Abstract
Background
Uric acid (UA) is known to be associated with excess adiposity and insulin resistance. Our aim was to investigate the relationship between UA and the factors associated with the metabolic syndrome and type 2 diabetes mellitus (T2DM), both initially and longitudinally.
Methods
Serum UA was assessed as a potential determinant of concurrent blood pressure, serum lipids, glucose regulation measured via an oral glucose tolerance test (OGTT), acute insulin response (AIR), and insulin action (M) measured with hyperinsulinemic–euglycemic clamps in 245 participants (72% Native American, 56% male). UA was also assessed as a predictor of the above variables in 60 participants with follow-up data available (median follow-up time=11.2 years [interquartile range (IQR)=8.1, 13.6 years]. The impact of UA on the risk of T2DM was determined as 36 of the 245 participants developed T2DM after the baseline visit.
Results
UA was negatively associated with both concurrent and future M, such that for every 1 mg/dL increase in serum UA, M decreased 7.6% (P<0.001) and future M decreased 6.3% (P=0.02). However, UA was not associated with AIR (P=0.7). UA concentrations were a predictor of T2DM [hazard risk ratio (HRR)=1.5; P=0.02]. UA was positively associated with both concurrent blood pressure and lipids and also predicted future increases in blood pressure and total cholesterol.
Conclusions
Not only did UA associate with concomitant insulin action, blood pressure, and lipids, it also predicted future declines in insulin action and T2DM. UA is a potential target for preventing decreases in insulin sensitivity and rises in blood pressure and cholesterol.
Introduction
Uric acid (UA) is the final oxidation product of purine metabolism generated during enzymatic degradation of hypoxanthine and xanthine. Uric acid levels often reflect dietary choices, including high intake of of purine-rich foods and fructose-containing foods.1,2 For example, the rise in UA levels observed in the American population has been attributed to the replacement of glucose with fructose in many foods,1 including many American soft drinks.3
Serum UA levels are known to be increased in people with excess adiposity, and recently, UA has also been shown to be associated with other cardiovascular risk factors, including hypertension and dyslipidemia.1,3–5 In a cross-sectional study, UA has been shown to be positively correlated with the number of metabolic syndrome components in an individual.5 Serum UA has been associated with an increased risk for development of worsening glucose status in a study in men.4 In an elderly population, people with high UA levels had an increased risk of future development of type 2 diabetes mellitus (T2DM).6 Hyperuricemic subjects have also been reported to have lower levels of insulin production, as assessed by the homeostasis model assessment (HOMA)-β index, implying a possible role for UA in β-cell function.7
Although there is good evidence that UA is associated with components of the metabolic syndrome, it is unclear if UA is a marker for concurrent lifestyle choices or is involved in the causal pathways leading to hypertension, dyslipidemia, or T2DM, either by affecting insulin secretion, insulin sensitivity, or both. To determine if UA is a longitudinal, as well as a cross-sectional, predictor of insulin action, insulin secretion, blood pressure or serum lipids, we investigated the relationship of UA to multiple measures in a longitudinal study of risk factors for the development of T2DM. We also used prospectively gathered data to determine if UA levels are a risk factor for development of T2DM.
Methods
Study population
From 1982 to 2007, we recruited healthy research volunteers, aged 18–55 years old, from the central Arizona area for our clinical research study on the metabolic determinants of obesity and T2DM. Only nondiabetic visits with serum creatinine levels <1.5 mg/dL, serum triglyceride level <400 mg/dL, serum cholesterol levels <300 mg/dL, serum alanine aminotransferase (ALT)<100 units/L, and serum aspartate aminotransferase (AST)<90 units/L were considered. The current analysis included 245 participants with data for serum UA available within 2 weeks of a 75-g oral glucose tolerance test (OGTT), body composition analysis, hyperinsulinemic–euglycemic clamp, blood pressure measurements, and a lipid panel. The lipid panel included high-density lipoprotein (HDL) measurements for 185 of the 245 subjects. Intravenous glucose tolerance tests (IVGTT) for assessment of acute insulin release (AIR) were available for 231 of the 245 subjects. Sixty of the initial subjects had longitudinal data available with at least one return visit with repeat UA levels, blood pressure assessment, lipid measures, and a hyperinsulinemic–euglycemic clamp.
For determination of development of T2DM, data were available from either repeat study visits at our clinical research unit or from a long-term longitudinal study of health in Native Americans from the Gila River Indian Community of Arizona in which many volunteers also participated.8 The latter study included biennial exams with chart review, history and physical exam, and a 75-g OGTT. Median follow-up time was 11.2 years [interquartile range (IQR)=8.1, 13.6 years] after the initial visit. Development of T2DM was determined from either the study-related OGTT according to 2003 American Diabetes Association (ADA) criteria8,9 or chart review. Both study protocols were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Written informed consent was obtained for all research subjects.
Data collection
Upon admission, blood was drawn for measurement of fasting serum uric acid. The ILAB 900 Analytical System (Instrumentation Laboratory, Bedford, MA) was used to determine serum uric acid concentrations before 1997. From 1997 to 2007, the Dade Dimension RxL analyzer (Siemens/Dade Behring, Deerfield, IL) was used. The reference range of the two systems was similar, and the method of analysis was not a significant covariate in any of the statistical models; therefore, data from the two systems were considered to be comparable.
After 3 days of stabilization on a weight-maintaining diet, volunteers underwent a 75-g OGTT with measurement of fasting and 2-hr glucose and insulin concentrations. The serum insulin concentrations were measured by radioimmunoassay using the Herbert modification10 of the method of Yalow and Berson11 or by an automated analyzer (Concept 4, ICN Radiochemicals Inc, Costa Mesa, CA). Regression equations were used to convert insulin concentrations to the earlier radioimmunoassay (RIA) values for comparability.
Body composition measurements were determined by underwater weighing with measurement of residual lung volume by helium dilution (53 participants) or by total body dual energy X-ray absorptiometry (DXA; DPX-L; Lunar Radiation, Madison, WI) (192 participants). DXA measurements were standardized to underwater values using a conversion equation.12 Blood pressure was taken with the volunteer in a seated position the same morning as the serum UA levels were drawn. Fasting serum total cholesterol, triglycerides, and HDL were measured by the automated immunoassay method using the Dade Behring dimension system analyzer (Siemens, NY).
AIR was measured as the initial response to a 25-g IVGTT. As previously described, AIR was calculated as the average increment in plasma insulin concentration above basal levels in samples obtained at 3, 4, and 5 min after injection of glucose.13 Insulin action (M) was assessed using the hyperinsulinemic–euglycemic clamp technique.13–15 After an overnight fast, a continuous insulin infusion was administered at a constant rate of 40 mU/m2 per min, and a variable infusion of 20% dextrose was used to maintain a constant plasma glucose level of 100 mg/dL. M, as a measure of insulin action, was the rate of dextrose required to maintain euglycemia during the last 40 min of the insulin infusion corrected for steady-state plasma insulin levels, glucose levels, and endogenous glucose production. M values were normalized to estimated metabolic body size defined as fat free mass plus 17.7 kg.13–15
Statistical analysis
Subject characteristics are presented as mean±standard deviation for continuously distributed data and median with 25th and 75th percentiles for skewed data. M and AIR were log10 transformed to meet the assumptions of linear regression. The degree of Pima heritage was determined by the self-identified number of great-grandparents of full Pima heritage. To determine the relationship of UA with metabolic syndrome components, including M, AIR, blood pressure, triglycerides, cholesterol, and HDL, three linear regression models were created. Model 1 included uric acid, age, sex, and degree of Pima heritage. Model 2 included all the covariates in model 1 plus percent body fat (PFAT), renal function (creatinine), and hepatic function (AST). Model 3 included all the covariates of model 2 plus blood pressure, total cholesterol, and triglyceride measurements (excluding the variable used as the dependent variable in the model). HDL was not included in model 3 because it limited the sample size, and sensitivity analyses confirmed that HDL did not substantially change the relationship of UA to any of the other variables. Model 2 was considered the most parsimonious of the statistical models. The AIR analysis was limited to those subjects of 100% Pima heritage (n=124) because there are large differences in insulin secretion rates between people of Pima heritage and others.16 In the 60 subjects with repeat measures, baseline UA and change in UA over time were evaluated as predictors of future M, AIR, blood pressure, cholesterol, triglycerides, percent body fat, and fasting and 2-hr glucose. Given the limited sample size of this subset of subjects, only the covariates from model 2 described above were included in the linear regression model along with the baseline value of the factor of interest and follow-up time.
Thirty-six of the 245 participants developed T2DM during the follow-up period. A Cox proportional hazards model was used to evaluate whether initial UA concentrations predicted the development of T2DM. Event time was the time from the initial measurement of UA to diagnosis of T2DM or the last available nondiabetic follow-up visit. The model was adjusted as above for model 1, model 2, and model 3. (Of note, including HDL in model 3 did not change our results.) To further explore the relationship of UA with risk of T2DM, a follow-up analysis was done with the proportional hazards model of model 2 but with UA expressed as a dichotomous variable, i.e., above versus below the median of 4.9 mg/dL, instead of as a continuous variable. Alpha was set at 0.05. Statistical analyses were performed using SAS version 9.2 and SAS Enterprise Guide 4.2. (SAS Institute Inc, Cary, NC).
Results
Subject characteristics
Characteristics of the 245 subjects (56% male) in the cross-sectional analysis are described in Table 1, and correlations between UA and the other variables are shown in Table 2. Mean serum UA levels were 5.0±1.3 mg/dL (median 4.9 mg/dL). Fifty-three percent of the study population was of full Pima heritage, 28% had no Pima heritage (20% Caucasian, 8% African-American), and 19% were of mixed heritage.
Table 1.
Demographics of the 245 Cross-Sectional Participants
| Variables | Values |
|---|---|
| Age (years) | 29.0±7.2 |
| Percent body fat (%) | 32.0±8.7 |
| Body mass index (kg/m2) | 33.6±7.9 |
| Systolic blood pressure (mmHg) | 117.4±16.2 |
| Diastolic blood pressure (mmHg) | 72.2±12.2 |
| Insulin action, M (mg/kg EMBS/min) | 2.6 (2.1–3.5) |
| Acute insulin response, AIR (pmol/l)a | 210.9 (133.8–367.4) |
| Fasting plasma glucose (mg/dL) | 86.5±10.8 |
| 2-hr plasma glucose (mg/dL) | 119.0±37.8 |
| Serum uric acid (mg/dL) | 5.0±1.3 |
| Total cholesterol (mg/dL) | 173±34 |
| Triglycerides (mg/dL) | 146±67 |
| AST (units/L) | 27.7±13.0 |
| ALT (units/L) | 38.4±22.2 |
| Serum creatinine (mg/dL) | 1.0±0.2 |
Values are expressed as mean±standard deviation (SD) or median (range 25th–75th percentile).
Full-heritage Pima Indians only (n=124).
EMBS, estimated metabolic body size (fat free mass +17.7), adjusted for mean glucose and insulin concentrations; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 2.
Spearman Correlations Between Serum Uric Acid and Covariates of the 245 Cross-Sectional Participants
| Variables | Spearman correlation coefficient (ρ) | P value |
|---|---|---|
| Age (years) | −0.01 | 0.9 |
| Degree of Pima heritage | −0.10 | 0.13 |
| Percent body fat (PFAT) (%) | −0.26 | <0.001 |
| Systolic blood pressure (mmHg) | 0.29 | <0.001 |
| Diastolic blood pressure (mmHg) | 0.33 | <0.001 |
| Insulin action, M (mg·kg−1(EMBS)·min−1) | −0.17 | 0.01 |
| Acute insulin response (AIR) (pmol/L)a | −0.03 | 0.7 |
| Fasting glucose (mg/dL) | 0.01 | 0.9 |
| 2-hr glucose (mg/dL) | −0.05 | 0.5 |
| Total cholesterol (mg/dL) | 0.16 | 0.01 |
| Triglycerides (mg/dL) | 0.21 | <0.001 |
| AST (units/L) | 0.24 | <0.001 |
| ALT (units/L) | 0.16 | 0.01 |
| Creatinine (mg/dL) | 0.4 | <0.001 |
Full-heritage Pima Indians only (n=124).
EMBS, estimated metabolic body size (fat free mass +17.7), adjusted for mean glucose and insulin concentrations; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
UA was higher in men (5.6±1.1 vs. 4.3±1.1 mg/dL; P<0.001). There were no racial differences in UA concentrations. Serum UA levels remained higher in men compared to women (P<0.001), even after adjustment for covariates. In linear regression models, UA was positively associated with PFAT [Table 3; unexplained variance of PFAT (partial r2) attributable to UA in model 2=3.4%].
Table 3.
Association of Uric Acid with Metabolic Syndrome Components After Adjustment for Covariates
| |
|
Predicted variables |
||||||
|---|---|---|---|---|---|---|---|---|
| Percent body fat (%) (n=245) | Insulin action (n=245)a | SBP (mmHg) (n=245) | DBP (mmHg) (n=245) | Triglycerides (mg/dL) (n=245) | Cholesterol (mg/dL) (n=245) | HDL (mg/dL) (n=185) | T2DM (HRR) (n=245; 36 events) | |
| Uric acid | β=−1.5; P<0.001 | β=−4.5%; P<0.01 | β=2.9; P<0.001 | β=2.2; P<0.001 | β=13.7; P<0.0001 | β=5.5; P<0.01 | β=−1.8; P=0.03 | NS |
| Model 1: UA, age, sex, degree of Pima Heritage | β=−1.3; P<0.0001 | β=−9.8%; P<0.0001 | β=2.3; P<0.01 | β=1.9; P<0.01 | β=19.2; P<0.0001 | β=8.3; P<0.0001 | β=−2.5; P<0.01 | HRR=1.4; P=0.03 |
| Model 2: model 1+% body fat2, creatinine, AST | β=−1.5; P<0.0001 | β=−7.6%; P<0.0001 | β=1.9; P=0.02 | β=1.5; P=0.03 | β=17.7; P<0.0001 | β=8.0; P<0.001 | β=−2.3; P=0.02 | HRR=1.5; P=0.02 |
| Model 3: model 2+other risk factorsb | β=−1.4; P<0.001 | β=−7.0%; P<0.0001 | β=1.9; P=0.03 | β=1.8; P=0.02 | β=14.3; P<0.001 | β=5.4; P=0.01 | β=−2.5; P=0.01 | HRR=1.5; P=0.03 |
Insulin action was log transformed to conform to the assumptions of linear regression, therefore, results are expressed as percent change in insulin action with each 1 mg/dl increase in uric acid.
Other risk factors included systolic blood pressure, diastolic blood pressure, triglycerides, and cholesterol. Models where one of these risk factors was the predicted variable did not include that variable as an explanatory variable.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; T2DM, type 2 diabetes mellitus; HRR, hazard risk ratio for T2DM determined by proportional hazards analyses; β, parameter estimates for the change in the predicted variable for each 1 mg/dL increase in uric acid as predicted by linear regression models; UA, uric acid; AST, aspartate aminotransferase.
M and AIR
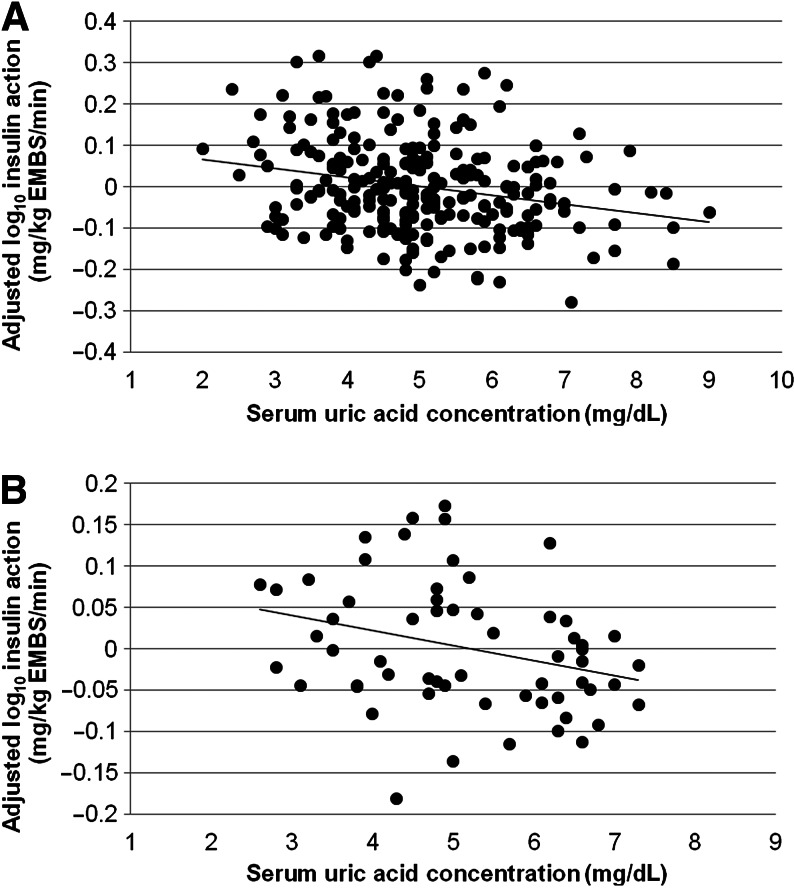
UA was a significant predictor of concurrent M in the 245 participants, even after adjustment for covariates (partial r2=5.5% in model 2; Fig. 1A and Table 3). In a subset analysis of subjects of 100% Pima heritage (n=124), the results were similar. UA was not significantly associated with AIR (P=0.7).
FIG. 1.
(A) The cross-sectional relationship between serum uric acid (UA) concentration and residual log10 insulin action (M) after adjustment for age, race, sex, percent body fat, and renal and liver function (n=245; partial r2=0.05; P<0.001). (B) The relationship between baseline serum UA and residual follow-up log10 insulin action (M) after adjustment for age, race, sex, percent body fat, baseline M, and follow-up time (n=60; partial r2=0.05; P=0.02). EMBS, estimated metabolic body size.
In the 60 participants with longitudinal data available, baseline serum UA predicted future M (partial r2=5%; P=0.02), even after controlling for the covariates in model 2, follow-up time, and baseline M (Fig. 1B). For every 1 mg/dL increase in initial serum UA, future M was decreased by 6.3% [95% confidence interval (CI) 2.7, 9.9%]. Neither baseline UA nor the change in UA predicted future AIR.
Thirty-six of 245 subjects were diagnosed with T2DM. In proportional hazards analyses, UA was associated with an increased risk of T2DM (Table 3). If UA was dichotomized into concentrations above or below the median value (4.9 mg/dL), those subjects with a ‘higher’ UA had a greater risk of T2DM [hazard risk ratio (HRR)=2.3; P=0.04]. In a subset analysis of subjects of 100% Pima heritage, 32 subjects were diagnosed with T2DM, and results were similar.
Hypertension and lipids
Serum UA correlated with both systolic and diastolic blood pressure (SBP and DBP) and lipids, even after adjustment for covariates (Table 3). UA was positively associated with SBP (partial r2=1% in model 2) and DBP (partial r2=1% in model 2). UA was also positively associated with total cholesterol (partial r2=2% in model 2) and triglycerides (partial r2=3% in model 2). UA was negatively correlated with HDL (partial r2=3% in model 2) in the subset of 185 subjects with this data available (Table 3). UA was not significantly associated with concurrent fasting glucose, 2-hr glucose, or serum insulin concentrations.
In participants with longitudinal data available, the change in UA over time predicted an increase in future SBP (partial r2=11%; P=0.007) and DBP (partial r2=9%; P=0.01) after controlling for the covariates of model 2 plus follow-up time and baseline blood pressure. For every 1 mg/dL rise in serum UA, future SBP increased by 6.9 mmHg (95% CI 2.0, 11.9 mmHg), and future DBP increased by 9.9 mmHg (95% CI 2.5, 17.4 mmHg). Change in UA also predicted an increase in total serum cholesterol (partial r2=6%; P=0.04) such that for every 1 mg/dL increase above initial serum UA, future cholesterol increased by 8.2 mg/dL. Neither baseline UA nor the change in UA was associated with future serum insulin concentrations, glucose levels, PFAT, triglycerides, or HDL.
Discussion
We confirmed the association of serum UA with insulin action, SBP and DBP measurements, and cholesterol in a population that included a high proportion of Native Americans. We were also able to demonstrate that the increased risk of developing T2DM seen with higher UA levels depends primarily on the association of UA with insulin action, as serum UA did not correlate with AIR. Interestingly, baseline UA measurements predicted future declines in insulin action, and rising UA over time was associated with future increases in blood pressure and cholesterol in longitudinal analysis.
The cross-sectional relationship between serum UA and insulin action has been described in other ethnicities.17–19 In one study, serum triglycerides and insulin action together explained 50% of the variation in serum UA level in a group of 37 subjects.19 In a comparison of obese individuals with lean individuals, insulin action was inversely associated with serum UA.18 A large Italian study also reported a negative correlation between hyperuricemia and insulin action.17 The relationship between UA and an increased risk for development of T2DM has also been found in larger studies.4,6 However, we now add to this prior literature by replicating the cross-sectional relationship between UA, insulin action, and T2DM in a younger and ethnically different population sample with a range of percent body fat. Our study extends the cross-sectional findings further by showing that serum UA also correlates with declines in future insulin action, as measured by repeated hyperinsulinemic–euglycemic clamps and independently from measures of adiposity.
It is unclear if higher UA levels directly lead to declines in insulin sensitivity and elevations in blood pressure and lipids or whether UA is a secondary marker for another pathophysiologic process. It is possible that UA may have direct effects because UA is the end product of purine degradation, a pathway that leads to the production of reactive oxygen species (ROS).20 ROS produced during the enzymatic reaction of xanthine oxidase, the protein that catalyzes the oxidation of xanthine to UA, have been hypothesized to induce endothelial dysfunction by reducing the bioavailability of the vasodilator nitric oxide, thus leading to vasoconstriction.21 This process has been implicated in the link between hyperuricemia and hypertension.20 A reduction in nitric oxide concentrations induced by elevated levels of ROS in tissues may also contribute to insulin resistance because central nervous system inhibition of nitric oxide synthase activity leads to decreased insulin action in rodents, and mice with genetic removal of the nitric oxide synthase protein have been shown to be insulin resistant.21,22
Inhibition of xanthine oxidase with allopurinol, a drug used to treat hyperuricemia, has been shown to improve endothelial dysfunction and reduce free radicals.20 In fructose-fed rodents, allopurinol has been shown to lower SBP, improve insulin sensitivity, and normalize triglyceride levels.2 Long-term allopurinol treatment in normotensive people with established T2DM has been shown to reduce hemoglobin A1c levels.23 The prediction of future insulin action by baseline UA levels, independent from measures of adiposity, supports a potential direct effect of UA.
However, because change in UA levels, rather than baseline UA levels, predicts future blood pressure and cholesterol levels, UA may also be an indicator for poor dietary choices, including excess consumption of high-fructose food and drink. A diet high in fructose increases purine production because fructose activates the rate-limiting step of de novo synthesis of purines in the liver,2 thus leading to overproduction of purines and a subsequent increase in UA levels. In subjects on purine-free diets supplemented with either glucose or fructose, those who received fructose supplementation had significantly higher serum and urine UA levels.24 Fructose is known to be more lipogenic than glucose and has been associated with both hypertriglyceridemia and insulin resistance.25 A recent animal model has shown that rats fed high-fructose diets exhibited a decreased number of insulin receptors in liver and skeletal muscle compared to controls.26 Insulin resistance in fructose-fed rodents is reversed by inhibition of peroxisome proliferator-activated receptor-γ coactivator-1β (PGC-1β), a master regulator of glucose and lipid metabolism.27 Because increased consumption of fructose in soft drinks has been shown to increase serum UA levels,28 lifestyle changes to decrease consumption of fructose may lead to lower serum UA and produce improvements in insulin sensitivity, risk of T2DM, blood pressure, and lipids.
Limitations of our study include the relatively small sample size, especially the small number of T2DM events. In addition, this is a secondary analysis of previously collected data in a study designed to understand the metabolic determinants of obesity and T2DM, and, as a result, two separate assays were used to measure UA. However, the data were collected prospectively and the risk of T2DM was determined with proportional hazards models. We did not perform ROC analyses, which would have been an alternate method to determine the additional risk prediction provided by UA beyond that of traditional risk factors and might have indicated cutoffs for uric acid in terms of T2DM risk. Given the long period of follow-up time, we considered proportional hazards analysis to be the more appropriate method for determining risk. The strengths of our study include the use of hyperinsulinemic–euglycemic clamps to assess insulin action, the availability of longitudinal data, and the replication of findings from prior studies in a healthy population with a high proportion of Native Americans, thereby, increasing the generalizability of this knowledge.
In conclusion, higher UA levels were associated with obesity, insulin resistance, higher blood pressure, and higher triglyceride and cholesterol concentrations. Baseline UA levels predicted future declines in insulin action, and an increase in UA over time was associated with increases in blood pressure and total cholesterol. There was an increased risk of T2DM with higher levels of UA, which is likely due mainly to the relationship of UA with insulin action. UA concentrations should be considered for routine measurement when assessing patients for metabolic syndrome, in part, for overall risk assessment and in part, as a tool for dietary counseling. It is conceivable that treatments that lower UA may improve insulin action, and also, possibly decrease the risk of T2DM. Future studies to observe the effects of reducing UA levels on insulin action and the risk of T2DM will be needed to validate this hypothesis.
Acknowledgments
We would like to thank the volunteers who participated in this study as well as the clinical research staff of the Phoenix Epidemiology and Clinical Research Branch. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. K.O. is supported by an American Podiatric Medical Association/American Diabetes Association (APMA/ADA) joint fellowship grant.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Glynn RJ. Campion EW. Silbert JE. Trends in serum uric acid levels 1961–1980. Arthritis Rheum. 1983;26:87–93. doi: 10.1002/art.1780260115. [DOI] [PubMed] [Google Scholar]
- 2.Schwarzmeier JD. Marktl W. Moser K. Lujf A. Fructose induced hyperuricemia. Effects of fructose on the de novo synthesis of adenine nucleotides in the liver and skeletal muscles of rats. Res Exp Med (Berl) 1974;162:341–346. doi: 10.1007/BF01851705. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA. Nielsen SJ. Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi N. Okamoto M. Yoshida H, et al. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 5.Yoo TW. Sung KC. Shin HS, et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J. 2005;69:928–933. doi: 10.1253/circj.69.928. [DOI] [PubMed] [Google Scholar]
- 6.Dehghan A. van Hoek M. Sijbrands EJ, et al. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 7.Simental-Mendia LE. Rodriguez-Moran M. Guerrero-Romero F. Failure of beta-cell function to compensate lack of insulin action in hyperuricemic subjects. Diabetes Metab Res Rev. 2009;25:535–541. doi: 10.1002/dmrr.988. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC. Pettitt DJ. Savage PJ. Bennett PH. Diabetes incidence in Pima indians: Contributions of obesity and parental diabetes. Am J Epidemiol. 1981;113:144–156. doi: 10.1093/oxfordjournals.aje.a113079. [DOI] [PubMed] [Google Scholar]
- 9.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 10.Herbert V. Lau KS. Gottlieb CW. Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 11.Yalow RS. Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tataranni PA. Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 13.Bunt JC. Krakoff J. Ortega E, et al. Acute insulin response is an independent predictor of type 2 diabetes mellitus in individuals with both normal fasting and 2-h plasma glucose concentrations. Diabetes Metab Res Rev. 2007;23:304–310. doi: 10.1002/dmrr.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogardus C. Lillioja S. Howard BV, et al. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984;74:1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lillioja S. Bogardus C. Obesity and insulin resistance: Lessons learned from the Pima Indians. Diabetes Metab Rev. 1988;4:517–540. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- 16.Lillioja S. Nyomba BL. Saad MF, et al. Exaggerated early insulin release and insulin resistance in a diabetes-prone population: A metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab. 1991;73:866–876. doi: 10.1210/jcem-73-4-866. [DOI] [PubMed] [Google Scholar]
- 17.Bonora E. Capaldo B. Perin PC, et al. Hyperinsulinemia and insulin resistance are independently associated with plasma lipids, uric acid and blood pressure in non-diabetic subjects. The GISIR database. Nutr Metab Cardiovasc Dis. 2008;18:624–631. doi: 10.1016/j.numecd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Muscelli E. Camastra S. Gastaldelli A, et al. Influence of duration of obesity on the insulin resistance of obese non-diabetic patients. Int J Obes Relat Metab Disord. 1998;22:262–267. doi: 10.1038/sj.ijo.0800580. [DOI] [PubMed] [Google Scholar]
- 19.Vuorinen-Markkola H. Yki-Jarvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab. 1994;78:25–29. doi: 10.1210/jcem.78.1.8288709. [DOI] [PubMed] [Google Scholar]
- 20.Khosla UM. Zharikov S. Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Wong WT. Wong SL. Tian XY. Huang Y. Endothelial dysfunction: The common consequence in diabetes and hypertension. J Cardiovasc Pharmacol. 55:300–307. doi: 10.1097/fjc.0b013e3181d7671c. [DOI] [PubMed] [Google Scholar]
- 22.Scherrer U. Sartori C. Defective nitric oxide synthesis: A link between metabolic insulin resistance, sympathetic overactivity and cardiovascular morbidity. Eur J Endocrinol. 2000;142:315–323. doi: 10.1530/eje.0.1420315. [DOI] [PubMed] [Google Scholar]
- 23.Dogan A. Yarlioglues M. Kaya MG, et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20:182–187. doi: 10.3109/08037051.2010.538977. [DOI] [PubMed] [Google Scholar]
- 24.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33:276–280. doi: 10.1136/ard.33.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sleder J. Chen YD. Cully MD. Reaven GM. Hyperinsulinemia in fructose-induced hypertriglyceridemia in the rat. Metabolism. 1980;29:303–305. doi: 10.1016/0026-0495(80)90001-3. [DOI] [PubMed] [Google Scholar]
- 26.Catena C. Giacchetti G. Novello M, et al. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens. 2003;16(11 Pt 1):973–978. doi: 10.1016/s0895-7061(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 27.Nagai Y. Yonemitsu S. Erion DM, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JW. Ford ES. Gao X. Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]