; Yamada et al., 2009), increased PR-ir was observed in the VMHvl and MTu of females during proestrus, but no changes were detected in PR-ir expression in the MeApd. These findings suggest that PMV neurons are responsive to changing levels of sex steroids across the estrous cycle.
Figure 1.
Regulation of sex steroid receptors in PMV neurons. A. Bar graphs showing the number of estrogen receptor α-immunoreactive (ERα-ir) neurons in the PMV of rats perfused during the afternoon of diestrus I (n = 5) or during the afternoon of the proestrus day (n = 4). B–C. Fluorescent photomicrographs of brain sections showing neurons that express ERα-ir in the PMV of rats perfused during diestrus (B) or the proestrus day (C). D. Bar graphs showing the number of progesterone receptor-immunoreactive (PR-ir) neurons in the PMV of rats perfused during diestrus or during the afternoon of the proestrus. E–F. Fluorescent photomicrographs of brain sections showing neurons that express progesterone receptor-immunoreactivity (PR-ir) in the PMV of rats perfused in the afternoon of proestrus. F is a higher magnification of the area highlighted in E. Data in the bar graph are expressed as mean ± SEM. *, statistically different (P < 0.05) from diestrus groups. We used unpaired two-tailed Student’s t tests. Abbreviations: 3v, third ventricle; Arc, arcuate nucleus; Scale bar: B–E = 160 μm; F = 40 μm.
Experiment 2: Requirement of PMV neurons for female sexual behavior
Sexual behavior, not high physiologic levels of sex steroids, induces Fos in PMV neurons
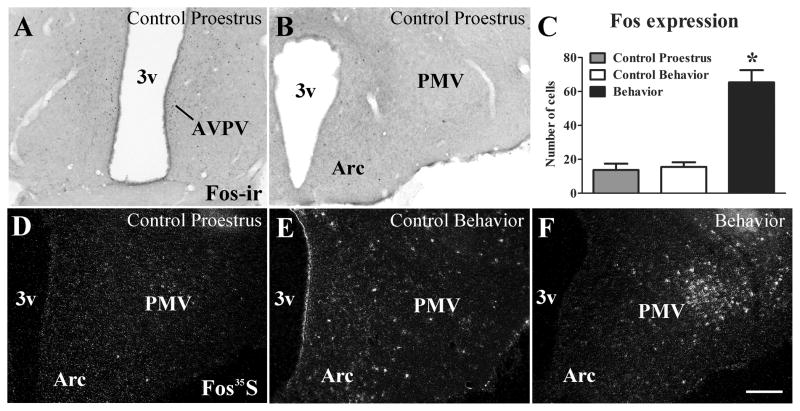
Previous studies have shown that sexual behavior or sex steroid treatment alone may induce Fos expression in PMV neurons (Pfaus et al., 1993; Coolen et al., 1996; Pfaus and Heeb, 1997). Thus, we assessed whether increased physiologic levels of sex steroids in cycling females induces Fos in the PMV. We observed that females perfused in the afternoon of the proestrus day (1h before lights off) showed increased Fos expression in the AVPV and PeN (Fig. 2A), in agreement with previous studies (Le et al., 1999; Donato et al., 2009). However, virtually no Fos expression (mRNA or protein) was detected in the PMV of females on proestrus (Fig. 2B, D). Likewise, a very low hybridization signal was detected in PMV neurons of rats perfused during the night of estrus (Control Behavior, Fig. 2E). In contrast, high densities of Fos mRNA were observed in the PMV of female rats perfused following sexual behavior (Fig. 2C, F). Our findings indicate that components of female sexual behavior (e.g., male odor, lordosis, etc.) contribute to the induction of Fos in PMV neurons independently of changes in sex steroids levels.
Figure 2.
Sexual behavior, not high physiologic levels of sex steroids, induces Fos in PMV neurons. A–B. Brightfield photomicrographs showing the distribution of Fos immunoreactivity (Fos-ir) in the anteroventral periventricular nucleus (AVPV, A) and in the ventral premammillary nucleus (PMV, B) of females on the afternoon of proestrus. Note the absence of Fos-ir in the PMV. C. Bar graphs showing the quantification of neurons that express Fos mRNA (Fos35S) in the PMV of rats perfused on the afternoon of proestrus (1h before light off, Control Proestrus, n = 4), of rats perfused 40 min after sexual behavior (3h after lights off, Behavior, n = 9) or control rats (3h after lights off, Control Behavior, n = 5). D–F. Darkfield photomicrographs showing Fos expression in the PMV of a rat perfused on proestrus, of a control rat (Control Behavior) and a rat perfused 40 min after expressing lordosis (Behavior). Of note is the increased Fos expression in the PMV only after sexual behavior. Data in bar graphs are expressed as mean ± SEM. *, statistically different (P < 0.05) from both control groups. For statistical analysis, we used the one-way ANOVA followed by the pairwise Newman-Keuls test. Abbreviations: 3v, third ventricle; Arc, arcuate nucleus. Scale bar: 160 μm.
Characterization of bilateral PMV lesions
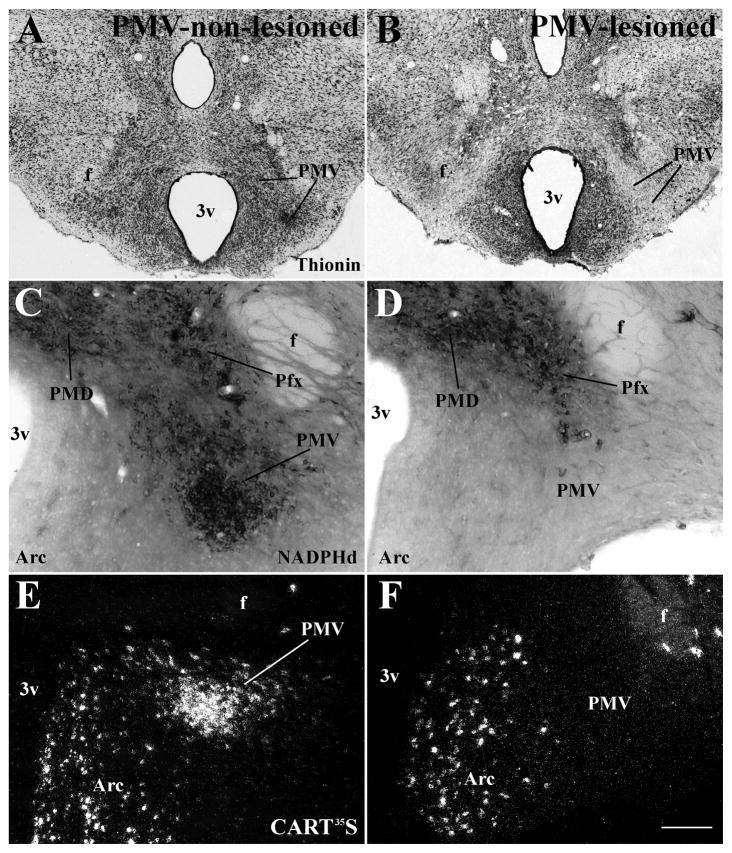
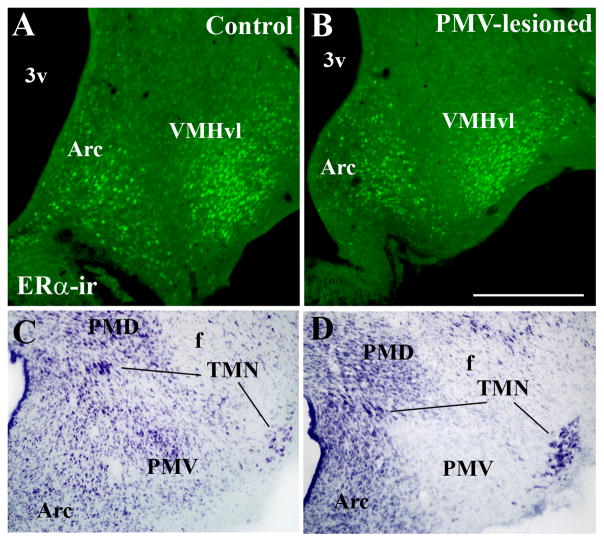
To further investigate the role played by the PMV in the expression of sexual behaviors, we produced bilateral lesions of the PMV in adult female rats. The extent of PMV lesions was determined by thionin staining of hypothalamic sections (Fig. 3A–B). In addition, two neurochemical markers (NADPHd activity and CART mRNA) that are highly expressed in PMV neurons and in surrounding nuclei were used to confirm the extent and the specificity of the lesions. We obtained 8 rats with specific and bilateral lesions of the PMV (PMV-lesioned group, Fig. 3B, D, F, Fig. 4). In these cases, we observed a marked decrease in the number of cells in the area comprising the PMV, and absence of NADPHd activity and CART mRNA expression bilaterally in the PMV. Because the VMHvl plays a critical role in the expression of female sexual behavior (Kow and Pfaff, 1988; Musatov et al., 2006) and the tuberomammillary nucleus (TMN) is essential for circadian and seasonal rhythms (Deurveilher and Semba, 2005; I’Anson et al., 2011), we performed a meticulous evaluation of putative contaminations or lesions of these sites. The VMHvl expresses a dense collection of ERα and the TMN is readily distinguished by its magnocellular characteristic. We used these two features to further determine whether injections of NMDA into the PMV compromised either one or both nuclei. Only rats with intact VMHvl defined by thionin staining and ERα immunoreactivity (Fig. 5A–B) were used in our analysis. As previously shown (Gerashchenko et al., 2004), we observed that TMN neurons are resistant to excitotoxic lesions as no difference in cell number and morphology was identified in any of the cases with good injections in the PMV (Fig. 5C–D). Morphology and cell density were also preserved in other adjacent nuclei including the MTu, the arcuate, the dorsal premammillary and the medial mammillary nuclei (Fig. 3). We obtained 11 control rats classified as PMV non-lesioned. These rats showed an intact PMV, as indicated by apparently normal densities of thionin-stained neurons, of NADPHd activity and expression of CART mRNA and no lesions of adjacent nuclei (Fig. 3A, C, E; Fig 4). In PMV-non lesioned rats, the normal distributions of NADPHd and CART mRNA, and normal neuronal density in the PMV, were defined by comparison with intact females perfused during proestrus (data not shown). Rats with partial lesions of the PMV or compromise of surrounding nuclei (n = 8) were excluded from all analysis.
Figure 3.
Characterization of bilateral excitotoxic lesions of the PMV. A–B. Brightfield photomicrographs of brain sections stained with thionin showing the PMV of a PMV-non-lesioned (A) and a PMV-lesioned rat (B). C–D. Brightfield photomicrographs of brain sections showing neurons that express NADPHd diaphorase (NADPHd) activity in a PMV-non-lesioned (C) and a PMV-lesioned rat (D). E–F. Darkfield photomicrographs showing the expression of cocaine and amphetamine regulated transcript (CART) mRNA (CART35S) in a PMV-non-lesioned (E) and a PMV-lesioned rat (F). The PMV neurons are intact in PMV-non-lesioned rats and absent in PMV-lesioned rats. Also, adjacent nuclei are preserved indicating the specificity of the lesions. Abbreviations: 3v, third ventricle; Arc, arcuate nucleus; f, fornix; Pfx, perifornical area; PMD, dorsal premammillary nucleus. Scale bar: A–B = 500 μm; C–D = 140 μm; E–F = 200 μm.
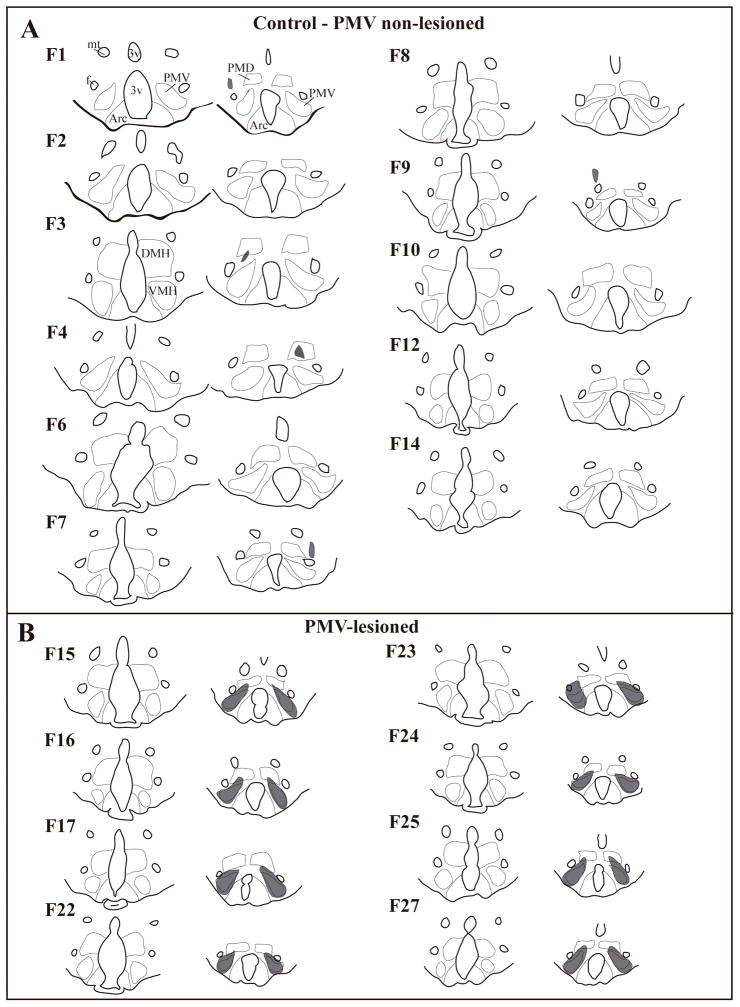
Figure 4.
Schematic illustration of control and excitotoxic lesions of the ventral premammillary nucleus (PMV). A, line drawings showing two rostro-to-caudal levels of brain sections from rats used as control (PMV non lesioned, small lesions are depicted in gray, F = individual rats). B, line drawing showing two rostro-to-caudal levels of brain sections from PMV-lesioned rats (lesions depicted in gray, F = individual rats). Only cases with intact ventromedial nucleus of the hypothalamus (VMH, rostral sections in B) were used. Please refer to cases F1 and F3 for abbreviations: 3v, third ventricle; Arc, arcuate nucleus; DMH, dorsomedial nucleus of the hypothalamus; f, fornix; mt, mammillothalamic nucleus; PMD, dorsal premammillary nucleus.
Figure 5.
Lack of contamination of the ventromedial and the tuberomammillary nuclei by the excitotoxic lesions (NMDA injections) of the ventral premammillary nucleus (PMV). A–B, Images showing distribution of estrogen receptor α (ERα) immunoreactivity in the arcuate nucleus (Arc) and in the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl) in a control (PMV non-lesioned, A) and in a PMV-lesioned (B) rat. Note the similarity of ERα-ir distribution in both cases, indicating lack of contamination of the VMHvl. C–D, Images showing the distribution of neurons of the tuberomammillary complex (dorsal and ventral tuberomammillary nuclei, TMN) in a control (PMV non-lesioned, C) and in a PMV-lesioned (D) rat. Note the similarity of TMN neurons (magnocellular and dark cytoplasm) distribution indicating lack of compromise of TMN neurons. Abbreviations: 3v, third ventricle; f, fornix; PMD, dorsal premammillary nucleus.
Lack of PMV inputs cause behavioral deficits
Following 8 weeks of recovery from surgery, we assessed changes in well-defined stereotypic behaviors indicative of sexual proceptivity and receptivity (Pfaff et al., 2006) in PMV-lesioned and PMV-non lesioned rats. We observed that lesions of the PMV caused no major changes in proceptive behaviors, as 60% of PMV-non lesioned rats and 50% of PMV-lesioned rats displayed similar pattern of darting, hopping and ear wiggling in the presence of the male. On the other hand, 50% of PMV-lesioned rats were non-receptive (no lordosis behavior in the first 10 attempts to mount), whereas only 18% of control rats failed to show lordosis. In those PMV-lesioned rats that show lordosis, we also report a decrease in the mean number of mounts resulting in lordosis compared to controls. PMV-lesioned rats also showed 40% decrease in the mean number of lordosis in 10 attempts to mount (3.5 ± 1.4 vs. 5.7± 1.2 in control rats), although this data did not reach statistical significance (P = 0.25).
Sexual proceptivity and receptivity can significantly affect aspects of the male’s sexual performance, including latency to mount and ejaculation rate (Landau and Madden, 1983; Erskine et al., 1989; Dewsbury, 1990). We did not observe a significant difference in the latency to mount between the PMV-lesioned and PMV-non lesioned groups. We observed that 30% of the males attained ejaculation during the first 10 attempts to mount when mating with PMV non-lesioned rats, whereas 12% of the males ejaculated when mating with PMV-lesioned rats.
Experiment 3: Neuroendocrine characterization of PMV-lesioned female rats following sexual behavior
Lesions of the PMV induce no changes in sex hormone levels after behavior test
To determine whether PMV neurons exert any effect on sex hormone levels after the behavioral test, we assessed the serum concentrations of LH, FSH, prolactin, estradiol, testosterone and progesterone in PMV-lesioned and PMV non-lesioned rats. No significant changes between the experimental groups were observed in serum levels of LH (PMV-non-lesioned: 46.4 ± 18.7 ng/mL; PMV-lesioned: 19.0 ± 9.0 ng/mL; P = 0.25), FSH (PMV-non-lesioned: 7.5 ± 1.4 ng/mL; PMV-lesioned: 8.1 ± 2.8 ng/mL; P = 0.82), prolactin (PMV-non-lesioned: 337 ± 100 ng/mL; PMV-lesioned: 657 ± 125 ng/mL; P = 0.06), estradiol (PMV-non-lesioned: 10.0 ± 1.1 pg/mL; PMV-lesioned: 8.4 ± 0.7 pg/mL; P = 0.27) or testosterone (PMV-non-lesioned: 0.14 ± 0.03 ng/mL; PMV-lesioned: 0.12 ± 0.02 ng/mL; P = 0.72). We also found no changes in mean levels of progesterone (PMV-non-lesioned: 99.6 ± 12.5 ng/mL; PMV-lesioned: 85.6 ± 21.3 ng/mL; P = 0.56). Nonetheless, we observed that progesterone levels were increased in females that showed lordosis compared to those that were non-receptive (120.7 ± 9.0 ng/mL vs. 35.0 ± 5.2 ng/mL in non-receptive; P < 0.0001). This pattern was observed in PMV-lesioned and PMV-non lesioned rats indicating that components of sexual behavior – perhaps uterine cervical stimulation – are the primary stimulus responsible for the observed increase in progesterone secretion (Adler et al., 1970).
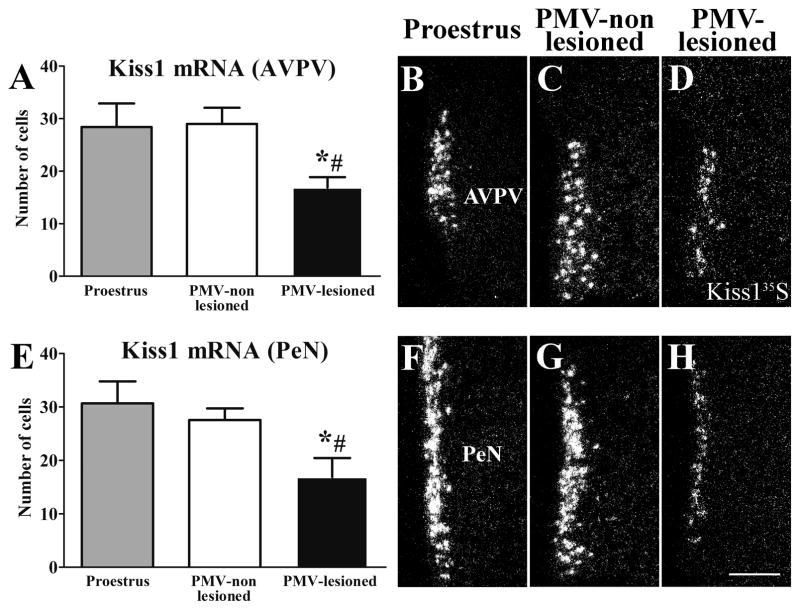
PMV-lesioned rats display suppressed Kiss1 mRNA expression in the AVPV and PeN
The PMV densely projects to the AVPV, a site involved in the preovulatory LH surge (Canteras et al., 1992; Rondini et al., 2004; Hahn and Coen, 2006). In addition, we have recently shown that fibers originating from PMV neurons are in close apposition to AVPV Kiss1 neurons (Donato Jr. et al., 2011b). As kisspeptin neurons are key players in reproductive physiology (de Roux et al., 2003; Seminara et al., 2003; Clarkson et al., 2008; Oakley et al., 2009; Clarkson et al., 2010; Pineda et al., 2010), we assessed the expression of Kiss1 mRNA by in situ hybridization in the AVPV and the anterior periventricular nucleus (PeN) of PMV-lesioned and PMV-non lesioned rats after the sexual behavior test. On the night of the behavioral estrus, PMV-non lesioned rats show similar number of neurons expressing Kiss1 mRNA (3× higher than background level) in the AVPV (Fig. 6A–C) and in PeN (Fig. 6E–G) compared to rats perfused in the afternoon of the proestrus day. On the other hand, we found a significant decrease in the number of neurons expressing Kiss1 mRNA (3× higher than background level) in the AVPV (Fig. 6A–D) and in PeN (Fig. 6E–H) of PMV-lesioned rats compared to PMV-non-lesioned rats and rats perfused in the afternoon of the proestrus, indicating a likely effect in intensity (mRNA levels) not absolute cell number.
Figure 6.
Lesions of the PMV suppressed Kiss1 mRNA expression in the anteroventral periventricular nucleus (AVPV) and the periventricular nucleus (PeN) during the behavioral test (night of estrus). A, E. Bar graphs showing quantification of the number of cells expressing Kiss1 mRNA (Kiss135S) of rats perfused in the afternoon of the proestrus (n = 5), of PMV-non-lesioned rats (n = 10) and of PMV-lesioned rats (n = 8) in the AVPV (A) and the PeN (E). PMV-lesioned rats showed a decreased number of Kiss1 cells in the AVPV and the PeN after the sexual behavior test compared to proestrus and PMV-non-lesioned rats. B–D, F–H. Darkfield photomicrographs showing the expression of Kiss1 mRNA in a proestrus (B, F), a PMV-non-lesioned (C, G) and a PMV-lesioned (D, H) rat in the AVPV (B–D) and the PeN (F–H). Data in bar graphs are expressed as mean ± SEM. *, statistically different (P < 0.05) from the proestrus group. #, statistically different (P < 0.05) from the PMV-non-lesioned group. For statistical analysis, we used the one-way ANOVA followed by the pairwise Newman-Keuls test. Scale bar = 200 μm.
The number of Kiss1 expressing cells in the arcuate nucleus of female rats is markedly reduced during proestrus and estrus compared with other phases of the estrous cycle (Smith et al., 2006). Accordingly, we found very low expression of Kiss1 mRNA in the arcuate nucleus of rats perfused on the night of estrus, and no difference between PMV-lesioned and PMV-non lesioned rats was detected (P = 0.68, data not shown).
PMV-lesioned rats show abnormally high GnRH mRNA expression on the night of the behavioral estrus
GnRH secretion or central infusion of GnRH analogs or antagonists significantly affects sexual behavior (Moss and Foreman, 1976; Sakuma and Pfaff, 1980, 1983). The PMV projects directly to GnRH neurons and likely modulates GnRH synthesis and/or secretion (Beltramino and Taleisnik, 1985; Rondini et al., 2004; Donato et al., 2009; Leshan et al., 2009; Donato Jr. et al., 2011b). Therefore, in order to assess the effects of lesions of the PMV on GnRH expression, we performed in situ hybridization for GnRH mRNA in brain sections of PMV-lesioned and PMV-non lesioned rats perfused after the sexual behavior test. We quantified the number of GnRH expressing neurons in a neuronal population located in the vicinity of the vascular organ of the lamina terminalis (OVLT). Unexpectedly, we observed that PMV-lesioned rats showed a higher number of neurons expressing GnRH mRNA (3× higher than background level) compared to PMV-non lesioned rats (P = 0.006; Fig. 7), indicating a likely effect in intensity (mRNA levels) not absolute cell number. Because GnRH mRNA expression is expected to decrease during the night of estrus compared to the expression level at the time of the LH surge (Park et al., 1990; Porkka-Heiskanen et al., 1994; Wang et al., 1995), we assessed the number of cells expressing GnRH mRNA in a group of rats perfused one hour before lights off on the proestrus day. As predicted (Park et al., 1990; Porkka-Heiskanen et al., 1994; Wang et al., 1995), PMV-non lesioned rats show a significant reduction in the number of cells expressing GnRH mRNA (3× higher than background) compared to proestrus rats, whereas PMV-lesioned rats displayed similar number of GnRH-expressing neurons compared to females perfused at the time of the LH surge (Fig. 7). Our findings indicate that lesions of the PMV disrupt the physiologic fluctuation of GnRH mRNA expression observed across the proestrus day and the night of estrus.
Figure 7.
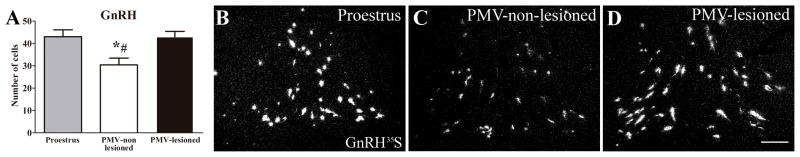
GnRH mRNA expression is abnormally increased in PMV-lesioned rats during the behavioral test (night of estrus). A. Bar graphs showing the quantification of the number of cells expressing GnRH mRNA (GnRH35S) of rats perfused in the afternoon of the proestrus (n = 5), of PMV-non-lesioned rats (n = 10) and of PMV-lesioned rats (n = 8). B–D. Darkfield photomicrographs showing the expression of GnRH mRNA in a proestrus (B), a PMV-non-lesioned (C) and a PMV-lesioned (D) rat. Data in bar graphs are expressed as mean ± SEM. *, statistically different (P < 0.05) from proestrus group. #, statistically different (P < 0.05) from the PMV-lesioned group. For statistical analysis, we used the one-way ANOVA followed by the pairwise Newman-Keuls test. Scale bar = 200 μm.
Discussion
In the present study, we demonstrate that PMV neurons are responsive to changing levels of sex steroid hormones and are activated during the expression of sexual behavior. Notably, lesions of the PMV altered the physiologic changes in Kiss1 and GnRH gene expression characteristic of the proestrus-to-estrus transition and decreased the occurrence of lordosis behavior.
The role played by PMV neurons in reproductive physiology was initially proposed by studies showing its involvement in odor-induced LH secretion (Beltramino and Taleisnik, 1985). Subsequently, it was made clear that the PMV is a putative target of sex steroid hormones, as it has a high concentration of ERα and androgen receptors, and ERβ to a lesser extent (Simerly et al., 1990; Merchenthaler et al., 2004). Using labeled progestin ligands, earlier studies also suggested that the PMV concentrates PR (Parsons et al., 1982; Rees et al., 1985), and recently it was demonstrated that PMV neurons express PR mRNA (Intlekofer and Petersen, 2011). Estrogen’s effects on ERα and PR expression are well described. Elevated levels of estrogen downregulate ERα and induce PR expression in a variety of brain areas such as the AVPV, the MPN and the mediobasal hypothalamus, whereas other sites (e.g., the medial amygdala) show no changes or more complex responses (MacLusky and McEwen, 1978; Simerly and Young, 1991; Simerly et al., 1996; Österlund et al., 1998; Yamada et al., 2009). Our findings indicate that, in physiologic conditions of high estrogens (proestrus), PMV neurons exhibit lower ERα-ir and higher PR-ir compared to diestrus. Although these differences indicate that the activity and/or gene expression of PMV neurons is modulated by changing levels of sex hormones across the estrous cycle, the number of cells positive to PR was very low in both conditions. Whether methodological issues (e.g., fixative use in the detection of PR immunoreactivity) decreased the labeling efficiency of PR immunoreactivity is not known. In any case, the physiological relevance of these findings needs further investigation.
The sustained high level of estrogen in proestrus is a crucial event; it triggers GnRH secretion and induces genetic and neurochemical modifications in specific neuronal populations involved in behavioral responses (Levine et al., 1982; Levine and Ramirez, 1982; Moenter et al., 1992; Pfaff et al., 2006; Herbison, 2008). The current literature supports the concept that the positive feedback action of estrogen upon GnRH secretion is mediated by interneurons (Wintermantel et al., 2006; Herbison, 2008). Of those interneurons, the kisspeptin cells located in the AVPV and PeN are of fundamental importance (Popolow et al., 1981; Wiegand and Terasawa, 1982; Herbison, 2008). The high estrogen levels characteristic of the afternoon of proestrus, or induced by hormone replacement, stimulate Kiss1 gene expression in AVPV and PeN neurons that, in turn, induces GnRH secretion (Gottsch et al., 2004; Navarro et al., 2005; Smith et al., 2005; Smith et al., 2006; Navarro et al., 2009). But, as expected from a highly redundant circuitry, other brain sites also play a role. In agreement with this notion, lesions of PMV neurons caused a condition of low levels of estrogen and LH and decreased activation of Kiss1 and GnRH neurons at the time of the LH surge (Donato et al., 2009). Herein, we investigated the consequences of this imbalanced condition on female sexual behavior. In cycling females, Kiss1 expression in the AVPV and PeN remains high during the transition from the afternoon of the proestrus day to the night of estrus (Kinoshita et al., 2005; Smith et al., 2006; Adachi et al., 2007). During the night of behavioral estrus, PMV-lesioned rats displayed decreased Kiss1 mRNA in the AVPV and PeN compared to PMV-non-lesioned rats. Whether these effects are the consequence of the lack of PMV inputs to Kiss1 cells or of the neuroendocrine imbalance caused by PMV lesions at the time of LH surge is unknown. However, lower expression of Kiss1 mRNA in the AVPV and PeN likely has a negative impact on preparation of the reproductive system for the night of behavioral estrus.
The GnRH gene expression is highly variable across the proestrus day (Park et al., 1990; Porkka-Heiskanen et al., 1994; Wang et al., 1995). At the time of the LH surge, GnRH neurons located adjacent to the OVLT are more transcriptionally active and exhibit high GnRH mRNA expression. However, during the night of estrus, this same neuronal population displays suppressed GnRH mRNA expression (Park et al., 1990; Porkka-Heiskanen et al., 1994; Wang et al., 1995). Accordingly, our control rats that were perfused during the estrus night had lower numbers of GnRH expressing neurons compared to rats perfused at the time of the LH surge. Notably, however, PMV-lesioned rats displayed abnormally high GnRH expression indicating that the expected suppression of GnRH mRNA observed during the night of estrus had not occurred. We postulate that the lack of excitatory inputs from PMV neurons caused a non-homeostatic steady state of GnRH expression across the transition from the proestrus day to the night of estrus.
Female sexual behavior relies on the rise and sustained high levels of estrogen (Davidson et al., 1968; Sodersten and Eneroth, 1981; Pfaff et al., 2006). Therefore, the low levels of estrogen during mid-proestrus (Donato et al., 2009) may be the major cause of the higher percentage of PMV-lesioned rats that did not express lordosis during the sexual behavior test. However, it is worth mentioning that GnRH also facilitates lordosis behavior (Moss and McCann, 1973; Pfaff, 1973; Sakuma and Pfaff, 1980), which raises the possibility that altered GnRH secretion or neurotransmission may have blunted the behavioral responses in this experimental model. Taken together, our findings suggest that removal of PMV inputs generates a vicious cycle in which decreased activation of GnRH and Kiss1 neurons in the AVPV/PeN results in decreased gonadotropins and estrogen secretion and, consequently, a deficient feedback action on GnRH neurons during the proestrus day. Ultimately, this condition may have a negative impact on female sexual behavior.
Of note, we have recently shown that PMV neurons play an essential role in mediating the effects of the adipocyte hormone leptin in the reproductive system (Donato Jr. et al., 2011a; Donato Jr. et al., 2011b). Interestingly, reports have suggested that leptin facilitates lordosis behavior in hamsters and rats (Wade et al., 1997; Donato Jr. et al., 2011a; García-Juárez et al., 2011), and these effects seem to be mediated by nitric oxide (Garcia-Juarez et al., 2012). Because the PMV houses the largest population of neurons that colocalizes nitric oxide and leptin receptors (Donato et al., 2010b; Leshan et al., 2012), the putative action of leptin on lordosis may be attained via direct stimulation of PMV neurons. Further studies will be necessary to test this hypothesis.
Highlights.
Changes in sex steroid levels modulate the expression of ERα in the PMV
Bilateral lesions of the PMV decrease receptive (lordosis) behavior
PMV-lesioned rats exhibit abnormal Kiss1 and GnRH mRNA expression in the estrus night
Acknowledgments
Financial support was provided by the NIH grants (HD061539 and HD69702) and Regent’s Scholar Research Award (UTSW) to C.F.E., and the São Paulo Research Foundation (FAPESP-Brazil) grants and fellowship (05/58997–4, 05/59286–4) to J.D.Jr, N.S.C. and C.F.E.. We thank Amanda Oliveira and Rogério Azevedo for technical assistance. We also thank Drs. R. Ahima (University of Pennsylvania, PA, USA), P. Couceyro (Finch University of Health Sciences, Chicago Medical School, North Chicago, IL, USA) and J.K. Elmquist (UT Southwestern Medical Center, Dallas, TX, USA) for supplying GnRH, CART and cFos cDNAs, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Adler NT, Resko JA, Goy RW. The effect of copulatory behavior on hormonal change in the female rat prior to implantation. Physiol Behav. 1970;5:1003–1007. doi: 10.1016/0031-9384(70)90155-1. [DOI] [PubMed] [Google Scholar]
- Anselmo-Franci JA, Franci CR, Krulich L, Antunes-Rodrigues J, McCann SM. Locus coeruleus lesions decrease norepinephrine input into the medial preoptic area and medial basal hypothalamus and block the LH, FSH and prolactin preovulatory surge. Brain Res. 1997;767:289–296. doi: 10.1016/s0006-8993(97)00613-6. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Ventral premammillary nuclei mediate pheromonal-induced LH release stimuli in the rat. Neuroendocrinology. 1985;41:119–124. doi: 10.1159/000124164. [DOI] [PubMed] [Google Scholar]
- Bond CT, Hayflick JS, Seeburg PH, Adelman JP. The rat gonadotropin-releasing hormone: SH locus: structure and hypothalamic expression. Mol Endocrinol. 1989;3:1257–1262. doi: 10.1210/mend-3-8-1257. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- Cavalcante JC, Bittencourt JC, Elias CF. Female odors stimulate CART neurons in the ventral premammillary nucleus of male rats. Physiol Behav. 2006;88:160–166. doi: 10.1016/j.physbeh.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 Signaling Is Essential for Preovulatory Gonadotropin-Releasing Hormone Neuron Activation and the Luteinizing Hormone Surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Han S-K, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Molecular and Cellular Endocrinology. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, MacConnell WP, van Straaten F, Verma IM. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983;3:914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JM, Rodgers CH, Smith ER, Bloch GJB. Stimulation of Female Sex Behavior in Adrenalectomized Rats with Estrogen Alone. Endocrinology. 1968;82:193–195. doi: 10.1210/endo-82-1-193. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Modes of estrus induction as a factor in studies of the reproductive behavior of rodents. Neurosci Biobehav Rev. 1990;14:147–155. doi: 10.1016/s0149-7634(05)80215-5. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The Ventral Premammillary Nucleus Links Fasting-Induced Changes in Leptin Levels and Coordinated Luteinizing Hormone Secretion. J Neurosci. 2009;29:5240–5250. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav. 2010a;99:67–77. doi: 10.1016/j.physbeh.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne) 2011;2:57. doi: 10.3389/fendo.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin Induces Phosphorylation of Neuronal Nitric Oxide Synthase in Defined Hypothalamic Neurons. Endocrinology. 2010b;151:5415–5427. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazão R, Elias CF. Hypothalamic Sites of Leptin Action Linking Metabolism and Reproduction. Neuroendocrinology. 2011a;93:9–18. doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011b;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS, Kornberg E, Cherry JA. Paced copulation in rats: effects of intromission frequency and duration on luteal activation and estrus length. Physiol Behav. 1989;45:33–39. doi: 10.1016/0031-9384(89)90163-7. [DOI] [PubMed] [Google Scholar]
- Freeman ME. Chapter 43: Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 2°. Amsterdam; Boston: Elsevier; 2006. pp. 2283–2388. [Google Scholar]
- Garcia-Juarez M, Beyer C, Gomora-Arrati P, Lima-Hernandez FJ, Dominguez-Ordonez R, Eguibar JR, Etgen AM, Gonzalez-Flores O. The nitric oxide pathway participates in lordosis behavior induced by central administration of leptin. Neuropeptides. 2012;46:49–53. doi: 10.1016/j.npep.2011.09.003. [DOI] [PubMed] [Google Scholar]
- García-Juárez M, Beyer C, Soto-Sánchez A, Domínguez-Ordoñez R, Gómora-Arrati P, Lima-Hernández FJ, Eguibar JR, Etgen AM, González-Flores O. Leptin facilitates lordosis behavior through GnRH-1 and progestin receptors in estrogen-primed rats. Neuropeptides. 2011;45:63–67. doi: 10.1016/j.npep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Chou TC, Blanco-Centurion CA, Saper CB, Shiromani PJ. Effects of lesions of the histaminergic tuberomammillary nucleus on spontaneous sleep in rats. Sleep. 2004;27:1275–1281. doi: 10.1093/sleep/27.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol. 2006;494:190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I’Anson H, Jethwa PH, Warner A, Ebling FJP. Histaminergic regulation of seasonal metabolic rhythms in Siberian hamsters. Physiology & Behavior. 2011;103:268–278. doi: 10.1016/j.physbeh.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Transmitter and peptide actions on hypothalamic neurons in vitro: implications for lordosis. Brain Res Bull. 1988;20:857–861. doi: 10.1016/0361-9230(88)90102-5. [DOI] [PubMed] [Google Scholar]
- Landau IT, Madden JE. Hormonal regulation of female proceptivity and its influence on male sexual preference in rats. Physiol Behav. 1983;31:679–685. doi: 10.1016/s0031-9384(83)80003-1. [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140:510–519. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012 doi: 10.1038/nm.2724. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo Y-H, Rhodes CJ, Munzberg H, Myers MG., Jr Direct Innervation of GnRH Neurons by Metabolic- and Sexual Odorant-Sensing Leptin Receptor Neurons in the Hypothalamic Ventral Premammillary Nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE, Pau K-YF, Ramirez VD, Jackson GL. Simultaneous Measurement of Luteinizing Hormone-Releasing Hormone and Luteinizing Hormone Release in Unanesthetized, Ovariectomized Sheep. Endocrinology. 1982;111:1449–1455. doi: 10.1210/endo-111-5-1449. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology. 1982;111:1439–1448. doi: 10.1210/endo-111-5-1439. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Gorski RA. Sexual behavior of male and female septal lesioned rats. Physiology & Behavior. 1980;24:569–573. doi: 10.1016/0031-9384(80)90253-x. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Moenter S, Brand R, Karsch F. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology. 1992;130:2978–2984. doi: 10.1210/endo.130.5.1572305. [DOI] [PubMed] [Google Scholar]
- Moss RL, Foreman MM. Potentiation of lordosis behavior by intrahypothalamic infusion of synthetic luteinizing hormone-releasing hormone. Neuroendocrinology. 1976;20:176–181. doi: 10.1159/000122481. [DOI] [PubMed] [Google Scholar]
- Moss RL, McCann SM. Induction of Mating Behavior in Rats by Luteinizing Hormone-Releasing Factor. Science. 1973;181:177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österlund M, Kuiper GJMG, Gustafsson J-Å, Hurd YL. Differential distribution and regulation of estrogen receptor-[alpha] and -[beta] mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Park O-K, Gugneja S, Mayo KE. Gonadotropin-Releasing Hormone Gene Expression during the Rat Estrous Cycle: Effects of Pentobarbital and Ovarian Steroids. Endocrinology. 1990;127:365–372. doi: 10.1210/endo-127-1-365. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow T, MacLusky N, McEwen B. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science. 1973;182:1148–1149. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y, Kow L, Lee AWL, Easton A. Chapter 34: Hormonal, neural, and genomic mechanisms for female reproductive behaviors, motivation, and arousal. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 2°. Amsterdam; Boston: Elsevier; 2006. pp. 1825–1920. [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kleopoulos SP, Mobbs CV, Gibbs RB, Pfaff DW. Sexual stimulation activates c-fos within estrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624:253–267. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Physiological Roles of the Kisspeptin/GPR54 System in the Neuroendocrine Control of Reproduction. In: Luciano M, editor. Prog Brain Res. Vol. 181. Elsevier; 2010. pp. 55–77. [DOI] [PubMed] [Google Scholar]
- Popolow HB, King JC, Gerall AA. Rostral medial preoptic area lesions’ influence on female estrous processes and LHRH distribution. Physiol Behav. 1981;27:855–861. doi: 10.1016/0031-9384(81)90053-6. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Urban J, Turek F, Levine J. Gene expression in a subpopulation of luteinizing hormone-releasing hormone (LHRH) neurons prior to the preovulatory gonadotropin surge. J Neurosci. 1994;14:5548–5558. doi: 10.1523/JNEUROSCI.14-09-05548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees HD, Bonsall RW, Michael RP. Localization of the synthetic progestin 3H-ORG 2058 in neurons of the primate brain: evidence for the site of action of progestins on behavior. J Comp Neurol. 1985;235:336–342. doi: 10.1002/cne.902350305. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. LH-RH in the mesencephalic central grey can potentiate lordosis reflex of female rats. Nature. 1980;283:566–567. doi: 10.1038/283566a0. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Modulation of the lordosis reflex of female rats by LHRH, its antiserum and analogs in the mesencephalic central gray. Neuroendocrinology. 1983;36:218–224. doi: 10.1159/000123459. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 Gene as a Regulator of Puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Carr AM, Zee MC, Lorang D. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Young BJ. Regulation of Estrogen Receptor Messenger Ribonucleic Acid in Rat Hypothalamus by Sex Steroid Hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Nunez AA, Thebert MM. Differential effects of electrolytic and chemical hypothalamic lesions on LH pulses in rats. Am J Physiol. 1988;255:E583–590. doi: 10.1152/ajpendo.1988.255.5.E583. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodersten P, Eneroth P. Serum levels of oestradiol-17{beta} and progesterone in relation to sexual receptivity in intact and ovariectomized rats. J Endocrinol. 1981;89:45–54. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- Wade GN, Lempicki RL, Panicker AK, Frisbee RM, Blaustein JD. Leptin facilitates and inhibits sexual behavior in female hamsters. Am J Physiol. 1997;272:R1354–R1358. doi: 10.1152/ajpregu.1997.272.4.R1354. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Hoffman GE, Smith MS. Increased GnRH mRNA in the GnRH neurons expressing cFos during the proestrous LH surge. Endocrinology. 1995;136:3673–3676. doi: 10.1210/endo.136.8.7628409. [DOI] [PubMed] [Google Scholar]
- Warembourg M, Logeat Fdr, Milgrom E. Immunocytochemical localization of progesterone receptor in the guinea pig central nervous system. Brain Res. 1986;384:121–131. doi: 10.1016/0006-8993(86)91227-8. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Noguchi D, Ito H, Yamanouchi K. Sex and regional differences in decrease of estrogen receptor [alpha]-immunoreactive cells by estrogen in rat hypothalamus and midbrain. Neurosci Lett. 2009;463:135–139. doi: 10.1016/j.neulet.2009.07.074. [DOI] [PubMed] [Google Scholar]