Abstract
Background
Primary central nervous system lymphomas, predominantly diffuse large B-cell lymphomas (PCNS-DLBCL), are aggressive malignancies, and no histopathological variables with independent prognostic value are currently available. The aim of this study is to determine the prognostic value of histopathological variables of PCNS-DLBCL.
Methods
Aggregative perivascular tumor cells (APVTs) and reactive perivascular T cell infiltrates (RPVIs) in tumor samples from 62 immunocompetent patients with PCNS-DLBCL were histopathologically and immunohistochemically studied. A mouse brain DLBCL model was established to confirm the special morphological features of PCNS-DLBCL. The therapy, overall response rate (ORR), and overall survival (OS) among patients were followed up.
Results
APVT was present in 54 (87%) of the 62 cases, whereas RPVI was present in 20 (32%). Patients with APVT-positive lesions exhibited significantly worse OS, with intermediate to high International Extranodal Lymphoma Study Group (IELSG) scores, compared with patients with RPVI-positive lesions. Among cases of APVT-positive lymphoma, the semiquantitative score of immunostaining of X-box–binding protein (XBP1) and CD44 demonstrated prognostic significance. Multivariate analysis confirmed independent associations between APVT and XBP1 and between CD44 staining and survival.
Conclusions
The presence of APVT and staining of XBP1 and CD44 are independently associated with survival among patients with PCNS-DLBCL. These features could be routinely assessed in histopathological and immunohistochemical specimens.
Keywords: central nervous system, diffuse large B-cell lymphoma, histopathology, prognosis
Primary central nervous system lymphomas (PCNSL) are aggressive malignancies confined to the central nervous system (CNS), mostly comprising diffuse large B-cell lymphomas (PCNS-DLBCL).1–3 The World Health Organization classification of tumors of hematopoietic and lymphoid tissues in 2008 defined it as a new DLBCL, because the incidence of PCNS-DLBCL is increasing and the majority of these tumors have special clinical and pathological features.4 The incidence of PCNS-DLBCL among immunocompetent and immunodeficient individuals has progressively increased over the past few years.4–8 Despite improved therapeutic interventions, the prognosis of PCNS-DLBCL remains poor. Therefore, determining the prognostic and predictive role of various biological and clinical parameters in this particular DLBCL entity is very important. In clinical pathological examinations, accumulation of malignant B cells around vessels is commonly found in PCNS-DLBCL; however, the histopathological prognostic factors of this feature are not well established.3,9,10 Unlike most other organs, the CNS is an immunoprivileged site with restricted access and a unique microenvironment that profoundly affects the capacity of tumor cells and reactive cells.3,11 PCNSL rarely involves extra-CNS tissues, and recurrence and dissemination occur only within the CNS. PCNS-DLBCL displays unique histopathological features, probably because of this special environment.12 Therefore, in the present study, we specifically focused on the PCNS-DLBCL microenvironment, explored its particular pathological features, and analyzed the potential parameters that may correlate the perivascular positioning of malignant B cells and reactive lymphocytes with the prognosis of PCNS-DLBCL. We examined aggregative perivascular tumor cells (APVTs) and reactive perivascular T cell infiltrates (RPVIs), as well as X-box–binding-protein (XBP1) and CD44 expression in the neoplastic B cell population and reactive T cells. We further analyzed the relationship between these parameters and PCNS-DLBCL prognosis.
Materials and Methods
Patients and Samples
Data on patients with PCNS-DLBCL over a 16-year period (January 1996–December 2011) were retrieved from the archives of the Department of Pathology of Changhai Hospital (Shanghai, China). A total of 62 patients with PCNS-DLBCL were included in this study, and all patients provided informed written consent for study sample collection and permission for use in research. The control groups comprised 10 cases of primary nodal DLBCL and 10 cases of glioblastoma. All patient tumor resections were collected by operation. Information about patient characteristics, clinical presentation, staging, sites of lesion, laboratory findings, primary treatment, objective response, site and date of relapse, and survival were collected from hospital information systems. Patients were evaluated with standard methods, including history, physical examination, and biochemical-hematological tests. Patients with human immunodeficiency virus–positive serology and those who received corticosteroids before surgery were excluded from the study. The clinical prognostic variables included in the present analysis were previously defined as International Extranodal Lymphoma Study Group (IELSG) prognostic scores in a study of an international series of patients with PCNS-DLBCL.13
Animal Model
Diffuse large B cell lymphoma cell lines Ly1 and Ly8 (donated by Dr. B. Hilda Ye, Albert Einstein College of Medicine, NY) were cultured in DMEM medium supplemented with 10% fetal bovine serum and maintained in an atmosphere of 5% CO2 in a humidified incubator at 37°C. Fresh cells were collected and counted, and 2.0 × 104 cells in 0.02 mL were intraperitoneally injected into male 5-week-old BALB/c nude mouse (SLAC Laboratory Animal Company, Shanghai, China) brain at an intended depth of 0.5 cm under anesthesia with 5% chloral hydrate. Mice were sacrificed at 21–28 days after cell injection, and samples were collected for future use. At least 10 mice were used per experimental group. As a control, cells were injected into the subcutaneous of nude mice to observe the tumor cell growth pattern.14,15 The animal protocol was approved by the Institutional Animal Care and Use Committee of the Second Military Medical University, Shanghai, China.
Morphological Analysis
All tissue samples were formalin fixed, paraffin embedded, and hematoxylin and eosin (HE) stained. The slides were reviewed by 3 pathologists for morphological features of diffuse B cell lymphomas according to the World Health Organization (WHO) classification.1,4 Two histopathological parameters of APVT and RPVI were evaluated in the context of small-to-medium–sized vessels within valuable surgical resection tissues.16,17 APVT was defined as the presence of at least 1 small-to-medium–sized vessel surrounded by an aggregation of tumor cells, regardless of scattered lymphocyte infiltration. These neoplastic cells displayed atypia and a B cell phenotype with multilayered (usually >3 layers) accumulation close to the vessel's wall. RPVI, defined as a small-to-intermediate–sized lymphocyte, displayed a T cell phenotype accumulating close to the vessel's wall or interposed between the vessel wall and a concentric rim of neoplastic lymphocytes.16,17
Immunohistochemistry
Immunohistochemistry was performed on 4-μm sections of formalin-fixed, paraffin-embedded samples by the Envision method using the following primary antibodies: CD20, CD3, CD34, XBP1, and CD44 (Dako, CA). The sections were counterstained with hematoxylin, dehydrated, and mounted in DePex. Negative controls were prepared by omitting the primary antibody, and normal controls were prepared using normal brain tissue from autopsy samples. To observe the immunostaining results, XBP1 staining was assessed in the cell nuclei; CD20, CD3, and CD44 were evaluated in the cell membrane; and CD34 was stained in the cytoplasm and cytoplasmic membrane. Any unequivocal staining, regardless of intensity and proportion of cells stained, was regarded as positive. The immunohistochemical results were scored using a semi-quantitative scoring system (Allred score).18,19 This system assessed the percentage of positive cells (none = 0; <1% = 1; 1% to 10% = 2; 10% to 33% = 3; 34% to 67% = 4; and >67% = 5) and the intensity of staining (none = 0; weak = 1; intermediate = 2; and strong = 3). The intensity and percentage scores were then added to obtain a final score of 0–8. According to the final score, the immunostaining signal was determined as negative (0–2), weak positive (3–4), positive (5–6), and strong positive (7–8).
Treatment and Follow-Up
Treatment consisted of radiotherapy and chemotherapy with radiotherapy. Radiotherapy was administered to the whole brain with a median dose of 34 ± 12 Gy in patients treated with primary chemotherapy or 41 ± 6 Gy in primary radiotherapy-treated patients. High-dose methotrexate (dose, ≥1 g/m2), the most commonly used drug, was administered to 38 patients. A chemotherapy regimen without high-dose methotrexate was used in 19 patients. A total of 15 patients were treated only by radiotherapy. Information on follow-up, including overall response rate (ORR) and overall survival (OS), was obtained from hospital information systems and the patients or patients' relatives. ORR was calculated from the first day of treatment to disease remission. OS was calculated from the date of pathological diagnosis to death or to the last date of follow-up.
Statistical Considerations
Prognostic factors were analyzed according to the IELSG prognostic score. Clinical characteristics, pathological features, and immunohistochemical parameters of the subgroups were compared by using the χ2 test for categorical variables. All of the probability values were 2-sided and considered to be statistically significant when P< .05. Survival curves were generated using the Kaplan-Meier method. Comparison of OS curves was performed using the log-rank test. Each patient was assigned to a group according to pathological features or immunohistochemical parameters. Analyses were performed using SPSS, version 13.0, statistical package for Windows (Stat Soft, Inc., Tulsa, OK).
Results
Clinical and Histopathological Characteristics
The detailed clinical and histopathological characteristics of the patients are summarized in Table 1. According to the IELSG clinical scores, of the 62 PCNS-DLBCL cases, 13, 23, and 26 patients had low, intermediate, and high risk, respectively. In terms of morphology, PCNS-DLBCL and the brain DLBCL model displayed an APVT pattern, whereas the nodal DLBCL and glioblastoma did not (Fig. 1A–D). APVT was easily recognizable and present in 54 (87%) of the 62 cases analyzed. In APVT-positive cases, tumor cells were mediated to large neoplastic cells with multiple layers around vessels. The tumor cells of PCNS-DLBCL expressed CD20, displaying B cell immunophenotype as nodal DLBCL and the mouse brain DLBCL model, whereas glioblastoma cells were CD20 negative (Fig. 1E–H). RPVI was present in 20 (32%) of the 62 cases of PCNS-DLBCL; among these cases, RPVI was focal and never extensive. In RPVI-positive cases, RPVI-forming cells expressed CD3 and were always smaller than the size of neoplastic cells. The nodal DLBCL, brain DLBCL model, and glioblastoma did not show RPVI morphology (Fig. 1I–L). The brain DLBCL model showed the presence of APVT morphological feature 20–25 days after neoplastic cells were implanted into the mouse brain tissue. By contrast, the morphological features of RPVI were not observed until the model mice had died and the tumor cell growth pattern was diffuse in the control group. In 62 cases of PCNS-DLBCL, the presence or absence of APVT (87% vs 13%; P< .01) and RPVI (32% vs 68%; P< .01) was statistically significant. RPVI occurred either in APVT-positive cases or APVT-negative cases without striking differences (P> .05). Other clinical and histopathological features of elevated lactate dehydrogenase and β2 microglobulin level were not statistically significant (P> .05).
Table 1.
Clinical and pathological characteristics of 62 patients with PCNS-DLBCL
| Characteristic | PCNS-DLBCL (%) |
|---|---|
| Number | 62 |
| Histopathological type (DLBCL)a | 62 |
| Ages (Years) | |
| Range | 26–70 |
| <60 | 33 (53) |
| >60 | 29 (47) |
| Sex | |
| Male | 26 (42) |
| Female | 36 (58) |
| Ocular diseaseb | 3/62 (5) |
| Multiple lesionsb | 10/62 (16) |
| Involvement of deep structuresc | 5/62 (8) |
| Positive CSF cytology examinationb | 8/62 (13) |
| Elevated lactate dehydrogenase levelb | 20/62 (32) |
| Elevatedβ2 microglobulin levelb | 26/62 (42) |
| IELSGb prognosis score | |
| Low (0-1) | 13 (21) |
| Intermediate (2-3) | 23 (37) |
| High (4-5) | 26 (42) |
| APVTb | |
| Positive | 54 (87) |
| Negative | 8 (13) |
| RPVIb | |
| Positive | 20 (32) |
| Negative | 42 (68) |
a According to the WHO classification.
b Relationship between the number of positive cases and the total number of assessed patients.
c Involvement of deep structures of the brain; ie corpus callosum and/or basal ganglia and/or brain stem and/or cerebellum.
Abbreviations: APVT, aggregative perivascular tumor cell; CSF, cerebrospinal fluid; IELSG, International Extranodal Lymphoma Study Group; RPVI, reactive perivascular T-cell infiltrate.
Fig. 1.
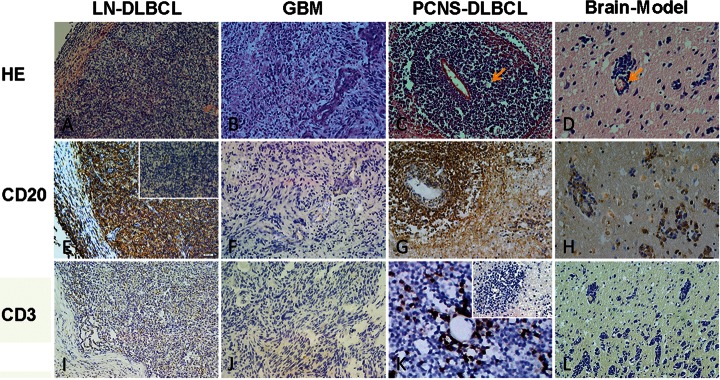
HE, CD20, and CD3 staining of the lymph node–diffuse large B cell lymphoma (LN-DLBCL), glioblastoma (GBM), primary central nervous system–diffuse large B cell lymphoma (PCNS-DLBCL), and Brain-Model. A, B, C, and D represent HE staining of LN-DLBCL, GBM, PCNS-DLBCL, and Brain-Model. PCNS-DLBCL and Brain-Model display clear APVT (yellow arrow), whereas both LN-DLBCL and GBM do not have APVT. E, F, G, H and I, J, K, L represent CD20 and CD3 staining of LN-DLBCL, GBM, PCNS-DLBCL, and Brain-Model, respectively. The cells with brown coloration are positive. In PCNS-DLBCL, CD3-positve T cells accumulated around the perivascular area interspersed within tumor cells (RPVI). The inserted figures are negative controls (magnification ×200).
XBP1 and CD44 Staining in PCNS-DLBCL Tissue
To further investigate the potential molecular pathogenesis of the tumor cell aggregative perivascular growth feature in PCNS-DLBCL, immunohistochemical analysis for XBP1 and CD44 staining in 62 PCNS-DLBCL cases were performed. CD34 was stained for displaying vessels. Normal brain tissue, nodal DLBCL, glioblastoma, PCNS-DLBCL, and the mouse brain DLBCL model all displayed vessels that were CD34 positive; the negative control was not stained (Fig. 2A–D). XBP1 staining in PCNS-DLBCL and the mouse brain DLBCL model showed a similarly strong pattern in the perivascular area and a broad distribution within the tumor microenvironment. The lymph node DLBCL, glioblastoma, negative control, and normal control were all negative for XBP1 (Fig. 2E–H). Moreover, tumor cells in the perivascular areas of PCNS-DLBCL and the mouse brain DLBCL model were all strongly positive for CD44. The lymph node DLBCL and glioblastoma were weakly positive for CD44, and the negative and normal controls were negative for CD44 (Fig. 2I–L). In APVT-positive PCNS-DLBCL, XBP1 and CD44 were positive in 87% (Allred score: weak positive, 5 cases; positive, 15 cases; strong positive, 27 cases) and 78% (Allred score: weak positive, 6 cases; positive, 17 cases; strong positive, 19 cases) of the 62 PCNS-DLBCL cases, respectively. In addition, in RPVI-positive PCNS-DLBCL, XBP1 and CD44 were positive in 8% (Allred score: weak positive, 1 case; positive, 1 case) and 25% (Allred score: weak positive, 2 cases; positive, 1 case; strong positive, 2 cases) of the cases, respectively. The staining and distribution of XBP1 and CD44 are displayed in Table 2. When the clinicopathological features were compared with XBP1 and CD44 expression, significant differences were found among XBP1, CD44 staining, and APVT (P< .05), RPVI (P< .01), and IELSG scores (P< .05). With different primary treatments, no significant differences between XBP1, CD44 expression and radiotherapy or chemotherapy (P> .05) and high-dose methotrexate (P> .05) were observed.
Fig. 2.
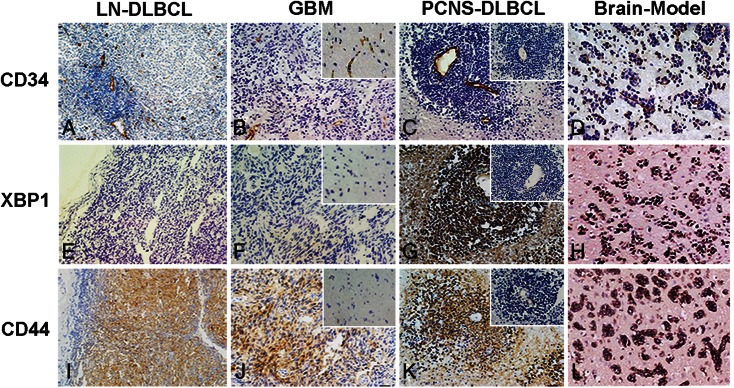
CD34, XBP1, and CD44 staining of LN-DLBCL, GBM, PCNS-DLBCL, and the mouse brain DLBCL model. The cells with brown coloration are protein positive. A, B, C, and D represent CD34 staining of showing vessels in LN-DLBCL, GBM, PCNS-DLBCL, and Brain-Model. E, F, G, H and I, J, K, L represent XBP1 and CD44 staining of LN-DLBCL, GBM, PCNS-DLBCL, and Brain-Model, respectively. PCNS-DLBCL and the mouse brain DLBCL model showed stronger signals of XBP1 (G and H) and CD44 (K and L) in aggregated perivascular tumor cells (APVT). LN-DLBCL and GBM were negative for XBP1 (E and F) and had weaker signals of CD44 (I and J). Inserted figures in B, F, and J represent normal control immunostaining of normal brain tissue slices. Inserted figures in C, G, and K show negative control immunostaining of a PCNS-DLBCL slice (omitting primary antibody) (magnification ×200).
Table 2.
Clinical-pathological characteristics and XBP1 and CD44 staining in 62 patients with PCNS-DLBCL
| Variable | Subgroup | No | XBP1 (%) | CD44 (%) | χ | P value |
|---|---|---|---|---|---|---|
| APVT | Positive | 54 | 47 (87) | 42 (78) | ||
| Negative | 8 | 2 (33) | 2 (33) | 13.848 | .0031 | |
| RPVI | Positive | 20 | 1 (8) | 5 (25) | ||
| Negative | 42 | 30 (73) | 35 (84) | 26.7697 | <.0001 | |
| IELSG prognosis score | Low | 13 | 4 (37) | 3 (25) | ||
| Intermediate | 23 | 17 (75) | 18 (81) | |||
| High | 26 | 20 (79) | 22 (86) | 15.0494 | .0199 | |
| Primary treatment | Radiotherapy | 31 | 16 (52) | 26 (84) | ||
| Chemotherapy | 57 | 45 (80) | 46 (82) | 4.4402 | .2177 | |
| High-dose methotrexate | Yes | 28 | 19 (70) | 21 (76) | ||
| No | 34 | 24 (71) | 27 (81) | 0.1168 | .989 |
Abbreviations: APVT, aggregative perivascular tumor cell; IELSG, International Extranodal Lymphoma Study Group; RPVI, reactive perivascular T cell infiltrate.
Treatment Response, Survival, and Prognostic Factors
Follow-up according to the analyzed variables are summarized in Table 3. The median follow-up time was 75 months (range, 1–140 months). The 3-year OS was 58% among all cases (median, 25 months; range, 2–48 months). The objective response after first-line treatment was complete response (CR) in 17 (28%) patients and partial response (PR) in 9 (15%) patients, with an ORR of 29%. A total of 27 patients experienced progressive disease (PD), and 3 patients died of toxicity. Sixteen of the 28 patients treated with chemotherapy containing high-dose methotrexate were alive at a median follow-up of 45 months, with a 3-year OS of 53% ± 6%. A total of 21 patients died of lymphoma (n = 15), treatment toxicity (n = 3), and neurological deterioration (n = 2) or unrelated causes while disease free (n = 1). The presence or absence of APVT (87% vs 13%; P< .01) was associated with higher progress rates, whereas the presence or absence of RPVI (32% vs 68%; P< .01) implied higher response rates. When analysis was limited to the 62 patients regarding APVT and RPVI with overall survival, patients with APVT-positive lymphoma survived shorter than did patients with RPVI-negative lymphoma (log-rank test, P= .0037) (Fig. 3A). Among the studied variables, the prognostic values of APVT and RPVI were more evident among patients with analyses performed both on the XBP1 and on the CD44 staining. The IELSG score had prognostic significance, but primary treatment and high-dose methotrexate without significant value (Table 3). The OS time of cases with XBP1- and CD44-positive staining was shorter than that among cases with XBP1- and CD44-negative staining (log-rank test, P= .0096). In addition, advanced-stage cases with strong staining of XBP1 and CD44 and those who relapsed had shorter survival. Fig. 3B displays the survival curves of the cases according to XBP1 and CD44 staining.
Table 3.
Multivariate analysis of ORR and OS with XBP1 and CD44 expression in 62 patients with PCNS-DLBCL
| Variable | No | XBP1 (%) | CD44 (%) | ORR (%) | 3-year OS (%) | χ | P value |
|---|---|---|---|---|---|---|---|
| APVI | |||||||
| Positive | 54 | 46 (87) | 42 (78) | 18 (34) | 31 ± 9 | ||
| Negative | 8 | 2 (33) | 2 (33) | 6 (83) | 64 ± 6 | 18.8162 | .0021 |
| RPVI | |||||||
| Positive | 20 | 1 (8) | 5 (25) | 15 (75) | 37 ± 9 | ||
| Negative | 42 | 30 (73) | 35 (84) | 19 (46) | 32 ± 7 | 29.5332 | .0001 |
| IELSG prognosis score | |||||||
| Low | 13 | 4 (37) | 3 (25) | 11 (88) | 69 ± 8 | ||
| Intermediate | 23 | 17 (75) | 18 (81) | 17 (75) | 41 ± 7 | ||
| High | 26 | 20 (79) | 22 (86) | 7 (29) | 6 ± 9 | 24.872 | .0056 |
| Treatment | |||||||
| Radiotherapy | 31 | 16 (52) | 26 (84) | 8 (26) | 24 ± 9 | ||
| Chemotherapy | 57 | 45 (80) | 46 (82) | 16 (29) | 43 ± 6 | 0.4714 | .4837 |
| High-dose Methotrexate | |||||||
| Yes | 38 | 26 (70) | 28 (76) | 28 (76) | 60 ± 7 | ||
| No | 24 | 17 (71) | 19 (81) | 12 (52) | 22 ± 8 | 2.4598 | .7825 |
Abbreviations: APVT, aggregative perivascular tumor cell; IELSG, International Extranodal Lymphoma Study Group; RPVI, reactive perivascular T cell infiltrate.
Fig. 3.
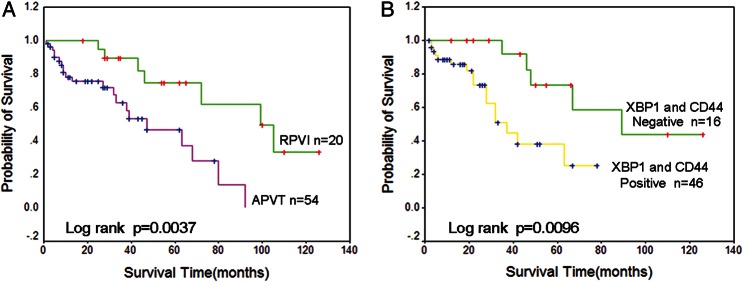
(A) OS according to the presence of APVT and RPVI in patients with PCNS-DLBCL. Patients with RPVI-positive lymphomas (green line) showed significantly better survival rates, compared with patients with APVT-positive lymphomas (red line). (B) OS associated with XBP1 and CD44 staining in patients with PCNS-DLBCL. Patients with XBP1 and CD44-positive lymphomas (yellow line) showed significantly worse survival rates, compared with patients with XBP1 and CD44-negative lymphomas (green line).
Discussion
During clinical pathological examination, accumulation of malignant B cells around vessels is typically found in PCNS-DLBCL. This study focuses on the relationship between distribution pattern of tumor cells and reactive infiltrating cells and PCNS-DLBCL prognosis. Our data showed that the presence of APVT is associated with higher progression rates and shorter survival, compared with the absence of APVT from the tumors. On the other hand, several reports show that RPVI correlates with improved survival in PCNS-DLBCL, which may be subject to high selective pressure mediated by the immune system.16,17 RPVI-forming cells in this study were CD3-positive, and their size was smaller than that of neoplastic cells. Lymphomas with RPVI are associated with improved survival, which is consistent with the findings in previous reports.16,17 We observed increased numbers of T cells in both APVT-positive and APVT-negative PCNS-DLBCL cases with respect to normal brain tissue, suggesting increased T cell immune reactions to tumor cells in a specific tumor microenvironment.
The presence of APVT and RPVI may confer specific features to PCNS-DLBCL; thus, we further determined the correlation of IELSG scores with the presence or absence of APVT and RPVI.13 According to the IELSG scores obtained, most patients (79%) belonged to intermediate and high-risk groups, which may be attributable in part to the inadequate or inappropriate treatment of the tumors in the present study. The perivascular position of APVT was seen in cases with intermediate to high IELSG scores; that of RPVI was seen in cases with low to intermediate IELSG scores. Assessment of APVT and RPVI combined with the IELSG scores could help distinguish subgroups of patients with different prognoses and offer practical advantages in routine diagnosis. These simple histological markers could be supplemented with IELSG scores to classify patients with lymphoma according to their aggressiveness.
Our data show that APVT and RPVI notably implicated tumor prognosis in PCNS-DLBCL; thus, determining the molecular pathogenesis of these special features is necessary. XBP1 is a major endoplasmic reticulum stress-linked transcriptional factor that is essential for survival under hypoxic conditions and positively regulates tumor growth and metastasis.20,21 Previous studies have revealed that XBP1 produces strong staining in dense populations of tumor cells and is closely associated with the vasculature in CNS lymphomas,22,23 partially regulated by paracrine interactions.24 Considering the microenvironment of the brain, tumor cells in the CNS could survive under insufficient oxygen conditions, and XBP1 over-expression may trigger the progression and form APVT in PCNS-DLBCL. CD44, also called lymphocyte-homing receptor, is a very important adhesive molecule that has important functions in lymphocyte migration, homing, and activation. CD44 is necessary for tumor progression and metastasis, and its high expression generally implies an unfavorable prognosis in DLBCL and several other hematological malignancies.25–28 Combining the special microenvironment of the CNS and the histopathological features of PCNS-DLBCL, we analyzed the staining of XBP1 and CD44 in APVT and RPVI and their prognostic significance. The patterns of XBP1 and CD44 staining were aggregated in the perivascular area in PCNS-DLBCL. DLBCL in the mouse brain model also showed strong XBP1 and CD44 staining. Hypoxia induces XBP1 at the transcriptional level and activates splicing of its mRNA, resulting in increased levels of activated XBP1 protein.20,24 Therefore, we believe that the expression pattern of XBP1 in the APVT area implicates XBP1 as an essential survival factor for hypoxic stress involving tumor cell aggregative perivascular growth pattern in PCNS-DLBCL. CD44 interferes with lymphocyte homing and the binding of lymphocytes to high endothelial venules in the lymph node.25,26 Thus, we believe that the strong expression of CD44 in perivascular tumor cells of PCNS-DLBCL may be an important factor for APVT. Our findings should be prospectively validated in a population that is uniformly treated. Actually, the primary therapy was different in this serial case of PCNS-DLBCL, and there were not significant differences between XBP1, CD44 expression, and radiotherapy or chemotherapy (such as high-dose methotrexate) (P> .05). We will continue to pay attention to the APVT and XBP1, CD44 expression with OS under the similar therapy of PCNS-DLBCL in the future. It will be more impressive if our findings will be prospectively confirmed in a population that is uniformly treated. Taken together, XBP1 and CD44 are complex interactions associated with the phenomenon of tumor cell angiotropism, which suggests that the vascular and perivascular microenvironments of PCNS-DLBCL may be responsible for the accumulation of malignant B cells by XBP1 and CD44. Cytokines, such as IL-4 (which is known to regulate the expression of XBP1 in B cells), L-selectin (which mediates lymphocyte-neoplastic cell interactions), and VEGF (which mediates hypoxia-inducible factor-mediated neovascularization), were also detected to analyze the special histopathological features of PCNS-DLBCL. However, the results obtained from the staining of these cytokines were not significantly different from those obtained in the tumor (data not shown).
No detailed descriptions on the correlation between histopathological features and tumor outcome are available in related reports.16,29,30 In the present study, we retrospectively analyzed the clinicopathological and morphological features of 62 cases of PCNS-DLBCL and found that PCNS-DLBCL could be characterized by the simultaneous presence of APVT and RPVI, which are significantly associated with prognosis. More cases need to be investigated to confirm these specific features. However, in the clinical setting, surgical collection of samples of PCNS-DLBCL is challenging.1,31–34 Of note, detection of APVT and RPVI, both of which are rapid, easy-to-assess, and valuable pathological parameters, could be used to better stratify PCNS-DLBCL cases. The presence of APVT with XBP1 and CD44 staining is independently associated with lower survival rates of PCNS-DLBCL. These parameters should be noted in daily practice to gain further understanding of this special kind of tumor.
Conflict of interest statement. None declared.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Project No. 39670733, 81172249 and 30930113).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. World Health Organization Classification of Tumors of the Central Nervous System. Lyon: IARC Press; 2007. [DOI] [PubMed] [Google Scholar]
- 2.Hagavathi S, Sharathkumar A, Hunter S, et al. Activated B-cell immunophenotype might be associated with poor prognosis of primary central nervous system lymphomas. Clin Neuropathol. 2008;27:13–20. doi: 10.5414/npp27013. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Okamoto H, Yin C, et al. Proteomic characterization of primary diffuse large B-cell lymphomas in the central nervous system. J Neurosurg. 2008;109:536–546. doi: 10.3171/JNS/2008/109/9/0536. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization classification of tumours: Pathology and genetics of tumors of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 5.Bhagavathi S, Wilson JD. Primary central nervous system lymphoma. Arch Pathol Lab Med. 2008;122:1830–1834. doi: 10.5858/132.11.1830. [DOI] [PubMed] [Google Scholar]
- 6.Montesinos-Rongen M, Siebert R, Deckert M. Primary lymphoma of the central nervous system: just DLBCL or not? Blood. 2009;113:7–10. doi: 10.1182/blood-2008-04-149005. [DOI] [PubMed] [Google Scholar]
- 7.Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin's lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- 8.Dubuisson A, Kaschten B, Lénelle J, et al. Primary central nervous system lymphoma report of 32 cases and review of the literature. Clin Neurol Neurosurg. 2004;107:55–63. doi: 10.1016/j.clineuro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Haene N, Catteau X, Maris C, et al. Endothelial hyperplasia and endothelial galectin-3 expression are prognostic factors in primary central nervous system lymphomas. Br J Haematol. 2008;140:402–410. doi: 10.1111/j.1365-2141.2007.06929.x. [DOI] [PubMed] [Google Scholar]
- 11.Kadoch C, Dinca EB, Voicu R, et al. Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model. Clin Cancer Res. 2009;15:1989–1997. doi: 10.1158/1078-0432.CCR-08-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coupland SE. The challenge of the microenvironment in B-cell lymphomas. Histopathology. 2011;58:69–80. doi: 10.1111/j.1365-2559.2010.03706.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal, Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 14.Tótth ÁA, Schnur J, Ladányi A, et al. Intracerebral human lymphoma – an experimental model. Pathol Oncol Res. 1996;2:174–176. doi: 10.1007/BF02903522. [DOI] [PubMed] [Google Scholar]
- 15.He MX, Wang JJ, Zhou XP, et al. Development of a nude mouse model for diffuse large B-cell lymphoma of brain. Zhonghua Bing Li Xue Za Zhi. 2011;40(12):840–842. Article in Chinese. [PubMed] [Google Scholar]
- 16.Ponzoni M, Berger F, Chassagne-Clement C, et al. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol. 2007;138:316–323. doi: 10.1111/j.1365-2141.2007.06661.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumari N, Krishnani N, Rawat A, et al. Primary central nervous system lymphoma: Prognostication as per international extranodal lymphoma study group score and reactive CD3 collar. J Postgrad Med. 2009;55:247–251. doi: 10.4103/0022-3859.58926. [DOI] [PubMed] [Google Scholar]
- 18.Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumour cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 19.O'Malley FP, Saad Z, Kerkvliet N, et al. The predictive power of semiquantitative immunohistochemical assessment of p53 and c-erbB-2 in lymph node-negative breast cancer. Hum Pathol. 1996;27:955–963. doi: 10.1016/s0046-8177(96)90224-5. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Ramirez L, Cao H, Nelson D, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto T, Onda M, Nagai H, et al. Upregulation and overexpression of human X-box binding protein 1 (hXBP-1) gene in primary breast cancers. Breast Cancer. 2003;10:301–306. doi: 10.1007/BF02967649. [DOI] [PubMed] [Google Scholar]
- 22.Iwakoshi NN, Lee AH, Vallabhajosyula P, et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:221–229. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 23.Maestre L, Tooze R, Cañamero M, et al. Expression pattern of XBP1(S) in human B-cell lymphomas. Haematologica. 2009;94:419–422. doi: 10.3324/haematol.2008.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenstein JL, Shen A, Batchelor TT, et al. Differential gene expression in central nervous system lymphoma. Blood. 2009;113:266–267. doi: 10.1182/blood-2008-04-152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mempel TR, Marangoni F. CD44 keeps tumor killers polarized. Immunity. 2008;29:843–845. doi: 10.1016/j.immuni.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Naor D, Wallach-Dayan SB, Zahalka MA, et al. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Seo DW, Kong SK, et al. TIMP1 induces CD44 expression and the activation and nuclear translocation of SHP1 during the late centrocyte/post-germinal center B cell differentiation. Cancer Lett. 2008;269:37–45. doi: 10.1016/j.canlet.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberth S, Schneider B, Rosenwald A, et al. Epigenetic regulation of CD44 in Hodgkin and non-Hodgkin lymphoma. BMC Cancer. 2010;10:517. doi: 10.1186/1471-2407-10-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreri AJ, Reni M. Prognostic factors in primary central nervous system lymphomas. Hematol Oncol Clin N Am. 2005;19:629–649. doi: 10.1016/j.hoc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Ferreri AJ, Reni M, Pasini F, et al. A multicentre study of treatment of primary CNS lymphoma. Neurology. 2002;58:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 31.Aho R, Ekfors T, Haltia M, et al. Pathogenesis of primary central nervous system lymphoma: invasion of malignant lymphoid cells into and within the brain parenchyme. Acta Neuropathol (Berl) 1993;86:71–76. doi: 10.1007/BF00454901. [DOI] [PubMed] [Google Scholar]
- 32.Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:2200–2210. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunn A, Montesinos-Rongen M, Strack A, et al. Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol. 2007;114:271–276. doi: 10.1007/s00401-007-0258-x. [DOI] [PubMed] [Google Scholar]
- 34.Bessell EM, Hoang-Xuan K, Ferreri AJ, et al. Primary central nervous system lymphoma: biological aspects and controversies in management. Eur J Cancer. 2007;43:1141–1152. doi: 10.1016/j.ejca.2006.12.011. [DOI] [PubMed] [Google Scholar]


