Abstract
Risks of introduction of lumpy skin disease (LSD) through traded Borena bulls to market chain and its consequences were assessed. The assessment used the framework that has been recommended by the World Animal Health Organization (OIE) for risk analysis. Likelihoods for release and exposure were estimated by a qualitative scale ranging from negligible to very high, whereas the consequences which resulted from disease occurrences were assessed quantitatively. The likelihood of the introduction of LSD to the market chain through traded Borena bulls is found to be high (medium uncertainty), whereas the probability of exposure is very high (medium uncertainty). From the total of 11,189 bulls observed during outbreak investigation of LSD in six sites of feedlot operation in and around Adama, 681(6.1 %) and 204 (1.8 %) bulls were found to be affected and dead with LSD, respectively. The total economic loss due to LSD was estimated to be 667,785.6 USD. The risk estimates for LSD are greater than negligible; therefore, disease prevention and control strategy along the chain should be carefully considered by the Ethiopian veterinary services.
Keywords: Borena bulls, Export, Risk assessments, Lumpy skin disease, Market chain, International trade
Introduction
Ethiopia is a resourceful country bestowed with the largest livestock resource in the African continent (FAOSTAT 2007) and the cattle population is estimated to be 53.4 million (CSA 2011) with the potential to export substantial numbers of live animals and their products. However, due to the prevalence of highly contagious transboundary animal diseases such as lumpy skin disease (LSD), the full potential of sub-sectors has not been realized in Ethiopia.
LSD is one of the most serious poxvirus diseases of cattle caused by lumpy skin disease virus (LSDV) within the genus Capripoxvirus (Babiuk et al. 2008). The World Organization for Animal Health (OIE) categorizes LSD as a notifiable disease because of the substantial economic impact of an outbreak. Restrictions to the global trade of live animals and animal products, costly control, and eradication measures such as vaccination campaigns as well as the indirect costs because of the compulsory limitations in animal movements cause significant financial losses on a national level (Tuppurainen and Oura 2012; Rich and Perry 2011).
Being one of the transboundary diseases, LSD could be spread through production and marketing chains (Rossiter and Hammadi 2009). In Ethiopia, live cattle market chain actors are not adequately informed of the importance of establishing the source of origin, traceability mechanisms, and related certification processes for marketed animals. Furthermore, they do not yet appreciate the importance of issues related to food quality, the link between animal health, meat quality and safety, and why documentation is fundamental in enhancing competitiveness in the global markets. For those reasons, diseases such as LSD can easily spread along the chain and it results in repeated outbreaks. This could affect particularly not only the economic well-being of the farmers and that of pastoral communities, but also more globally the country's economy.
Quantitative and qualitative estimates of the risk, the spatial variation in the risk, and the factors associated with the risk for LSD virus introduction and spread into the market chain are a prerequisite for the development of differential policies for prevention and eventual control of epidemics. Information on risk assessment of LSD in livestock market chain for export from Ethiopia in general and Borena live cattle market chain for export in particular is highly scanty. Hence, there is a need for risk assessment of this disease in Borena bull market chain for export. Therefore, the aim of this study was to assess the risk of introduction of LSD in Borena bull market chain for export.
Materials and methods
Study area
The study was conducted in and around Adama. Adama (Nazret/Nazareth) is situated about 99 km away from the nation's capital Addis Ababa following the famous and ever busy port highway, Djibouti road, going eastern direction. It is a bowl-like sinking sight surrounded by small hills just in the heart of the great East-African Rift Valley. It is located in the East Shewa Zone of Oromia, at 8°33′ N, 39°16′ E, 8.55° N, and 39.27° E at an elevation of 1,712 m, southeast of Addis Ababa. Adama and surrounding areas host a total of 200 feedlots of various sizes (MoA 2010).
Studied population and sources of information
The studied population consisted of bulls at finishing phase for export in Adama district. The origins of the bulls were mainly Borena pastoral system and very small proportion from Bale lowlands. All animals used for study were males with 3–5 years age category and vaccinated for LSD. All bulls found in 20 export-oriented feedlots in and around Adama were used to follow-up the occurrences of new case of LSD between June and December 2011. From 18,864 bulls registered in the study sites during the outbreak, 11,189 bulls were clinically examined.
Data for the risk assessment parameters were obtained from secondary data, interview with 31 feedlot operators, and personal field observations and from scientific literatures. Checklists were used to estimate level of biosecurity in all feedlots.
Methods of risk assessments
This study used the framework that has been recommended by the OIE (2004) for risk analysis. The framework outlines four key steps that should be covered systematically (Fig. 1). In this risk assessment, the hazard is defined as LSDV. The probabilities were assessed and described textually (Table 1).
Fig. 1.
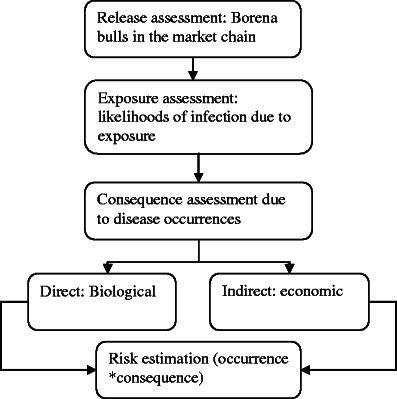
General scheme of the risk assessment (OIE method)
Table 1.
Interpretation of probability categories
| Probability category | Interpretation |
|---|---|
| Negligible | Event is so rare that does not merit to be considered |
| Very low | Event is very rare but cannot be excluded |
| Low | Event is rare but does occur |
| Medium | Event occurs regularly |
| High | Event occurs very often |
| Very high | Event occurs almost at certainly |
Adapted from EFSA (2006)
In this study, uncertainty was classified as low (solid and complete data available), medium (some but not complete data available), and high (scarce or no data available) (Pfeiffer 2007). Uncertainties were demonstrated within brackets after likelihood estimates.
Release assessment
The risk question posed for release was “What is the probability of introduction of lumpy skin disease into feedlots by Borena bull moving along market chain?” Scenario tree was designed to describe and evaluate the pathway of introduction of LSD through traded bulls from the point of production until the feedlots in Central Ethiopia. Scenario trees contain nodes that describe the events from which probabilities of an event derive; these probabilities were qualified according to the aforementioned terms. The overall probability of a release pathway was then arrived at by combining the probabilities of the various stages.
Exposure assessment
The risk question for exposure was described as: “What is the probability that bulls in value chain become exposed to LSDV?” The exposure pathway which describes the transmission of LSDV was designed. Overall probability of exposure pathway was arrived at by combining the probabilities of the various stages.
Consequence assessments
Clinical and epidemiological investigation of LSD outbreak
In all participated cattle feedlots, bulls in all pens were observed by a veterinarian and any suspects of LSD case were examined. Bulls with suspected case of LSD were examined for physical status, temperature, superficial lymph node, and skin lesions according to Rodostits et al. 2007.
Direct economical loss estimation
The direct economic losses caused by LSD were calculated based on mortality rate and rejection rate due to LSD from international markets. Since there were no markets for LSD-rejected bulls, the economic loss attributed to LSD rejection and death was estimated with similar parameters. Estimated total economical losses due to LSD outbreak were calculated thus:
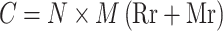 |
Where:
C = Total economical losses estimated due to LSD outbreak
N = Total number of bulls at risk of LSD in and around Adama feedlots during outbreak
M = Average terminal market price
Rr = Rejection rate
Mr = Mortality rate
Risk estimation
The risk estimate for the LSD occurrence in the value chain was made according to the combination matrix presented in Table 2. The consequence estimation was based on biological and economical impact of LSD in the feedlots.
Table 2.
Risk category combination matrix
| Parameter 2/exposure risk category | ||||||
|---|---|---|---|---|---|---|
| Parameter 1/release risk category | Very low | Low | Medium | High | Very high | |
| Very low (VL) | VL | VL | VL | VL | VL | |
| Low (L) | VL | L | L | L | L | |
| Medium (M) | VL | L | M | M | M | |
| High (H) | VL | L | M | H | H | |
| Very high (VH) | VL | L | M | H | VH | |
Adapted from Zepeda (2007)
Data management and analysis
Data were classified, filtered, coded using MS Excel 5, and were transferred to Statistical Package for Social Sciences software version 16. Thereafter, they were analyzed according to the different variables.
Results
Release assessment
The pathway in which LSD could be introduced to feedlots through Borena bull in market chain is shown in Fig. 2.
Fig. 2.
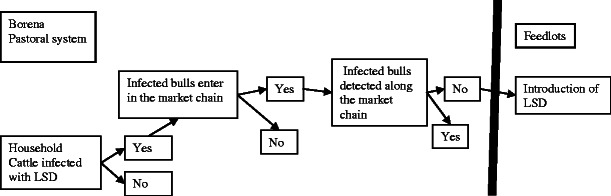
Scenario tree outlining LSD pathway along the Borena bull market chain
Probability of Borena cattle in the market chain infected with LSD
Lumpy skin disease is maintained endemically in Borena pastoral system and outbreaks occurred almost every year (Gari et al. 2010; Rufael et al. 2008). All ages and types of cattle are susceptible to LSD (Radostits et al. 2007; Vorster and Mapham 2008). There is no national control and prevention strategy designed for LSD control (MoA 2010). The bulls can get infected at markets, collection sites, and during transportation, increasing the probability of bulls reaching the feedlot in and around Adama shedding the virus. Taking into consideration the prevalence of LSD in Borena Zone and susceptibility of Borena bulls to the disease, the probability that an infected bull enters to the market chain is high (medium uncertainty).
Probability of infected Borena bull not detected in the market chain
The incubation period in natural outbreaks is estimated to be 1–4 weeks (Tuppurainen and Oura 2012). According to semi-structured interviewee (SSI) results, the average time spent on transportation from production areas to feedlots in Central Ethiopia is 1.65 days. Since bulls from production areas to the feedlots spent less than 7 days, the likelihood of an infected bull without clinical sign undetected along the market chain is very high. There was no veterinarian performing pre-purchase inspection and selection for quality assurance and certification for live cattle along the chain. The bulls were not subjected to any test before they were moved into feedlots. About 87.1 % interviewed feedlot operators have a trend of buying animals as batch which increase the probability of an infected bull pass undetected. The probability of not detecting the infected bull under the specified conditions is therefore assessed to be very high (low uncertainty).
Probability of lack of containment of the LSD virus
Pre-purchase inspection involves a visual and physical evaluation of bulls while moving freely in the markets. In 93.5 % of feedlots observed, dead bodies were disposed where insects have easy accesses. Infected bulls were isolated in non-insect proof isolation pen with very high probability of viral transmission through mechanical vectors (Chihota et al. 2001; Ali et al. 1990). In 100 % of surveyed feedlots, staffs do not use any protective cloths while handling the cattle and also do not use sanitary and disinfection facilities to avoid contamination. Generally, there could be an introduction of unobservable LSD infection via traded bulls, mechanical vectors, contaminated equipments, and contaminated staffs. Thus, due to lack of biocontainment of the virus along the value chain, the probability of undetected infection entered to the feedlots is considered to be high (low uncertainty).
Summary of release assessment for LSD
The analysis results revealed that the overall risk for the release of LSDV in feedlots is estimated to be high (medium uncertainty). Release pathway probabilities vary from high to very high (Table 3). Therefore, according to Table 1 interpretation, the probability of LSDV release into feedlots by traded bulls in the market chain occurs very often.
Table 3.
Summary of release assessment for LSD in Borena bull market chain
| Risk pathway | Risk category | Uncertainty |
|---|---|---|
| Probability of Borena bull in the market chain infected with LSD | High | Medium |
| Probability of infected Borena bull not detected while passing along the market chain | Very high | Low |
| Probability of lack of biocontainment of the virus within the facility along the value chain | High | Low |
| Overall risk estimate for release | High | Medium |
Exposure assessment
The pathway through which bulls in the feedlots could be exposed to LSDV is presented in Fig. 3.
Fig. 3.
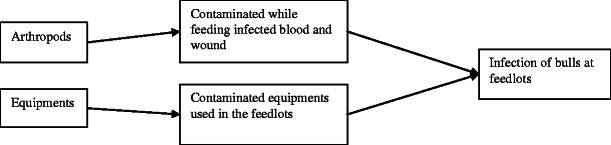
Exposure pathway of LSD in feedlots
Exposure pathway 1: exposure of bull to LSDV from blood-feeding arthropods
The transmission of LSDV is believed to occur mainly by blood-feeding arthropods (Chihota et al. 2001; Yeruham et al. 1995). According to SSI results, vaccination of bulls started a week after the final bull that was purchased has been entered in the feedlots and registered by quarantine service. However, the duration of time from introduction of the first batch of bulls to vaccination is sufficient for transmission of the disease to susceptible bulls by biting insects. Furthermore, 71 % interviewed feedlot operators have doubt on the efficacy of the LSD vaccine. Infected bulls were isolated in non-insect proof isolation pen with very high probability of transmission. Since temporal LSD occurrence was associated with an increase in the biting-fly population (Gari et al. 2010), the probability of infection of bulls in a feedlot after exposure to LSDV from blood-feeding arthropods during rainy season is very high (medium uncertainty).
Exposure pathway 2: exposure of bulls to LSD from equipments
As part of sanitary and phytosanitary (SPS) requirements and rules and regulations of animal quarantine, all bulls destined for export must be vaccinated. SSI indicated that 71 % operators get veterinary service from mobile veterinarians with higher probability using contaminated equipments between feedlots. LSDV is remarkably stable and surviving for long periods at ambient temperature, especially in dried scabs (Rovid 2008). If proper needle hygiene was not practiced, needles contaminated with virulent LSDV during the actual vaccination and treatment procedure serve as vehicle for transmission of the virus (Magori-Cohen et al. 2012; Tuppurainen and Oura 2012). Almost in all feedlots, the equipments were not disinfected. The likelihood of exposure of bulls in the feedlots to LSDV through unsterilized needles is therefore considered to be high (low uncertainty).
Summary of exposure assessment for LSD
The risk estimates for all the exposure pathways are presented in Table 4. The probabilities in the pathways range from high to very high. The overall risk estimate for exposure of bulls is thus very high (medium uncertainty). Therefore, exposure of bulls to LSDV occurs almost certainly (Table 1).
Table 4.
Summary of exposure assessment for LSD in Boren bull market chain
| Risk pathway | Risk category | Uncertainty |
|---|---|---|
| Probability of infection of bulls in the feedlots after exposure to LSDV from contaminated blood-feeding arthropods | Very high | Medium |
| Probability of infection of bulls in the feedlots after exposure to LSDV from contaminated equipments | High | Low |
| Overall risk estimate for exposure | Very high | Medium |
Consequence assessment
Clinical and epidemiological investigation of LSD outbreak
In each feedlot, animals manifesting the characteristic signs of LSD-like nodules on the skin and mucous membranes and rise in temperature were considered as clinically affected by LSD. From the total of 11,189 bulls observed during outbreak investigation of LSD in six sites of feedlot operation in and around Adama, 681 (6.1 %) bulls showed clinical signs and lesions suggestive of LSD and 204 (1.8 %) bulls were found dead with LSD (Table 5).
Table 5.
Morbidity, mortality, and case fatality rates of LSD in relation to site in and around Adama
| Site | PAR | New case | Dead | Morb. (%) | Mort. (%) | CFR (%) |
|---|---|---|---|---|---|---|
| Boku | 817 | 89 | 25 | 10.9 | 3.1 | 28.1 |
| Dera | 1,050 | 15 | 5 | 1.4 | 0.5 | 33.3 |
| Koshe | 2,263 | 269 | 109 | 11.9 | 4.8 | 40.5 |
| Migra | 1,771 | 74 | 24 | 4.2 | 1.4 | 32.4 |
| Jogo | 4,055 | 158 | 25 | 3.9 | 0.6 | 15.8 |
| Wanji | 1,233 | 76 | 16 | 6.2 | 1.3 | 21.1 |
| Total | 11,189 | 681 | 204 | 6.1 | 1.8 | 30 |
PAR population at risk, Morb. morbidity, Mort. mortality, CFR case fatality rate
Direct economic loss estimation
Direct economic loss was estimated based on LSD mortality rate (1.8 %), rejection rate (4.1 %), and total number of bulls at risk of LSD in and around Adama feedlots during an outbreak (18,864). Terminal market survey indicated that a bull with 375 kg average weight was sold with 600 USD average price (1.60 USD/kg). Accordingly, the total economic loss due to LSD outbreak was estimated to be 667,785.6 USD which is equivalent to 11,325,643.8 ETB (1 USD = 16.96 ETB).
Risk estimation
Risk assessment result revealed that likelihood of introduction of LSD to feedlots from infected bull passing along market is high. The likelihood of infection, as a consequence of exposure, is considered to be very high. Therefore, the probability of LSD occurrence in the feedlots from the release and exposure is also considered to be high. As a consequence, the overall prevalence of LSD is more than negligible that needs control intervention along the value chain.
Discussions
In recent years, application of risk analysis is relatively new and evolving discipline in the animal and veterinary public health fields that has become increasingly important after the establishment of the agreement on the application of sanitary and phytosanitary measures (SPS agreement), being largely employed to provide importing countries with an objective method of assessing disease risks associated with the importation of animal products (OIE 2004). Nevertheless, it is also applicable to other areas of animal disease control and, more widely, to other risky decision contexts (Wooldridge et al. 2006; Fahrion et al. 2008; FAO 2010; FAO 2011).
In this assessment, the overall risk of LSD is considered to be high as a result of high risk (medium uncertainty) of introduction of LSD to market chain and very high (medium uncertainty) exposure of bulls to LSDV in the value chain. This might be due to their place of origin, certification system in the market chain, facilities in the chain to exclude the risk, awareness of the actors in the chain towards the risk, and biosecurity measures in feedlots.
As a biological consequence, the presence of characteristic skin nodules and outstanding features of the LSD are best indicative of the occurrence of LSD at affected feedlots. The clinical picture was in accord with those documented by Coetzer (2004), Brenner et al. (2006), Gari et al. (2010), Kumar (2011), Salib and Osman (2011), and Body et al. (2012).
The observed LSD prevalence at animal level found in this study was 6.1 % which was in close agreement with the previous findings from Nekemt area of 7.02 % (Regassa 2003) and 8.1 % reported by Gari et al. (2010) in the country. Mortality (1.8 %) observed during this outbreak was similar with previous reports by Salib and Osman (2011) in Egyptian cattle.
The total economic loss due to LSD death and rejection was estimated to be 667,785.6 USD. The total estimated economic losses caused by LSD in this study were 6.1 % of the total income of the business owners from finished bulls. This loss estimated for individual business owner might be more than estimated since each feedlot operator incurred additional costs associated with treatment of sick bulls and maintenance of the rejected animals. Hence, the introduction of trade-sensitive diseases into feedlots can cause severe financial hardship and lead to the failure of the business. Furthermore, an outbreak of diseases such as LSD in the feedlots increases the risk facing individuals or firms considering entering the live cattle export industry, even in time when the disease may not be present.
Live cattle export from Ethiopia is currently largely feedlot based. The introductions of LSD into feedlots certainly affect access to specific markets. For longer time, Middle East markets are the traditional destination of Ethiopian live bulls. However, the current health status of Borena bulls in market chain unquestionably becomes a challenge for the country's future live cattle export opportunities to those countries. The repeated bans imposed by importing countries on livestock and meat export trade of Ethiopia signify a lack of confidence in the country's export systems. Therefore, the results of this risk assessment were used as a tool for supporting future regulations and policy in LSD prevention and control along cattle market chain.
Acknowledgments
The authors are so much thankful to the Faculty of Veterinary Medicine, Addis Ababa University for funding this research. Feedlot operators are highly appreciated for their all round cooperation during LSD outbreak investigation.
References
- Ali AA, Attia EH, Selim A, Abdul-Hamid YM. Clinical and pathological studies on lumpy skin disease in Egypt. Veterinary Record. 1990;127:549–550. [PubMed] [Google Scholar]
- Babiuk S, Bowden TR, Boyle DB, Wallace DB, Kitching RP. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transboundary Emerging Diseases. 2008;55:263–272. doi: 10.1111/j.1865-1682.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- Body, M. K., Singh, P., Hussain, M.H., Al-Rawahi, A. Al-Maawali, M., Al-Lamki, K.and Al-Habsy, S., 2012. Clinico-histopathological findings and PCR based diagnosis of lumpy skin disease in the Sultanate of Oman. Pakistan Veterinary Journal, 32(2), 206–210.
- Brenner, J., M Haimovitz, E. Oron, Y. Stram, O. Fridgut, V. Bumbarov, L. Kuznetzova, Z. Oved, A., Waserman, S., Garazzi, S., Perl, D., Lahav, N., Edery and Yadin, H., 2006. Lumpy skin disease (LSD) in a large dairy herd in Israel. Israeli Journal of Veterinary Medicine, 61, 73–77.
- Chihota CM, Rennie LF, Kitching RP, Mellor PS. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae) Epidemiology and Infection. 2001;126:317–321. doi: 10.1017/S0950268801005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzer J.A.W., 2004. Lumpy skin disease. In: Infectious Diseases of Livestock. 2nd Ed. Coetzer, J.A.W. and RC Justin (eds). Oxford University Press, Cape Town, South Africa, 1268–1276.
- CSA, 2011. Agricultural sample survey 2010/2011, Report on livestock and livestock characteristics. Central Statistical Agency of Ethiopia. Statistical Bulletin, 505.
- EFSA, 2006. Scientific Report on Migratory Birds and their Possible Role in the Spread of Highly Pathogenic Avian Influenza.
- Fahrion A, Richa K, Jamir L, Begum S, Rutsa V, Ao S, Padmakumar V, Grace D. Risk assessment in the pork meat chain in Nagaland. India: International Livestock Research; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2010. Value chain analysis as a tool for avian influenza control. Situation Update 71. Rome.
- FAO, 2011. A value chain approach to animal diseases risk management. Technical foundations and practical framework for field application. Animal Production and Health Guidelines 4, Rome.
- FAOSTAT, 2007. Database (http://faostat.fao.org). Rome. Accessed July 2011.
- Gari G, Waret-Szkuta A, Grosbois V, Jacquiet P, Roger F. Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiology and Infection. 2010;138:1657–1666. doi: 10.1017/S0950268810000506. [DOI] [PubMed] [Google Scholar]
- Kumar SM. An outbreak of lumpy skin diseases in Holstein dairy herd in Oman. A clinical case report. Asian journal of animals and veterinary Advances. 2011;6(8):851–859. doi: 10.3923/ajava.2011.851.859. [DOI] [Google Scholar]
- Magori-Cohen, R., Louzoun, Y., Herziger,.Y, Oron, E.,, Arazi, A., Tuppurainen, E., Shpigel, N., and Klement, E., 2012. Mathematical modelling and evaluation of the different routes of transmission of lumpy skin disease virus. Veterinary Research, 43, 1, [DOI] [PMC free article] [PubMed]
- MoA . Ethiopia Animal Health Year book (2009/10). Ministry of Agriculture Animal and Plant Health Regulatory Directorate. Ethiopia: Addis Ababa; 2010. [Google Scholar]
- OIE, 2004. Handbook on import risk analysis for animals and animal products. Vol. 1, Introduction and qualitative risk analysis. Paris, World Organisation for Animal Health.
- Pfeiffer, D.U., 2007. Assessment of H5N1 HPAI risk and the importance of wild birds. Journal of Wildlife Diseases 43(3):S47–S50.
- Radostits, O. M. Gay, C.C., Hinchcliff, K. W and Constable, P. D., 2007. Veterinary Medicine: A textbook of diseases of cattle, horses, sheep, pigs and goat. 10th Ed. WB Saunders Co., Philadelphia, USA.
- Regassa C., 2003. Preliminary study of major skin diseases of cattle coming to Nekemt Veterinary Clinic (unpublished DVM Thesis, Addis Ababa University).
- Rich KM, Perry BD. The economic and poverty impacts of animal diseases in developing countries: new roles, new demands for economics and epidemiology. Preventive Veterinary Medicine. 2011;101(3–4):133–147. doi: 10.1016/j.prevetmed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Rossiter, P. B. and Hammadi, N. L, 2009. Living with transboundary animal diseases (TADs). Tropical Animal Health and Production, 41, 999–1004. [DOI] [PubMed]
- Rovid, S.A., 2008. Lumpy Skin Disease. The center for food security and public health, Iowa State University. College of Veterinary Medicine. http://www.cfsph.iastate.edu. Accessed at April 2012.
- Rufael, T., Catley, A., Bogale, A., Sahle, M. and Shiferaw., Y.,2008. Foot and Mouth Disease in Borana Pastoral system, Southern Ethiopia. Tropical Animal Health and Production, 40, 29–38. [DOI] [PubMed]
- Salib FA, Osman AH. Incidence of lumpy skin disease among Egyptian cattle in Giza Governorate, Egypt. Veterinary World. 2011;4(4):162–167. [Google Scholar]
- Tuppurainen ESM, Oura CAL. Review: Lumpy skin disease: An Emerging Threat to Europe, the Middle East and Asia. Transboundary and Emerging Diseases. 2012;59:40–48. doi: 10.1111/j.1865-1682.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- Vorster, J.H. and Mapham, P.H., 2008. Lumpy skin disease. Livestock health and production Review. Jaargang, 10 (1). www.cpdsolutions.co.za
- Wooldridge M, Hartnett E, Cox A, Seaman M. Quantitative risk assessment case study: smuggled meats as disease vectors. Revue Scientifique et Technique de l’Office International des Epizooties. 2006;25(1):105–117. doi: 10.20506/rst.25.1.1651. [DOI] [PubMed] [Google Scholar]
- Yeruham I, Nir O, Braverman Y, Davidson M, Grinstein H, Haymovitch M, Zamir O. Spread of lumpy skin disease in Israeli dairy herds. Veterinary Record. 1995;137:91–93. doi: 10.1136/vr.137.4.91. [DOI] [PubMed] [Google Scholar]
- Zepeda C., 2007. Highly pathogenic avian influenza in domestic poultry and wild birds: a risk analysis framework. Journal of Wildlife Diseases, 43:S51-S54.


