Abstract
Background
We conducted a phase I study to estimate the maximum tolerated dose and describe the dose-limiting toxicities and pharmacokinetics of oral capecitabine rapidly disintegrating tablets given concurrently with radiation therapy to children with newly diagnosed brainstem or high-grade gliomas.
Methods
Children 3–21 y with newly diagnosed intrinsic brainstem or high-grade gliomas were eligible for enrollment. The starting dose was 500 mg/m2, given twice daily, with subsequent cohorts enrolled at 650 mg/m2 and 850 mg/m2 using a 3 + 3 phase I design. Children received capecitabine at the assigned dose daily for 9 wks starting from the first day of radiation therapy (RT). Following a 2-wk break, patients received 3 courses of capecitabine 1250 mg/m2 twice daily for 14 days followed by a 7-day rest. Pharmacokinetic sampling was performed in consenting patients. Six additional patients with intrinsic brainstem gliomas were enrolled at the maximum tolerated dose to further characterize the pharmacokinetic and toxicity profiles.
Results
Twenty-four patients were enrolled. Twenty were fully assessable for toxicity. Dose-limiting toxicities were palmar plantar erythroderma (grades 2 and 3) and elevation of alanine aminotransferase (grades 2 and 3). Systemic exposure to capecitabine and metabolites was similar to or slightly lower than predicted based on adult data.
Conclusions
Capecitabine with concurrent RT was generally well tolerated. The recommended phase II capecitabine dose when given with concurrent RT is 650 mg/m2, administered twice daily. A phase II study to evaluate the efficacy of this regimen in children with intrinsic brainstem gliomas is in progress (PBTC-030).
Keywords: brainstem glioma, capecitabine, pediatric, pharmacokinetic, phase I
Primary brain tumors constitute the most common solid tumor of childhood, accounting for about 20% of all childhood malignancies.1 Although survival for pediatric cancer has improved significantly over the past 30 years, children with brainstem and high-grade gliomas continue to have a dismal prognosis. Children with intrinsic brainstem gliomas have 1- and 2-year progression-free survival (PFS) of less than 25% and 10%, respectively.2 The 5-year overall survival (OS) for children with incompletely resected high-grade gliomas is also less than 10%.2–4
Intrinsic brainstem gliomas are astrocytic neoplasms that typically occur in the pons, midbrain, or medulla. They have a characteristic MRI appearance that usually obviates the need for histological diagnosis in children with an appropriate clinical history.5 Radiation therapy (RT) provides symptomatic improvement and delays the time to progression; however, attempts to intensify radiation or add concurrent chemotherapy as a radiosensitizing agent have not further improved the outcome for children with intrinsic brainstem gliomas.2,6–11
High-grade gliomas of childhood, most commonly anaplastic astrocytomas and glioblastoma multiforme, are clinically aggressive and regionally invasive tumors. The role of chemotherapy in children with this disease is not clear. The use of temozolomide given concurrently and following RT is widely accepted for adults with high-grade gliomas based on improvement in PFS and OS in a large randomized trial.12 However, in the most recently completed Children's Oncology Group study of chemoradiotherapy with temozolomide in children with high-grade gliomas (ACNS0126), 3-year OS was only 22% ± 5%, with a 3-year event-free survival rate for children with glioblastoma of only 7% ± 4%.13 Thus, treatment of children with newly diagnosed brainstem and high-grade gliomas in clinical trials designed to maximize the impact of RT is appropriate.
Capecitabine (N4-pentyloxycarbonyl-5′-deoxy-5-fluorocytidine) is an oral fluoropyrimidine carbamate rationally designed to generate 5-fluorouracil (FU) preferentially in tumor tissue through exploitation of high intratumoral concentrations of thymidine phosphorylase (TP), an enzyme present at significantly increased concentrations in a wide range of tumor types. Studies in adult human pharmacokinetics (PK) have shown that after oral administration, capecitabine is rapidly and almost completely absorbed through the gastrointestinal wall with a time-to-peak-concentration of 2 hr and a short elimination half-life of 0.55 to 0.89 hr.14 Capecitabine is then metabolized to 5-FU via a 3-step enzymatic cascade, with the final stage of this conversion mediated by TP. In a second 3-step sequence, 5-FU is catabolized to dihydrofluorouracil (FUH2) by the enzyme dihydropyrimidine dehydrogenase (DPD) and then to fluoro-beta-ureidopropionate (FUPA) and fluoro-beta-alanine (FBAL), neither of which has any antiproliferative activity.
Cancer cells expressing high TP are very sensitive to capecitabine, and radiation is also a potent inducer of TP.15–17 Studies in U87 glioblastoma murine xenografts demonstrate a 70-fold induction of TP expression following a single dose of 5-Gy radiation; these levels increase initially after radiation completion and then decline over time.16 Because TP is expressed at high levels in malignant gliomas and increases in response to RT, the combination of capecitabine plus RT should lead to preferential conversion to the cytotoxic moiety in the glioma cells. In addition, capecitabine is a radiation sensitizer potentially enhancing the effects of RT.18,19 Further, capecitabine has been reported as an effective treatment for CNS parenchymal and leptomeningeal metastases in patients with breast cancer. A phase I study of capecitabine given concomitantly with RT in adults with glioblastoma demonstrated that the combination of capecitabine + RT was well tolerated, with a maximum tolerated dose (MTD) of 640 mg/m2 given twice daily.20
The primary objectives of this trial were to estimate the MTD and to describe dose-limiting toxicities (DLTs) of capecitabine rapidly disintegrating tablets (RDTs) administered concurrently with RT to children with newly diagnosed nondisseminated, intrinsic brainstem gliomas or newly diagnosed nondisseminated incompletely resected high-grade gliomas. The RDTs were made specifically to facilitate dosing in young children unable to swallow intact tablets. Secondary objectives were to describe the safety profile and antitumor activity of capecitabine RDTs in combination with RT in this patient population and to characterize the PK of capecitabine RDTs.
Materials and Methods
Children 3–21 years of age with newly diagnosed diffuse intrinsic brainstem gliomas or incompletely resected high-grade gliomas (anaplastic astrocytoma, glioblastoma multiforme, or other high-grade glioma) were eligible for this study. A histopathologic diagnosis was not required for patients with brainstem gliomas with characteristic MRI findings and clinical history. No prior therapy other than surgery or corticosteroids was allowed. Patients with high-grade gliomas were required to start therapy within 28 days of definitive surgery. Other eligibility criteria included: Karnofsky (for patients aged >16 y) or Lansky performance status (for patients aged ≤16 y) of ≥50; adequate hematologic function (absolute neutrophil count ≥1000/mm3, platelets ≥100 000/mm3, hemoglobin ≥8 g/dL); age-appropriate renal function; and adequate hepatic function (bilirubin ≤1.5× and serum glutamic pyruvic transaminase ≤5× the institutional upper limit of normal for age). Patients were excluded if they were receiving other anticancer or experimental therapy; had uncontrolled infection or significant cardiac, hepatic, gastrointestinal, renal, pulmonary, or other systemic disease; had a known hypersensitivity to capecitabine or its components; had known DPD deficiency; were receiving warfarin, sorivudine, or chemically related analogs, such as brivudine; or were pregnant or lactating. Patients were required to agree to use medically acceptable forms of birth control. The institutional review boards of each Pediatric Brain Tumor Consortium institution approved the protocol prior to initial patient enrollment, and continuing approval was maintained throughout the study. Patients or their legal guardians signed written informed consent, and assent was obtained as appropriate at the time of enrollment.
Treatment Plan
Study design
We aimed to maximize the cytotoxicity of capecitabine by administering it concurrently with radiation because preclinical studies demonstrate that radiation can selectively increase TP in tumor tissue. Because capecitabine can also serve as a radiosensitizing agent, the doses and schedules of administration were different during the “radiation” and “postradiation” therapeutic phases of our study.
Drug administration and radiation therapy
Capecitabine was provided as 125-, 175-, 250-, and 350-mg strawberry-flavored film-coated RDTs by Hoffmann–La Roche. Tablets could be swallowed whole with an 8-oz glass of water or after complete dispersion in room-temperature water. Administered doses did not deviate from the doses based on body surface area by more than 7%.
Capecitabine RDTs were administered twice daily without interruption for 9 weeks, beginning within 24 h of the initiation of RT. The starting dose was 500 mg/m2 (1000 mg/m2/day) with subsequent dose escalations in increments of 30%. No drug was administered during weeks 10 through 11. During the postradiation phase, the capecitabine RDT dose was 1250 mg/m2 (2500 mg/m2/day) for 14 consecutive days followed by a 7-day break. Patients were to receive a total of 3 postradiation courses. The entire duration of protocol treatment was 20 weeks.
Conventional or conformal RT was administered once daily 5 days a week in fractions of 180 cGy/day to a total dose of 5580 cGy. Treatment was directed to the maximal tumor dimensions demonstrated by either T1- or T2-weighted MRI, whichever was larger, plus a 1.5-cm anatomic margin in all dimensions (clinical target volume). The planning target volume was an additional 3–5 mm to the clinical target volume depending on the type of head immobilization and institutional preference.
Monitoring
Baseline assessment for all patients included a history, physical exam, and laboratory studies, with a pretreatment pregnancy test for females of childbearing potential. Routine testing for DPD deficiency was not required but was recommended if a patient developed significant toxicity during therapy. During the radiation phase, patient assessments included weekly history, physical exam, and laboratory studies. During the postradiation phase, patients had a history, physical exam, and laboratory monitoring at the start of each course as well as weekly complete blood counts. Neuro-imaging (MRI) was performed at diagnosis, week 11, week 20, and then every 3 months thereafter.
Trial design
A traditional 3 + 3 dose escalation scheme was used to estimate the MTD. Patients who came off therapy for reasons other than toxicity prior to the end of the 11-week dose-finding period were replaced for the purpose of estimating the MTD. Similarly patients who did not experience DLTs but who took <75% of their prescribed dose during the dose-finding period were deemed inevaluable for estimating the MTD. Six additional patients with intrinsic brainstem glioma were enrolled at the MTD, to further assess the toxicity profile at the recommended phase II dose.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.21 DLT was defined as any of the following events occurring during the 11-week dose-finding period: any event leading to interruption of planned radiation for 5 consecutive days or 10 days total; grade 4 neutropenia or thrombocytopenia; grade 3 thrombocytopenia that required a platelet transfusion on 2 or more occasions; any grade 3 or 4 nonhematologic toxicity (with the exception of grade 3 nausea or vomiting of <5 days duration, grade 3 transaminases that returned to baseline value within 7 days of study drug interruption and did not recur upon rechallenge, and/or grade 3 fever or infection of <5 days duration); grade 2 nonhematologic toxicities that persisted for >7 days and required treatment interruption, or any other capecitabine-related adverse events that required need for dose reduction, permanent cessation of therapy, or interruption of study drug for >7 days or that recurred on rechallenge with capecitabine RDTs.
Standard 2-dimensional imaging criteria were used for response assessment. Response findings for stable disease or better had to be maintained for at least 12 weeks and accompanied by a stable or decreasing dexamethasone dose and a stable or improving neurological exam. Patients could continue to receive capecitabine RDTs despite a radiographic increase in tumor size (25%–49%) if the investigator and patient/family thought that the patient was benefiting from therapy, due to the frequent difficulties in radiographically distinguishing radiation necrosis from tumor progression.
Pharmacokinetics
All patients were asked to participate in the PK substudy on days 1 and 14 of the first course. Patients were assigned to 1 of 2 sampling schedules, which were utilized to minimize the number of samples required and maximize the information obtained. In schedule A, samples were obtained predose, 10 min, 30 min, and 1, 2.5, 6, 8, and 10 h after dosing. In schedule B, samples were obtained at baseline, 15 min, 45 min, and 1.5, 4, 8, and 10 h after dosing. Additionally, samples were obtained 1–4 h after the morning dose on days 7 ± 2 and 21 ± 2.
Determination of plasma concentrations of capecitabine and its metabolites (5′-deoxy-5-fluorocytidine [DFCR], 5′-deoxy-5-fluorouridine [DFUR], 5-FU, and FBAL) was done using a validated liquid chromatography (LC) method with mass spectrometry detection. Capecitabine, 5′-DFCR, 5′-DFUR, 5-FU, and FBAL were extracted from EDTA human plasma samples by protein precipitation followed by solid phase extraction, which was performed in 2 steps, resulting in 2 sample extract fractions (A and B). Fraction A was transferred into 2 separate vials, one for derivatization with 2-methoxy-2,4-dephenyl-3(2H)-furanone and the other for 5-FU analysis by atmospheric pressure chemical ionization LC/tandem mass spectrometry (MS/MS) in the negative ion mode. The derivatized fraction was then analyzed for FBAL by negative ion spray LC/MS/MS. Fraction B sample extracts were analyzed for capecitabine, 5′-DFCR, and 5′-DFUR by turbo ion spray LC/MS/MS in the positive, positive, and negative ion modes, respectively.
The assay has a lower limit of quantitation of 10 ng/mL for capecitabine and 5′-DFCR, 50 ng/mL for 5′-DFUR, 2 ng/mL for 5-FU, and 15 ng/mL for FBAL. The calibration-curve ranges were linear from 10 to 5000 ng/mL for capecitabine and 5′-DFCR, from 50 to 25 000 ng/mL for 5′-DFUR, from 2 to 1000 ng/mL for 5-FU, and from 15 to 7500 ng/mL for FBAL.
Precision and accuracy quality control samples were prepared at concentrations of 30, 2500, and 4000 ng/mL for capecitabine and 5′-DFCR; 150, 12 500, and 20 000 ng/mL for 5′-DFUR; 6, 500, and 800 ng/mL for 5-FU; and 45, 3700, and 6000 ng/mL for FBAL. The intra-assay precision (coefficient of variation) results calculated from quality control samples ranged from 2.9% to 5.0% for capecitabine, from 1.9% to 6.4% for 5′-DFCR, from 1.6% to 7.8% for 5′-DFUR, from 2.5% to 7.9% for 5-FU, and from 2.4% to 10.7% for FBAL. The intra-assay accuracy (relative error) calculated from quality control samples ranged from −2.5% to 5.0% for capecitabine, from −2.8% to 3.3% for 5′-DFCR, from −1.5% to 2.0% for 5′-DFUR, from −4.9% to −1.7% for 5-FU, and from −1.7% to 5.1% for FBAL.
PK assessments were based on a previously described population (pop)PK model in adults for the main capecitabine metabolites (5′-DFUR, 5-FU, and FBAL).22 The expected PK concentration profiles (90% confidence interval [CI]) for an adult population receiving 500, 650, or 850 mg/m2 doses were simulated, and observed pediatric PK concentrations at the respective dose levels were superimposed for comparison. Individual PK parameter estimates for the pediatric patients were obtained using a Bayesian feedback approach by fixing the parameters to those from the adult popPK model. PK simulation and parameter estimation were conducted in NONMEM version V.1 software.23
Study Results
A total of 24 eligible children were enrolled from May 2007 through October 2009. Twenty patients were fully assessable for toxicity. Four patients were not assessable for toxicity: 1 withdrew prior to start of therapy, 1 withdrew because of researchers’ inability to get the child to take the first full dose of medication, 1 had significant clinical deterioration after enrollment and therefore did not meet the criteria to start therapy, and 1 was taken off therapy due to severe noncompliance but did not experience any unusual or severe capecitabine RDT-related toxicity. Patient characteristics are outlined in Table 1.
Table 1.
Patient characteristics
| Characteristic | No. of Patients |
|---|---|
| Total patients enrolled | 24 |
| Evaluable | 20 |
| Inevaluable | 4 |
| Diagnosis | |
| Brainstem glioma | 15 |
| High-grade glioma | 9 |
| Glioblastoma | 6 |
| Anaplastic astrocytoma | 3 |
| Gender | |
| Male | 9 |
| Female | 15 |
| Age, y | |
| Median | 9 |
| Range | 5–17 |
| Ethnicity | |
| Hispanic or Latino | 5 |
| Non-Hispanic | 17 |
| Unknown | 2 |
| Race | |
| Asian | 1 |
| Black | 6 |
| Pacific Islander | 1 |
| White, Non-Hispanic | 13 |
| Unknown | 3 |
Adverse Events
Capecitabine RDTs were generally well tolerated. The primary toxicities included palmar plantar erythrodysesthesia (hand-foot syndrome) with or without desquamation and moderate reversible elevations of serum transaminases. Grade 2 or higher adverse events that were possibly, probably, or definitely related to capecitabine RDTs are reported in Table 2.
Table 2.
Toxicities grade ≥2 noted during the radiation (dose-finding) period attributed to capecitabine RDTs
| Adverse Events | Grade 2 |
Grade 3 |
||||
|---|---|---|---|---|---|---|
| 500 mg/m2 (n = 3) | 650 mg/m2 (n = 12) | 850 mg/m2 (n = 5) | 500 mg/m2 (n = 3) | 650 mg/m2 (n = 12) | 850 mg/m2 (n = 5) | |
| Hemoglobin | 2 | |||||
| Leukopenia | 4 | 1 | ||||
| Neutropenia | 3 | 1 | 1 | |||
| Sweating | 1 | |||||
| Desquamation | 1 | |||||
| Hand-foot syndrome | 4 (3 DLT) | 3 (1 DLT) | 1 (DLT) | |||
| Constipation | 1 | |||||
| Nausea | 1 | 2 | ||||
| Vomiting | 1 | |||||
| Infection | 1 | |||||
| ALT (SGPT) | 2 | 1 (DLT) | 1 (DLT) | |||
| AST (SGOT) | 1 | |||||
| Creatinine | 1 | |||||
Abbreviations: SGPT, serum glutamic pyruvic transaminase; AST, aspartate aminotransferase; SGOT, serum glutamic oxaloacetic transaminase.
During the dose-escalation phase, there were no DLTs in the 3 patients enrolled at the first dose level, 500 mg/m2. One of the first 3 patients enrolled at the second dose level, 650 mg/m2, developed dose-limiting grade 2 palmar plantar erythrodysesthesia and prolonged elevation of alanine aminotransferase (ALT); however, the patient delayed notifying the treating physician. As a result, capecitabine was not discontinued in a timely fashion and the toxicity became dose limiting. The cohort was expanded and there were no additional DLTs. Three of the 5 evaluable patients treated at the third dose level, 850 mg/m2, developed DLTs that included palmar plantar erythrodysesthesia that did not resolve to baseline within 7 days (grade 2 [n = 1] and grade 3 [n = 1]) and grade 2 ALT elevation that did not resolve to baseline within 7 days. The MTD and recommended phase II dose of capecitabine RDTs administered concurrently with RT was therefore 650 mg/m2 administered every 12 h. An additional 6 patients with brainstem gliomas were enrolled at the 650 mg/m2/dose level to further evaluate the toxicity profile of capecitabine RDT with RT. Two patients in this expanded cohort developed grade 2 palmar plantar erythrodysesthesia that required dose reduction and was considered dose limiting. After brief interruption in treatment, the symptoms improved and both patients resumed treatment after dose reduction without significant toxicity.
Of the 17 patients who completed the dose-finding phase, 2 had disease progression and 1 was removed from treatment for noncompliance prior to starting the postradiation treatment phase. Thus, 14 patients were eligible to start the postradiation phase during which capecitabine was administered at the recommended adult dose (1250 mg/m2 twice daily for 14 d followed by a 1-wk rest) for a planned total of 3 courses. Capecitabine was generally well tolerated during this phase, with a similar toxicity profile to that of the dose-finding phase. Three additional patients came off treatment due to clinical or radiographic progression prior to the planned completion of 3 courses of postradiation therapy. One-year PFS and OS for the 8 patients with high-grade gliomas who received at least 1 dose of capecitabine were estimated to be 37.5% ± 14.8% and 50% ± 17.7%, respectively.
There were 3 deaths on or within 30 days of treatment. Two patients died of progressive disease, one during the postradiation phase and one 29 days after completing protocol therapy. The third patient died 24 days after completing capecitabine therapy, having developed diarrhea during the final week of therapy. Both progression of brainstem glioma and diarrhea with an associated Clostridium dificile infection and toxic megacolon were felt to have contributed to the patient's death. Table 3 outlines the additional adverse events that were noted during the postradiation phase.
Table 3.
Adverse events ≥grade 2 attributed to capecitabine RDTs in the postradiation phase (n = 14 patients)
| Adverse Event | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Leukopenia | 2 | ||
| Lymphopenia | 6 | 2 | |
| Neutropenia | 2 | 1 | |
| Fatigue | 1 | ||
| Rash | 1 | ||
| Desquamation | 2 | ||
| Hand-foot syndrome | 3 | ||
| Diarrhea | 1 | 1 | |
| Limb edema | 1 | ||
| ALT (SGPT) | 1 | ||
| Hyperglycemia | 1 | ||
| Pain | 1 |
Abbreviations: SGPT,serum glutamic pyruvic transaminase.
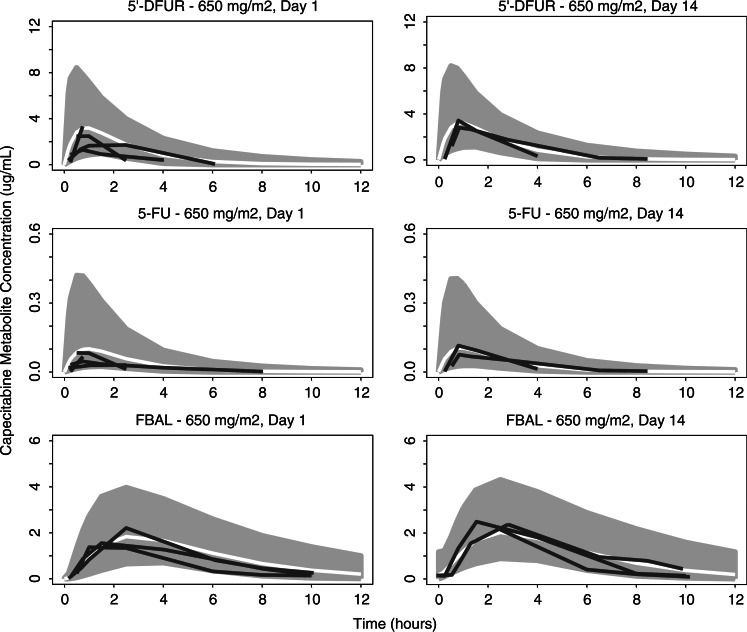
PK plasma concentrations for capecitabine and its metabolites (5′-DFCR, 5′-DFUR, 5-FU, and FBAL) were available from 9 patients, aged 6–17 years, who consented to participation in the PK substudy. The apparent clearance for the capecitabine main metabolites 5′-DFUR (CL1), 5-FU (CL2), and FBAL (CL3) in these pediatric patients tended slightly higher than expected in an adult population. Apparent volume of distribution of 5′-DFUR (V1) and of FBAL (V3) tended to be lower than previously observed in adult patients. Thus, the plasma exposures (area under the curve) for capecitabine and its metabolites in pediatric patients were similar or slightly lower than in adults at equivalent dose levels. This finding is illustrated in Fig. 1 for the 4 patients with PK data at the 650 mg/m2 dose (PK parameters are summarized in Table 4). As a result of the large interpatient variability and small number of patients with PK data, definitive conclusions about the apparent lower exposure in pediatric patients cannot be drawn at this time.
Fig. 1.
Simulated PK time courses for the capecitabine metabolites 5′-DFUR, 5-FU, and FBAL for adults receiving 650 mg/m2/dose with superimposed observed PK time courses for 4 pediatric patients at the same dose level. Gray areas represent the simulated 90% confidence intervals, white lines the simulated median PK time course for adults, and black lines the observed data in pediatric patients receiving capecitabine at the 650 mg/m2 dose level.
Table 4.
Summary of PK parameters by dose level
| Dose (mg/m2) | Analyte |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | 5′-DFUR |
5-FU |
FBAL |
|||||
| Median | Range | Median | Range | Median | Range | |||
| V (L) | 500 | 2 | 72 | 45–100 | 18 | (fixed) | 63 | 52–75 |
| 650 | 4 | 74 | 62–91 | 18 | (fixed) | 54 | 50–62 | |
| 850 | 3 | 86 | 75–94 | 18 | (fixed) | 124 | 74–126 | |
| Adult* | 91 | 18 | 74 | |||||
| CL (L/h) | 500 | 2 | 102 | 91–113 | 1305 | 1030–1580 | 26 | 22–30 |
| 650 | 4 | 85 | 76–104 | 1327 | 1256–1394 | 21 | 19–35 | |
| 850 | 3 | 94 | 88–107 | 1265 | 1260–1283 | 35 | 24–37 | |
| Adult* | 76 | 1190 | 28 | |||||
| Cmax (μg/mL) | 500 | 2 | 2.7 | 1.7–3.7 | 0.14 | 0.05–0.22 | 1.6 | 1.1–2.0 |
| 650 | 4 | 2.8 | 2.6–3.4 | 0.10 | 0.08–0.13 | 2.0 | 1.3–2.4 | |
| 850 | 3 | 5.5 | 4.2–7.4 | 0.21 | 0.15–0.33 | 2.4 | 2.4–2.9 | |
| AUC (μg/mL*h) | 500 | 2 | 3.9 | 3.0–4.7 | 0.16 | 0.14–0.18 | 6.6 | 6.3–6.9 |
| 650 | 4 | 5.8 | 3.7–7.9 | 0.21 | 0.14–0.24 | 9.1 | 7.3–11.9 | |
| 850 | 3 | 11.9 | 10.4–12.6 | 0.47 | 0.46–0.47 | 13.8 | 13.1–20.1 | |
| Adult* | 10.6 | 0.36 | 12.8 | |||||
Abbreviations: V (L), volume, liters; CL (L/h), clearance, liters/hr; Cmax, peak concentration; AUC, area under the curve.
*Population model parameters for adults receiving 650 mg/m2 are provided for comparison.22
Discussion
Clinical trials of multimodal therapy should be designed to maximize the effects of the combined therapy. In this trial, we built on the results of preclinical studies, which demonstrated that radiation resulted in a significant induction of TP in glioblastoma xenografts with subsequent increased antitumor activity of capecitabine, and that cells that express high levels of TP respond more favorably to therapy with capecitabine.15–17,24,25 In addition, capecitabine has been used effectively as a single agent to treat brain metastases in patients with breast cancer.24,26 These data suggest that a favorable therapeutic index may be achieved by 2 mechanisms: chemosensitization with increased conversion of capecitabine to the active form by induction of TP by RT as well as by sensitization of cancer cells to radiation by the radiosensitizing effects of capecitabine.16,27
Phase I trials of oral formulations are frequently difficult to perform in the pediatric population because young children in particular are not able to swallow pills or capsules. In addition, studies of oral formulations may be difficult or impossible to perform if there are a limited number of fixed dosing strengths because the actual deliverable dose may differ markedly from the prescribed dose. In this study, both of these limitations were overcome, as a variety of dosing strengths were available and capecitabine RDTs were specifically developed to allow rapid dissolution in a small quantity of water for children unable to swallow tablets.
The preliminary analysis of the PK data from the radiation phase of therapy was performed prior to enrollment of patients in the postradiation phase. The results suggest that the clearance of capecitabine and its metabolites in children was similar to or slightly more rapid than that in adults. As a result, there were no safety concerns about administering the recommended phase II adult dose in the postradiation phase of therapy. Additional PK data are currently being collected in the ongoing phase II trial of capecitabine RDTs in children with newly diagnosed brainstem gliomas, and once this study is complete, a full popPK analysis of the combined data will be performed to further investigate and assess the PK disposition in children.
The toxicity profile in the postradiation phase of therapy was also comparable to that of adults. Interestingly, 1 patient in the expanded cohort who experienced a DLT during the radiation phase of therapy subsequently received postradiation capecitabine (1250 mg/m2 twice daily) without subsequent DLT despite the marked difference in dose.
The combination of capecitabine RDTs and RT was generally well tolerated without unexpected neurological or gastrointestinal toxicities. In fact, the majority of the adverse events that occurred during the radiation and postradiation phases were similar. The MTD in this study was similar to that established in an adult study of radiation with capecitabine as initial therapy for glioblastoma where 625 mg/m2/dose given twice daily (1250 mg/m2/d) was the MTD. In adults, the DLTs were hand-foot syndrome and diarrhea.20 Similarly, hand-foot syndrome was a DLT in this study, as was prolonged mild elevation of ALT. In contrast, diarrhea was infrequent in our study, with the exception of a single patient, in whom late onset of diarrhea associated with C. dificile was severe.
In conclusion, the combination of capecitabine RDTs and RT was relatively well tolerated in this patient population. Capecitabine-related toxicities were generally mild and resolved with cessation of capecitabine therapy, with the exception of 1 patient who developed diarrhea associated with C. dificile. A Pediatric Brain Tumor Consortium phase II trial (PBTC-030) to further evaluate the efficacy of the combination of capecitabine with RT in pediatric patients with intrinsic brainstem gliomas is now open. Based on the toxicity and PK results of this trial, patients are receiving 650 mg/m2 twice daily with radiation and 1250 mg/m2 twice daily postradiation. Children with brainstem gliomas from this study, who were treated at the MTD and meet the eligibility criteria for the phase II study will be included in the analyses of the current phase II trial to maximize the use of available data from children with this rare and devastating disease and to facilitate the timely completion of the trial.
Funding
This work was supported in part by the National Institutes of Health grant no. K12 CA090433-06, grant no. U01 CA81457 for the Pediatric Brain Tumor Consortium, and grant no. 5M01RR000188, and by the American Lebanese Syrian Associated Charities, NIH grant no. 5M01RR000188.
Aknowledgments
The authors and the PBTC acknowledge clinical research assistant support by Joyson Pekkattil.
Conflict of interest statement. None declared.
References
- 1.Pollack IF. Brain tumors in children. N Engl J Med. 1994;331(22):1500–1507. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- 2.Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9(2):197–206. doi: 10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- 3.Wisoff JH, Boyett JM, Berger MS, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group trial no. CCG-945. J Neurosurg. 1998;89(1):52–59. doi: 10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- 4.Finlay JL, Zacharoulis S. The treatment of high grade gliomas and diffuse intrinsic pontine tumors of childhood and adolescence: a historical—and futuristic—perspective. J Neurooncol. 2005;75(3):253–266. doi: 10.1007/s11060-005-6747-7. [DOI] [PubMed] [Google Scholar]
- 5.Albright AL, Packer RJ, Zimmerman R, et al. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children's Cancer Group. Neurosurgery. 1993;33(6):1026–1029. doi: 10.1227/00006123-199312000-00010. discussion 1029–1030. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group phase I/II trial. Cancer. 1993;72(4):1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Bradley KA, Pollack IF, Reid JM, et al. Motexafin gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a Children's Oncology Group phase I study. Neuro Oncol. 2008;10(5):752–758. doi: 10.1215/15228517-2008-043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner CD, Chi S, Marcus KJ, et al. Phase II study of thalidomide and radiation in children with newly diagnosed brain stem gliomas and glioblastoma multiforme. J Neurooncol. 2007;82(1):95–101. doi: 10.1007/s11060-006-9251-9. [DOI] [PubMed] [Google Scholar]
- 9.Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children's Oncology Group study. Cancer. 2007;110(11):2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 10.Broniscer A, Iacono L, Chintagumpala M, et al. Role of temozolomide after radiotherapy for newly diagnosed diffuse brainstem glioma in children: results of a multiinstitutional study (SJHG-98) Cancer. 2005;103(1):133–139. doi: 10.1002/cncr.20741. [DOI] [PubMed] [Google Scholar]
- 11.Sanghavi SN, Needle MN, Krailo MD, et al. A phase I study of topotecan as a radiosensitizer for brainstem glioma of childhood: first report of the Children's Cancer Group-0952. Neuro Oncol. 2003;5(1):8–13. doi: 10.1093/neuonc/5.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 13.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85–104. doi: 10.2165/00003088-200140020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Andreetta C, Puppin C, Minisini A, et al. Thymidine phosphorylase expression and benefit from capecitabine in patients with advanced breast cancer. Ann Oncol. 2009;20(2):265–271. doi: 10.1093/annonc/mdn592. [DOI] [PubMed] [Google Scholar]
- 16.Blanquicett C, Gillespie GY, Nabors LB, et al. Induction of thymidine phosphorylase in both irradiated and shielded, contralateral human U87MG glioma xenografts: implications for a dual modality treatment using capecitabine and irradiation. Mol Cancer Ther. 2002;1(12):1139–1145. [PubMed] [Google Scholar]
- 17.Kim TD, Li G, Song KS, et al. Radiation-induced thymidine phosphorylase upregulation in rectal cancer is mediated by tumor-associated macrophages by monocyte chemoattractant protein-1 from cancer cells. Int J Radiat Oncol Biol Phys. 2009;73(3):853–860. doi: 10.1016/j.ijrobp.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 18.Vermund H, Gollin FF. Mechanisms of action of radiotherapy and chemotherapeutic adjuvants. A review. Cancer. 1968;21(1):58–76. doi: 10.1002/1097-0142(196801)21:1<58::aid-cncr2820210110>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhu AX, Willett CG. Chemotherapeutic and biologic agents as radiosensitizers in rectal cancer. Semin Radiat Oncol. 2003;13(4):454–468. doi: 10.1016/S1053-4296(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 20.Grunda JM, Fiveash J, Palmer CA, et al. Rationally designed pharmacogenomic treatment using concurrent capecitabine and radiotherapy for glioblastoma: gene expression profiles associated with outcome. Clin Cancer Res. 16(10):2890–2898. doi: 10.1158/1078-0432.CCR-09-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute. Common Terminology for Adverse Events (CTCAE) Bethesda, MD: US Deparment of Health and Human Services, National Institutes of Health; 2006. Version 3.0. Vol NIH Pub. No 03–5410. [Google Scholar]
- 22.Gieschke R, Burger HU, Reigner B, et al. Population pharmacokinetics and concentration-effect relationships of capecitabine metabolites in colorectal cancer patients. Br J Clin Pharmacol. 2003;55(3):252–263. doi: 10.1046/j.1365-2125.2003.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beal SL, Sheiner LB, Boeckmann AJ, editors. NONMEM User's Guides (1989–2006) Ellicott City, MD: Icon Development Solutions; 2006. [Google Scholar]
- 24.Endo M, Shinbori N, Fukase Y, et al. Induction of thymidine phosphorylase expression and enhancement of efficacy of capecitabine or 5′-deoxy-5-fluorouridine by cyclophosphamide in mammary tumor models. Int J Cancer. 1999;83(1):127–134. doi: 10.1002/(sici)1097-0215(19990924)83:1<127::aid-ijc22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Meropol NJ, Gold PJ, Diasio RB, et al. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24(25):4069–4077. doi: 10.1200/JCO.2005.05.2084. [DOI] [PubMed] [Google Scholar]
- 26.Fabi A, Vidiri A, Ferretti G, et al. Dramatic regression of multiple brain metastases from breast cancer with capecitabine: another arrow at the bow? Cancer Invest. 2006;24(4):466–468. doi: 10.1080/07357900600705805. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Lee P, Bidard FC, Pierga JY. Contemporary issues and the potential uses of capecitabine in metastatic breast cancer. Cancer Treat Rev. 2009;35(7):582–589. doi: 10.1016/j.ctrv.2009.06.003. [DOI] [PubMed] [Google Scholar]