Abstract
Many significant advances in our understanding of intestine development, intestinal stem cell homeostasis and differentiation have been made in recent years. These advances include novel techniques to culture primary human and mouse intestinal epithelium in three-dimensional matrices, and de novo generation of human intestinal tissue from embryonic and induced pluripotent stem cells. This short review will focus on the directed differentiation of human pluripotent stem cells into intestinal tissue, highlight novel uses of this tissue, and compare and contrast this system to primary intestinal epithelial cultures.
Keywords: Organoid, Enteroid, Intestine, Pluripotent, Stem cell, Crypt

Introduction
Undifferentiated stem cells, which drive embryogenesis and development, are often responsible for adult tissue maintenance and regeneration in response to injury [1]. Stem cells are defined by their long-term ability to divide and self-renew and their ability to give rise to specialized cell types through differentiation. Stem cells are classified by the range of their potential to differentiate into specialized cells. For example, pluripotent stem cells are able to give rise to any embryonic cell type whereas multipotent stem cells can give rise to smaller subsets of more closely related cell types. Adult stem cells are specialized to specific tissues and are often multipotent. They are responsible for tissue homeostasis and regeneration after damage. While they are capable of self-renewal and differentiation, their “stemness” is reduced over time when grown in vitro [1–4]. Unlike adult stem cells, pluripotent and embryonic stem cells can give rise to all tissues found in the human body and are virtually unlimited in their ability to proliferate in vitro, making them attractive for use in biomedical research and regenerative medicine.
Human pluripotent stem cells (hPSCs) broadly refer to all pluripotent human stem cell types, including human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). hESCs are most efficiently derived from the inner cell mass of human blastocytes generated during in vitro fertilization procedures [5]. They are commonly grown in culture on either inactivated feeder layers of murine embryonic fibroblasts or under feeder-free conditions on tissue culture plates coated with a basement membrane protein substrate [5–7]. The development of conditions for feeder-free derivation and growth of hESCs [6, 8–11] has enabled standardized production of cells more amenable for use in therapeutic applications (reviewed elsewhere [12]).
One major challenge when using hESC-derived cells for therapeutic purposes is generating tissue for transplantation that will not be rejected by the patient’s immune system. Another challenge for the use of hESCs has been the ethical opposition to destroying human embryos during hESC isolation and accordingly, strict rules exist regarding derivation and use of new hESC lines in projects reliant on US federal funding. Recently, methods to reprogram human or mouse “adult-like” somatic cells into “embryonic-like” stem cells was described by Shinya Yamanaka, a seminal discovery awarded the Nobel Prize in Medicine in 2012 [13, 14]. This method, called cellular reprogramming, was used to generate mouse and human iPSCs from fibroblast cells through the forced expression of a specific set of transcription factors (Oct4, Sox2, Klf4, c-Myc) [15–18]. These reprogrammed cells resemble hESCs in morphology and stem cell-defining characteristics, although detailed comparative gene expression studies suggest that there may be important differences between hESCs and iPSCs (reviewed elsewhere [19]). The original protocol for iPSC generation involved use of retroviral vectors which leave a “genetic footprint” in the reprogrammed cell, raising concerns for the safety of using iPSCs for transplantation [16]. In recent years, the methods have been further refined to eliminate the use of retroviruses [18], thus making it possible to generate patient-specific iPSCs without changing the genetic composition of the cells.
hPSCs, embryonic and induced, can now be used to study and recapitulate many embryonic developmental stages in vitro. Translational embryology is an emerging field in which researchers are able to use what we know about different stages of embryonic development to direct differentiation of pluripotent stem cells into specialized cells and tissues (reviewed elsewhere [20, 21]). Directed differentiation is achieved by treating cells with recombinant proteins or small molecules that regulate important developmental signaling pathways, thereby mimicking key events during in vivo development. This approach has been used to generate a wide spectrum of cell types derived from all three primary germ layers (ectoderm, mesoderm, and endoderm), holding great promise for studies and treatments of many diseases [21, 22]. This review focuses on recent advances that used directed differentiation to generate intestinal tissue from hPSCs.
Growing the Intestine In Vivo
Differentiation of hESCs or iPSCs into intestinal tissue requires a step-wise process mimicking major developmental events including definitive endoderm differentiation, gut specification and morphogenesis, and intestine development, growth, and homeostasis. We will briefly highlight these major developmental milestones. The three primary germ layers, generated in the process of gastrulation during embryogenesis, all contribute to the development of the intestine. The enteric nervous system that innervates the gut arises from the ectoderm; the smooth muscle, connective tissue, and vasculature of the gut arise from the mesoderm; and intestinal epithelium arises from the endoderm [23–26]. For the purpose of this review, we will focus on the development of the intestinal epithelium.
The TGF-β superfamily member Nodal is essential for the specification of endoderm. Variable levels of exposure to this growth factor control anterior–posterior (A–P) patterning of the endoderm [27]. Fibroblast growth factor (FGF), Wnt, bone morphogenetic protein (BMP), and retinoic acid signaling are also involved in A–P patterning and induction of tissue-specific transcription factors (reviewed elsewhere [23, 28]). Simultaneous with formation of the gut tube, the endoderm is specified into future intestinal epithelium primarily through the induction of the transcription factor Cdx2 [29–32]. At this stage, the intestine is a single layer of Cdx2+ cuboidal epithelial cells that transitions to become a pseudostratified epithelium. The subsequent rapid expansion and thickening of the pseudostratified epithelium is responsible for increasing the length and girth of the intestine [33, 34]. Substantial remodeling then takes place, forming a single layer of columnar epithelium. The formation of the columnar epithelium is coincident with the emergence of villi and highly proliferative intervillus regions [35–37]. The mechanistic details of villus morphogenesis and formation of the intervillus regions is not entirely known; nevertheless, evidence suggests crosstalk between the epithelium and mesenchyme through the Hedgehog, BMP, platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, and Wnt pathways are important for the process (reviewed elsewhere [24, 38]). Transcription factors such as Gata4 and Gata6 are later involved in the specification of proximal versus distal intestine [39–41], although other less well understood factors are also likely involved.
After villus morphogenesis is complete, epithelial proliferation becomes restricted to the intervillus regions of the embryonic intestine. It is generally speculated that the cells in the intervillus region may give rise to the adult stem cells residing in the crypts of Lieberkühn, however, this has not been formally demonstrated. Notably, many of the best characterized adult intestinal stem cell (ISC) markers are not expressed in the proliferative embryonic intervillus domain [42, 43], suggesting that proliferating embryonic progenitor cells and adult stem cells in the crypt are not molecularly equivalent. Five differentiated cell types appear during and after emergence of villi: enterocytes (or colonocytes in the large intestine), goblet, tuft, enteroendocrine, and M-cells [44, 45]. In the mouse, Paneth cells appear in the small intestine only after crypts develop postnatally [42].
Once the crypts emerge during intestinal maturation, Wnt/β-catenin and Notch signaling are two critical pathways involved in the regulation of intestinal proliferation and cytodifferentiation (reviewed elsewhere [46]). Since many molecular markers of ISC are Wnt target genes, it is likely that Wnt signaling is essential for ISC maintenance [47]. Wnt signaling also controls Paneth cell differentiation, consistent with the spatial co-localization of ISCs and Paneth cells at the base of the crypts where Wnt signals are high [48]. The Notch pathway is critical for ISC self-renewal and differentiation [49–51] (reviewed in [52]). During differentiation, Notch/Hes1 signaling in absorptive cells opposes expression of the secretory transcription factor Atoh1/Math1 [50, 53, 54]. Further specification of secretory cell types occurs downstream of Atoh1/Math1 through Neurogenin3 [55, 56] for enteroendocrine cells, Gfi1, and SPDEF [57–59] for goblet cells, and Gfi1, Sox9, and SPDEF for Paneth cells [58–62]. Crosstalk between the Wnt/β-catenin and Notch signaling pathways needs to be further elucidated given reports of Wnt also playing a role in cell fate decisions [63–66].
Growing Intestinal Tissue In Vitro
Primary Intestinal Epithelial Culture Methods and Nomenclature
Major breakthroughs in our understanding of ISC regulation have been made over the last few years [36, 49, 67–71]. These advances have, in turn, had several positive consequences including the identification of novel ISC markers, an explosion in the development of new genetic tools to identify and study ISCs in mice [72–76], opening new avenues to develop therapeutic drugs to treat intestinal cancer [77], and technological breakthroughs enabling researchers to grow robust primary epithelial cultures from mouse and human intestine [78–80]. To date, many groups have published methodologies for growing primary intestinal epithelial cultures [78–83], which all include the core tenets that three-dimensional (3D) structure and high levels of Wnt signaling are critical to maintain long-term growth in vitro. Due to their robustness, development of these 3D culture methods have yielded powerful, physiologically relevant systems that facilitate the study of intestinal homeostasis [48, 51], gene regulation and function [48, 84, 85], and have additional promise for the performance of biochemical assays and drug screens. High Wnt signaling and 3D intestinal culture conditions [78, 79] enabled the expansion of human embryonic gut-like tissue derived from hPSCs into more mature human intestinal “organoids” [31, 86] (see “Generating Induced Human Intestinal Organoids (iHIOs)” section).
Given the diverse methods and tissue sources for primary intestinal cultures, there has been a recent initiative for the gastrointestinal research community to adopt a common nomenclature [87] (Table 1), although to date, a consensus has not been reached. This may be due, in part, to the fact that one of the original papers describing intestinal epithelial culture conditions referred to the resulting 3D structures as “intestinal organoids” [78]. These “organoids,” described by Sato et al. [78] are epithelial in nature, and contain no mesenchyme. Further, these epithelial-only cultures have been published and referred to as organoids in more than 30 publications since 2009. Stelzner et al. [87] proposed that these epithelial cultures be re-coined “enteroids,” since they are solely derived from the enteric epithelium and that the term “organoid” be reserved for tissue that more closely resembles the organ proper, composed of multiple tissue types including the epithelial and mesenchymal components characteristic of native intestine. Stelzner et al. also suggest referring to in vitro generation of intestinal tissue from non-intestinal sources as “induced” intestine. For the purposes of this review, we will refer to intestine derived from human embryonic or iPSCs as “induced human intestinal organoids” (iHIOs). As the suggested system for nomenclature implies, iHIOs contain epithelial and mesenchymal tissue. We caution readers not to confuse iHIOs with epithelial-only cultures derived from isolated intestinal epithelium, crypts or single ISCs (Table 1).
Table 1.
Description of terms and abbreviations
| Abbreviations | Full name | Description |
|---|---|---|
| hESC | Human embryonic stem cell | Pluripotent cells derived from human embryo |
| iPSC | Induced pluripotent stem cells | Pluripotent cells derived from reprogrammed somatic cells |
| Spheroid | Intestinal spheroid or mid/hindgut spheroid | 3D structure generated from human endoderm, resembling early embryonic gut tissue. Spheroids expand and give rise to iHIOs |
| Organoid | Organoid | 3D organ-like structure grown in vitro that resembles a complex organ in vivo, including multiple cell and tissue types |
| iHIO | Induced human intestinal organoid | 3D intestinal tissue generated from pluripotent stem cells, comprised of intestinal epithelial and mesenchymal tissue |
| Enteroid | Enteroid | Primary intestinal epithelium grown in culture, can be generated from mouse or human intestinal epithelium |
Generating Induced Human Intestinal Organoids (iHIOs)
As already highlighted, human embryonic and iPSCs have been highly touted for their potential to treat or cure disease through cell-based transplantation therapies. Nevertheless, the potential for hPSCs goes far beyond that of transplantation. hPSCs also hold amazing potential to accurately mimic human development, homeostasis, and disease in vitro. Work over the past decade has focused on understanding regulation of pluripotency and differentiation in hPSCs. More recently, directed differentiation has emerged as the most efficient approach to achieving in vitro generation of a cell or tissue of interest [48, 82, 83, 88–102]. Many breakthroughs in pluripotent stem cell differentiation have occurred using two-dimensional (2D) culture conditions directed at generating a single cell type of interest. In contrast, generation of 3D organ units (organoids) or more complex tissue from hPSCs has only recently been recognized as a viable approach for differentiation [31, 103, 104]. Such 3D models will offer complex multi-lineage, multi-cellular systems that can more closely recapitulate both normal physiology as well as pathological conditions in vitro, forming the basis for new in vitro human models designed to help understand normal homeostasis, complex multigenic diseases, perform drug screens, and validate the efficacy of new drugs prior to clinical trials [105].
Using directed differentiation, we were able to successfully generate 3D intestinal tissue by recapitulating embryonic development of the intestine in vitro [31, 86]. We employed a step-wise differentiation protocol that included endoderm induction, A–P patterning, intestinal lineage commitment, and intestinal growth and differentiation (Fig. 1). In the embryo, as cells migrate through the primitive streak, they are exposed to Nodal, a TGFß family member. Depending on the concentration and time of exposure to Nodal signaling, cells adopt either a mesodermal or endodermal fate en route to forming their proper germ layer [106–117]. In hPSCs, robust and efficient endoderm induction is achieved by mimicking Nodal signaling using Activin A [20, 88, 118]. We are routinely able to generate human endoderm cultures with >85 % efficiency, determined by co-staining of the transcription factors SOX17 and FOXA2 using a well-established 3-day differentiation protocol [31, 88]. Following induction, we consider the resulting human FOXA2+/SOX17+ endodermal tissue to be naive, capable of giving rise to all endodermal lineages including pancreatic (PDX1+), hepatic (ALB+), biliary (SOX17+), and intestinal (CDX2+) [31, 119].
Fig. 1.
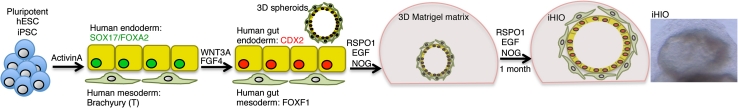
Schematic of human pluripotent stem cell-derived intestinal organoids. Human-induced pluripotent (iPSC) or embryonic (hESC) stem cells are differentiated into FOXA2/SOX17 positive endoderm with >85 % efficiency. A small proportion of cells (~2–5 %) also differentiate into Brachyury (T)-positive mesoderm. Induction of the intestinal epithelial transcription factor CDX2 is achieved by activating FGF and WNT signaling for 4 days. The mesenchymal population expands and expresses the intestine–mesenchyme transcription factor FOXF1. During this 4-day induction, 3D spheroids comprised of CDX2+ epithelial and FOXF1+ mesenchymal layers form, and delaminate from the tissue culture dish. Spheroids are then cultured in a 3D matrix (Matrigel) in “high WNT” conditions (WNT3A and/or RSPO1) along with EGF and Noggin (NOG). During the first month in culture, spheroids expand drastically in size, giving rise to iHIOs. iHIOs can be split and re-cultured, and maintained for many months in vitro
During endoderm induction, the embryo simultaneously undergoes complex morphogenetic movements and patterning events to give rise to the early gut tube, which is patterned into different domains along the A–P and dorsal–ventral (D–V) axes with the different domains giving rise to different subsets of endodermal organs [23, 109, 120–124]. Of note, work done in a host of vertebrate organisms has shown that an increasing anterior-to-posterior gradient of FGF, WNT and BMP signaling acts to posteriorize the endoderm [28, 125–129]; WNT and/or FGF signaling is able to induce human endoderm towards CDX2+ intestinal lineages [31, 32]. In FGF4 + WNT3A treated induced human endoderm, we observed robust and stable induction of CDX2 in ~95 % of cells after 96 h of treatment. Remarkably, we also observed dramatic morphogenetic movements in the tissue culture dish, which gave rise to gut-like “spheroids,” which were small 3D clusters of cells that budded from the underlying monolayer. These spheroids were comprised of an inner epithelial layer and outer mesenchymal layer. Although the mechanisms downstream of FGF and/or WNT signaling that govern these complex in vitro morphogenetic tissue movements are unclear, this system will likely be an excellent tool to study how complex tissue movements and tube formation occurs.
Following spheroid formation, we took advantage of pro-intestinal conditions established by Sato et al. [78] and continued to culture spheroids embedded in Matrigel and medium supplemented with recombinant human growth factors that promoted high levels of WNT signaling (WNT3A and/or RSPO1). Spheroid size and complexity increased remarkably over 1 month, giving rise to an epithelial and mesenchymal layer. The epithelium expressed molecular markers typical of many small intestinal cell types, including enteroendocrine cells (chromogranin A), goblet cells (mucin2), Paneth cells (lysozyme), and enterocytes (dipeptidyl peptidase (DPP)4, villin). We also observed that when iHIOs were cultured for >2 months, expression of ISC markers such as achaete-scute complex homolog 2 (ASCL2) and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) were observed. Using iHIOs, we have published a limited number of experiments to show that the epithelium appears to be functional and behaves in a normal physiological manner. For example, the epithelium in iHIOs is turned over every 6–7 days, similar to intestinal cell turnover in vivo [84, 130–132]. We have also demonstrated that iHIOs have a functional enterocyte peptide transport system by visualizing transport of a fluorescently labeled dipeptide [31, 133].
Experimental Utility of iHIOs and Enteroids
Epithelial-only enteroids generated from adult mouse or human intestinal epithelium have a proven track record as a highly useful tool for studying physiologically relevant events, such as ISC regulation and differentiation. In this light, it is important to highlight some of the advantages that iHIOs have to offer, as well as to point out potential disadvantages compared to enteroid cultures. One of the most significant advantages iHIOs offer is the ability to use this model to study human embryonic events in vitro. We have demonstrated that, much like the developing intestine in vivo, iHIOs transition from an early hindgut-like stage into a pseudostratified epithelium, which then undergoes tissue rearrangements that give rise to a columnar epithelium that has villus-like structures and proliferating intervillus-like domains [31, 38]. Further, development and differentiation of specialized cell types in the normal intestine are reflected in iHIOs. For example, we demonstrated that knockdown or overexpression of Neurog3 led to a respective decrease or increase of enteroendocrine cells in iHIOs, consistent with the described role of Neurog3 in humans and mice [31, 55, 134–136]. Recently, it has also been demonstrated that the transcription factor Arx is also critical for proper enteroendocrine development in the developing mouse intestine and in iHIOs [137].
A second advantage of iHIOs is their multi-lineage composition. That is, iHIOs have an endodermally derived epithelial layer, and a mesodermally derived mesenchymal layer. The presence of both germ layers is a byproduct of the ActivinA endoderm differentiation protocol used in the first step in iHIO generation. While differentiation is very efficient, and routinely generates ~90 % endoderm, a small population of cells differentiate into Brachyury (T)-positive mesodermal progenitors that give rise to FoxF1+ intestinal mesenchymal progenitors. The iHIO mesenchymal layer, while disorganized, is comprised of multiple cell types, including smooth muscle (smooth muscle actin +), intestinal subepithelial myofibroblasts (ISEMF), and fibroblasts [31]. Since iHIOs possess this mesenchymal layer, they can be used to study intestinal mesenchymal biology in addition to epithelial-mesenchymal interactions.
A third advantage of iHIOs is the ability to generate patient-specific intestinal tissue for the study of human disease. With the recent advances in generating induced pluripotent stem cell lines [15, 16], it is now possible to generate intestinal tissue from patients with specific diseases. Although likely possible, it is currently unclear whether enteroids (epithelium only) can be generated from diseased or damaged epithelium.
Coupling these two in vitro systems (iHIOs and enteroids) to study intestinal disease will be ideal given their distinct advantages. For example, iHIOs will enable the study of “healthy” intestinal tissue from an individual whereas enteroids from the same individual will have been exposed to the disease environment in vivo. For example, iHIOs generated from individuals with inflammatory bowel disease (IBD) will have “healthy,” non-inflamed intestinal tissue, with no exposure to the immune system whereas enteroids from the same patient will have had exposure to inflammation and the immune system. Therefore, intestinal tissue that has been in a disease-state (enteroids) and a non-disease state (iHIOs) from this patient can be examined, compared and experimentally manipulated. In this light, iHIOs will be a very powerful tool to elucidate the pathogenesis of complex multigenic human diseases.
Perspectives
Emerging evidence suggests that iHIOs will be useful for a wide variety of studies, such as infectious diseases [138]. As iHIOs are more widely adopted as a model to study human intestinal biology and pathobiology, their true utility and limitations will become more apparent. The significance of having complex, multi-lineage (mesenchyme, epithelium) 3D human intestinal tissue as a model experimental system will likely be unparalleled. In this light, it will be exciting to see how the scientific community puts this novel system to use.
Acknowledgments
We thank Dr. Deneen Wellik and Dr. Linda Samuelson for reading and commenting on the manuscript. Ms. Sha Huang kindly provided the iHIO image in Fig. 1. J.R.S. is supported by the University of Michigan Center for Organogenesis, the University of Michigan Biological Sciences Scholar Program and the NIDDK (KO1-DK091415). S.R.F. is supported by an NIDDK training grant, “Training in Basic and Translational Digestive Sciences” (1T32DK094775-01).
Conflict of interest
JRS is a co-inventor of intellectual property held over generating iHIOs. SRF declares no conflict of interest.
References
- 1.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/S0092-8674(00)81692-X. [DOI] [PubMed] [Google Scholar]
- 2.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 3.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Bigdeli N, Andersson M, Strehl R, et al. Adaptation of human embryonic stem cells to feeder-free and matrix-free culture conditions directly on plastic surfaces. J Biotechnol. 2008;133:146–153. doi: 10.1016/j.jbiotec.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 10.Rosler ES, Fisk GJ, Ares X, et al. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev Dyn. 2004;229:259–274. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- 11.Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–1641. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 13.Daley GQ. Cellular alchemy and the golden age of reprogramming. Cell. 2012;151:1151–1154. doi: 10.1016/j.cell.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MH, Cohen J. Reprogramming rewarded: the 2012 Nobel prize for Physiology or Medicine awarded to John Gurdon and Shinya Yamanaka. Reprod Biomed Online. 2012;25:549–550. doi: 10.1016/j.rbmo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narsinh KH, Plews J, Wu JC. Comparison of human induced pluripotent and embryonic stem cells: fraternal or identical twins? Mol Ther. 2011;19:635–638. doi: 10.1038/mt.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spence JR, Wells JM. Translational embryology: using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev Dyn. 2007;236:3218–3227. doi: 10.1002/dvdy.21366. [DOI] [PubMed] [Google Scholar]
- 21.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Williams LA, Davis-Dusenbery BN, Eggan KC. SnapShot: directed differentiation of pluripotent stem cells. Cell. 2012;149:1174–1174.e1. [DOI] [PubMed]
- 23.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–2091. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 25.de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60:1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 27.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 28.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 29.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial–mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grainger S, Savory JGA, Lohnes D. Cdx2 regulates patterning of the intestinal epithelium. Dev Biol. 2010;339:155–165. doi: 10.1016/j.ydbio.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherwood RI, Maehr R, Mazzoni EO, Melton DA. Wnt signaling specifies and patterns intestinal endoderm. Mech Dev. 2011 doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grosse AS, Pressprich MF, Curley LB, et al. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walton KD, Kolterud A, Czerwinski MJ, et al. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc Natl Acad Sci. 2012;109:15817–15822. doi: 10.1073/pnas.1205669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 37.Bondow BJ, Faber ML, Wojta KJ, Walker E, Battle MA. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol. 2012;371:1–12. doi: 10.1016/j.ydbio.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501–520. doi: 10.1002/dvdy.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battle MA, Bondow BJ, Iverson MA, et al. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–1686.e1. [DOI] [PMC free article] [PubMed]
- 40.Beuling E, Awuah NYAB, Stapleton KA, et al. GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology. 2011;140:1219–1229.e2. [DOI] [PMC free article] [PubMed]
- 41.Bosse T, Piaseckyj CM, Burghard E, et al. Gata4 is essential for the maintenance of jejunal–ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim T-H, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dehmer JJ, Garrison AP, Speck KE, et al. Expansion of intestinal epithelial stem cells during murine development. PLoS One. 2011;6:e27070. doi: 10.1371/journal.pone.0027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanaya T, Hase K, Takahashi D, et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat Immunol. 2012;13:729–736. doi: 10.1038/ni.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Lau W, Kujala P, Schneeberger K, et al. Peyer’s patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol Cell Biol. 2012;32:3639–3647. doi: 10.1128/MCB.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 47.Schepers A, Clevers H. Wnt signaling, stem cells, and cancer of the gastrointestinal tract. Cold Spring Harb Perspect Biol. 2012;4:a007989. doi: 10.1101/cshperspect.a007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farin HF, van Es JH, Clevers H. Redundant sources of wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 49.Vandussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2011 doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Es JH, van Gijn ME, Riccio O, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 51.Durand A, Donahue B, Peignon G, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noah TK, Shroyer NF. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu Rev Physiol. 2012 doi: 10.1146/annurev-physiol-030212-183741. [DOI] [PubMed] [Google Scholar]
- 53.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 54.Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 55.Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen G, Korfhagen TR, Xu Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregorieff A, Stange DE, Kujala P, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345.e1–3. [DOI] [PubMed]
- 60.Bastide P, Darido C, Pannequin J, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mori Akiyama Y, van den Born M, van Es JH, et al. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Shroyer NF, Wallis D, Venken KJT, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peignon G, Durand A, Cacheux W, et al. Complex interplay between β-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166–176. doi: 10.1136/gut.2009.204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuchiya K, Nakamura T, Okamoto R, Kanai T, Watanabe M. Reciprocal targeting of Hath1 and β-catenin by Wnt glycogen synthase kinase 3β in human colon. Cancer. 2007;132:208–220. doi: 10.1053/j.gastro.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 65.Leow CC. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64:6050–6057. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- 66.Rodilla V, Villanueva A, Obrador-Hevia A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinto D. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haramis APG. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 69.Muncan V, Sansom OJ, Tertoolen L, et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol. 2006;26:8418–8426. doi: 10.1128/MCB.00821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pellegrinet L, Rodilla V, Liu Z, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240.e1–7. [DOI] [PMC free article] [PubMed]
- 72.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 73.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montgomery RK, Carlone DL, Richmond CA, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powell AE, Wang Y, Li Y, et al. The Pan–ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeda N, Jain R, Leboeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011 doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fearon ER, Spence JR. Cancer biology: a new RING to Wnt signaling. Curr Biol. 2012;22:R849–R851. doi: 10.1016/j.cub.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 79.Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 81.Ramalingam S, Daughtridge GW, Johnston MJ, Gracz AD, Magness ST. Distinct levels of Sox9 expression mark colon epithelial stem cells that form colonoids in culture. Am J Physiol Gastrointest Liver Physiol. 2012;302:G10–G20. doi: 10.1152/ajpgi.00277.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012 doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 83.Fuller MK, Faulk DM, Sundaram N, Shroyer NF, Henning SJ, Helmrath MA. Intestinal crypts reproducibly expand in culture. J Surg Res. 2012;178:48–54. doi: 10.1016/j.jss.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koo B-K, Stange DE, Sato T, et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods. 2012;9:81–83. doi: 10.1038/nmeth.1802. [DOI] [PubMed] [Google Scholar]
- 85.Koo B-K, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 86.McCracken KW, Howell JC, Spence JR, Wells JM. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stelzner M, Helmrath MA, Dunn JCY, et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 89.Pankratz MT, Li X-J, LaVaute TM, Lyons EA, Chen X, Zhang S-C. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan Y, Yang D, Zarnowska ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu B-Y, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zahabi A, Shahbazi E, Ahmadideh H, et al. A new efficient protocol for directed differentiation of retinal pigmented epithelial cells from normal and retinal disease induced pluripotent stem cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0599. [DOI] [PubMed] [Google Scholar]
- 93.Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci USA. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomson M, Liu SJ, Zou L-N, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kane NM, Meloni M, Spencer HL, et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010;30:1389–1397. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 96.Shim JH, Kim SE, Woo DH, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 97.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 98.Basma H, Soto-Gutiérrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999.e4. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 100.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 101.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2009;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeLaForest A, Nagaoka M, Si-Tayeb K, et al. HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development. 2011;138:4143–4153. doi: 10.1242/dev.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Klos M, Wilson GF, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang A, Sander M. Generating cells of the gastrointestinal system: current approaches and applications for the differentiation of human pluripotent stem cells. J Mol Med. 2012;90:763–771. doi: 10.1007/s00109-012-0923-y. [DOI] [PubMed] [Google Scholar]
- 105.Zhu H, Lensch MW, Cahan P, Daley GQ. Investigating monogenic and complex diseases with pluripotent stem cells. Nat Rev Genet. 2011;12:266–275. doi: 10.1038/nrg2951. [DOI] [PubMed] [Google Scholar]
- 106.Lawson KA, Meneses JJ, Pedersen RA. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev Biol. 1986;115:325–339. doi: 10.1016/0012-1606(86)90253-8. [DOI] [PubMed] [Google Scholar]
- 107.Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- 108.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 109.Tam PPL, Kanai-Azuma M, Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev. 2003;13:393–400. doi: 10.1016/S0959-437X(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 110.Aoki TO, David NB, Minchiotti G, et al. Molecular integration of Casanova in the Nodal signalling pathway controlling endoderm formation. Development. 2002;129:275–286. doi: 10.1242/dev.129.2.275. [DOI] [PubMed] [Google Scholar]
- 111.Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- 112.Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–3662. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- 113.Tremblay KD, Hoodless PA, Bikoff EK, Robertson EJ. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development. 2000;127:3079–3090. doi: 10.1242/dev.127.14.3079. [DOI] [PubMed] [Google Scholar]
- 114.Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- 115.Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takahashi S, Yokota C, Takano K, et al. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development. 2000;127:5319–5329. doi: 10.1242/dev.127.24.5319. [DOI] [PubMed] [Google Scholar]
- 117.Yasuo H, Lemaire P. A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr Biol. 1999;9:869–879. doi: 10.1016/S0960-9822(99)80391-1. [DOI] [PubMed] [Google Scholar]
- 118.Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 119.Spence JR, Lange AW, Lin S-CJ, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robb L, Tam PPL. Gastrula organiser and embryonic patterning in the mouse. Semin Cell Dev Biol. 2004;15:543–554. doi: 10.1016/j.semcdb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 121.Lewis SL, Tam PPL. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235:2315–2329. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- 122.Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 123.Franklin V, Khoo P-L, Bildsoe H, Wong N, Lewis S, Tam PPL. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech Dev. 2008;125:587–600. doi: 10.1016/j.mod.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 124.Murtaugh LC, Wells JM. Understanding endoderm development: more than just a series of tubes. Dev Dyn. 2011;240:461–462. doi: 10.1002/dvdy.22587. [DOI] [PubMed] [Google Scholar]
- 125.Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 126.Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior–posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 127.Smith DM, Nielsen C, Tabin CJ, Roberts DJ. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut–foregut boundary. Development. 2000;127:3671–3681. doi: 10.1242/dev.127.17.3671. [DOI] [PubMed] [Google Scholar]
- 128.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 129.Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Creamer B, Shorter RG, Bamforth J. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 1961;2:110–118. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Creamer B. The turnover of the epithelium of the small intestine. Br Med Bull. 1967;23:226–230. doi: 10.1093/oxfordjournals.bmb.a070561. [DOI] [PubMed] [Google Scholar]
- 132.Macdonald WC, Trier JS, Everett NB. Cell proliferation and migration in the stomach, duodenum, and rectum of man: radioautographic studies. Gastroenterology. 1964;46:405–417. [PubMed] [Google Scholar]
- 133.Groneberg DA, Döring F, Eynott PR, Fischer A, Daniel H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am J Physiol Gastrointest Liver Physiol. 2001;281:G697–G704. doi: 10.1152/ajpgi.2001.281.3.G697. [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Cortina G, Wu SV, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- 135.Cortina G, Smart CN, Farmer DG, et al. Enteroendocrine cell dysgenesis and malabsorption, a histopathologic and immunohistochemical characterization. Hum Pathol. 2007;38:570–580. doi: 10.1016/j.humpath.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 136.Rubio-Cabezas O, Jensen JN, Hodgson MI, et al. Permanent neonatal diabetes and enteric anendocrinosis associated with biallelic mutations in NEUROG3. Diabetes. 2011;60:1349–1353. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Du A, McCracken KW, Walp E, et al. Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol. 2012 doi: 10.1016/j.ydbio.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Finkbeiner SR, Zeng X-L, Utama B, Atmar RL, Shroyer NF, Estes MK. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 2012;3:e00159–12. [DOI] [PMC free article] [PubMed]


