Abstract
Background
To assess management patterns and outcome in patients with glioblastoma multiforme (GBM) treated during 2008–2010 in Spain.
Methods
Retrospective analysis of clinical, therapeutic, and survival data collected through filled questionnaires from patients with histologically confirmed GBM diagnosed in 19 Spanish hospitals.
Results
We identified 834 patients (23% aged >70 years). Surgical resection was achieved in 66% of patients, although the extent of surgery was confirmed by postoperative MRI in only 41%. There were major postoperative complications in 14% of patients, and age was the only independent predictor (Odds ratio [OR], 1.03; 95% confidence interval [CI],1.01–1.05; P = .006). After surgery, 57% received radiotherapy (RT) with concomitant and adjuvant temozolomide, 21% received other regimens, and 22% were not further treated. In patients treated with surgical resection, RT, and chemotherapy (n = 396), initiation of RT ≤42 days was associated with longer progression-free survival (hazard ratio [HR], 0.8; 95% CI, 0.64–0.99; P = .042) but not with overall survival (HR, 0.79; 95% CI, 0.62–1.00; P = .055). Only 32% of patients older than 70 years received RT with concomitant and adjuvant temozolomide. The median survival in this group was 10.8 months (95% CI, 6.8–14.9 months), compared with 17.0 months (95% CI, 15.5–18.4 months; P = .034) among younger patients with GBM treated with the same regimen.
Conclusions
In a community setting, 57% of all patients with GBM and only 32% of older patients received RT with concomitant and adjuvant temozolomide. In patients with surgical resection who were eligible for chemoradiation, initiation of RT ≤42 days was associated with better progression-free survival.
Keywords: glioblastoma multiforme, older patients, practice patterns, radiotherapy delay, surgical complications
Glioblastoma multiforme (GBM) is the most common and malignant glioma, with an annual incidence of 3–4 new cases per 100,000 inhabitants.1,2 Despite advances in diagnosis and treatment, the prognosis of GBM remains poor, with a probability of survival of 12% at 4 years.3 The current standard of care, established in 2005, includes postsurgical external beam radiotherapy (RT) with concomitant and adjuvant temozolomide (TMZ) therapy.4 However, because of the extension of the standard treatment to the general nonselected population outside of clinical trials and the differences between countries, it has been less reported.5–8
The main goal of the present study was to evaluate the routine clinical treatment of patients with GBM in Spain to determine the implementation of the standard treatment and to evaluate outcome. We also analyzed the impact of surgical complications on outcome, the influence on survival of delay in initiation of RT, and the treatment of patients >70 years of age, a segment of patients with GBM that will increase in the next decade and was excluded in the seminal article that established the standard treatment for GBM.4,9
Methods
All medical records from patients with newly diagnosed, histologically confirmed GBM from January 2008 through December 2010 were reviewed at 19 Spanish hospitals. Patients with a history of low-grade glioma and 32 patients who were lost to follow-up immediately after surgery were excluded. Seventeen of the hospitals are reference centers for brain tumors in the Spanish Public Health System and serve around 11 540.000 inhabitants, 24% of the total Spanish population. The 2 other participant hospitals were a private and a community hospital that included 2.3% of patients.
Data were collected retrospectively using a specific data form. Each form was identified by a number and sent to the coordinating study center (Hospital Clínic, Barcelona). Queries and inconsistencies were communicated to hospital investigators for resolution before the data were included in the computerized data base. The study was approved by the Institutional Ethical Committee of the coordinating center and, subsequently, by each participating center.
The data form included information on clinical presentation, comorbidities, tumor location (with no specification on the degree of eloquency10), type of surgery (gross total, partial resection, and biopsy), postoperative complications, Karnofsky performance status (KPS) at the time of discharge from the neurosurgery ward (recorded in only 53% of the medical records), starting and ending dates for RT, total and fraction doses, type of RT and chemotherapy, use of antiepileptic drugs for seizure prophylaxis, date of progression, salvage treatment, and survival. Tumor location was based on the MRI reports, and central review was not performed. Postoperative complications were defined as major if, according to the local investigator, the event prevented or significantly delayed the starting of subsequent treatment.
Statistical Analysis
Survival and time-dependent variables were estimated using the Kaplan-Meier method, and log-rank test was used for comparisons. Overall survival (OS) and progression-free survival (PFS) were defined as the time from surgery to death or censored at the date of last follow-up and to radiological or clinical criteria of progression, respectively. The Cox regression model (backward stepwise method) was used to determine independent predictors of OS. Relevant clinical factors entered into the multivariate model were sex, age, KPS, tumor location, type of surgery, and complementary treatments. A multivariate logistic binary regression analysis was used to identify predictors of type of surgery, surgical complications, and mortality. The variables introduced using the backward stepwise method were hospital, age, sex, neurological symptoms, lesion location, time from onset of symptoms to surgery, and type of surgical resection. To analyze the impact of RT delay on OS, we used a cutoff of 42 days, because it was the median waiting time and previous studies showed that RT delay within the period of 42 days does not influence OS.11
The descriptive information analysis presented categorical variables as observed counts and weighted percentages and continuous variables as mean or median with the corresponding standard error or range, depending on the nature of variable. A χ2, Student's t, or Mann-Whitney U test was used to identify differences between groups. All calculations were performed using SPSS software, version 18.0 (SPSS), and P <.05 was considered to be statistically significant.
Results
Patient Characteristics and Surgical Management
The clinical and management characteristics of the 834 patients included in the study are shown in Table 1. The median age was 62 years (range, 20–85 years), and 23% of the patients were >70 years of age. Type of surgery included gross total resection in 37.7% of patients, partial resection in 28.4%, and biopsy in 33.9%. The extent of resection was assessed using early postoperative MRI (median, 3 days after surgery; range, 1–7 days) in 41% of patients who underwent surgical resection. Postoperative complications were reported in 224 (26.8%) patients, and 115 (13.7%) were considered to be major. Major complications were regional in 95 (82.6%) patients and included hematomas (51 patients), local infections (14), immediate postoperative acute neurological deterioration of unclear cause (9), cerebral infarction (7), uncontrollable cerebral edema (7), CSF leaks (5), and seizures with neurological deterioration (2). Seventeen (14.8%) patients had major systemic complications, and the cause of the complication was unknown in 3 (2.6%). We did not find a relationship between the number of major complications and the total number of patients with GBM who underwent surgery at each center (annual median number of patients undergoing surgery per center: 18; range, 6–42). In the multivariate analysis, older age was the only independent predictor of major postoperative complications (odds ratio [OR], 1.03; 95% confidence interval [CI],1.01–1.05; P = .006).
Table 1.
Clinical characteristics of the whole series and by type of surgery
| Characteristics | Whole series n (%) 834(100) | Surgerya n (%) 549 (66.1) | Biopsy n (%) 282 (33.9) | P |
|---|---|---|---|---|
| Age* | 62 (20–85) | 61 (20–85) | 64 (26–85) | <.001 |
| >70 years | 192 (23.0) | 104 (19.0) | 88 (31.2) | <.001 |
| Sex | ||||
| Male | 511 (61.3) | 337 (61.4) | 172 (61.0) | .94 |
| Female | 323 (38.7) | 212 (38.6) | 110 (39.0) | |
| Symptoms | ||||
| Focal | 571 (68.5) | 372 (67.9) | 196 (69.5) | .69 |
| Cognitive | 313 (37.5) | 185 (34.1) | 127 (45.5) | <.001 |
| Epilepsy | 205 (24.6) | 137 (25.0) | 66 (23.4) | .67 |
| MRI location | ||||
| Single lesion | 700 (83.9) | 505 (92.0) | 192 (68.1) | <.001 |
| Lobarb | 555 (79.3) | 451 (89.3) | 101 (52.6) | <.001 |
| Right side | 351 (50.1) | 283 (56.0) | 67 (34.9) | <.001 |
| Delay 1st imaging/surgery* | 16 (0–177) | 16 (0–177) | 18 (0–152) | .021 |
| <31 days | 669 (82.1) | 454 (84.7) | 212 (76.8) | |
| 31–60 days | 109 (13.4) | 61 (10.8) | 51 (18.5) | |
| >60 days | 37 (4.5) | 21 (4.5) | 13 (4.7) | |
| Major surgical complications | 115 (13.7) | 80 (14.6) | 33 (11.7) | .31 |
| 30-days mortality | 66 (7.9) | 24 (4.4) | 40 (14.2) | <.001 |
| Epilepsy prophylaxis | 286 (48.4) | 217 (56.1) | 68 (33.5) | <.001 |
| Postoperative KPS | 70 (10–100) | 80 (10–100) | 60 (10–100) | <.001 |
| ≥70 | 284 (63.5) | 217 (73.6) | 67 (44.4) | |
| 60 | 82 (18.3) | 42 (14.2) | 40 (26.5) | |
| <60 | 81 (18.3) | 36 (12.2) | 44 (29.1) | |
| Treatment | ||||
| Yes | 648 (77.7) | 483 (88.0) | 164 (58.2) | <.001 |
| No | 186 (22.3) | 66 (12.0) | 118 (41.8) | |
In 3 patients, the type of surgical resection was not registered.
aGross total resection in 263 patients as assessed by postoperative MRI.
bTumor confined to 1 or 2 lobules.
*Values expressed as median and range.
The overall 30-day postoperative mortality rate was 7.9%. Cause of death was related to postoperative complications in 38 patients, and neurological deterioration was probably attributable to tumor progression in 28. The multivariate analysis disclosed that biopsy was the only independent predictor of surgical mortality (OR, 4.76; 95% CI, 2.31–9.80, P < .001).
Complementary Oncological Treatment and Outcome
The oncological treatment is shown in Table 2. A total of 186 patients (22.3%) were not treated after surgery. The main reasons were low postoperative KPS, because of surgical complications (40.9%), tumor-related symptoms (31.7%), or probable tumor progression (16.2%) and, less commonly, patient decision (5.4%). The reason was unknown for the rest (5.8%). Among the 648 treated patients, the standard protocol of RT with concomitant and adjuvant TMZ was initiated in 478 (73.8%), including 43 patients who also received BCNU wafers at the time of surgery (Table 2). The majority of patients (94%) finished the RT and concomitant TMZ treatment, and 79% of them received at least 1 cycle of adjuvant TMZ. Despite being the most prescribed treatment, only 57% of patients could receive the standard protocol in the whole series.
Table 2.
First-line treatment options and outcome expressed in months for the 834 patients
| Treatment | N | PFSa | Median OSa | % 2y survival |
|---|---|---|---|---|
| RT + TMZ regimens | 509 | 8.8 (8.1–9.5) | 16.1 (14.9–17.4) | 25.30% |
| Concomitant/adjuvant TMZ | 435 | 8.9 (8.0–9.7) | 16.4 (15.0–17.8) | 25.50% |
| BCNU wafers plus TMZb | 53 | 8.7 (7.1–10.4) | 18.8 (13.5–24.1) | 30.10% |
| Adjuvant TMZ alone | 21 | 6.6 (4.4–8.9) | 10.7 (5.7–15.7) | 8.00% |
| RT alonec | 70 | 3.4 (2.6–4.3) | 7.2 (5.6–8.9) | 2.30% |
| RT plus BCNU wafers | 23 | 6.0 (4.0–8.1) | 8.7 (4.7–12.8) | 12.90% |
| Clinical Trials | 46 | 6.5 (5.2–7.7) | 16.1 (9.6–22.6) | 26.00% |
| No treatment | 186 | – | 2.0 (1.7–2.4) | 0.00% |
aExpressed as median, in months, (95% C.I.).
b43 patients received concomitant/adjuvant TMZ and 10 only adjuvant TMZ.
c47.1% of patients received hypofractioned schedules or did not complete the total 60 Gy standard dose.
At the end of the study, 511 (78.8%) treated patients had tumor progression or relapse that was identified as only radiological (26.2%), clinically confirmed by imaging (62.7%), and only clinical (11.1%). Bevacizumab alone or with irinotecan was given to 159 patients (31.2%) and intensive TMZ regimens to 92 (18.0%). Fourteen (2.7%) patients had surgery alone or radiosurgery, 14 (2.7%) were included in clinical trials, and 232 (45.4%) did not receive any further treatment.
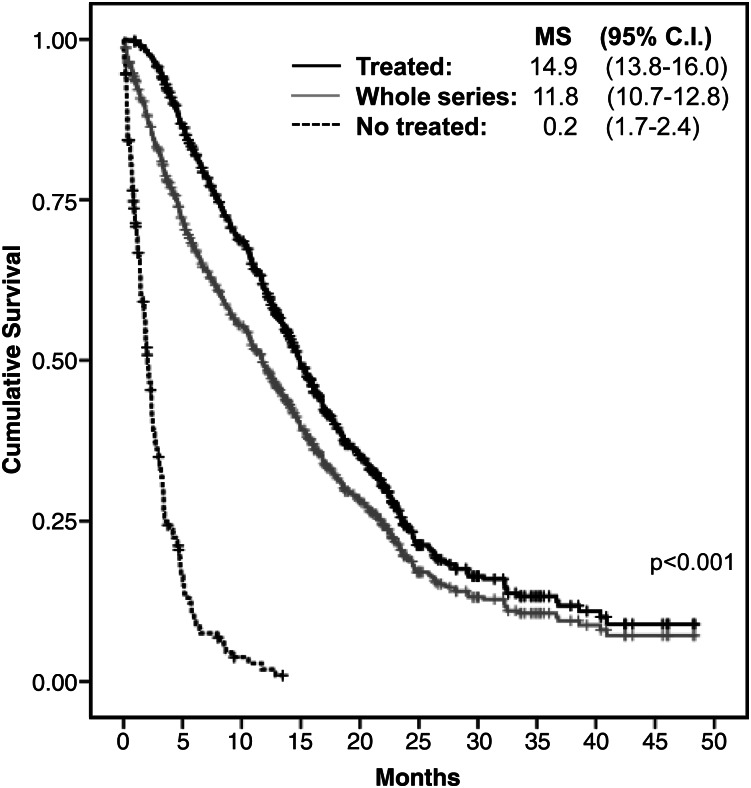
On January 31, 2012, 129 patients (15.5%) were alive, and 82 (9.8%) were lost to follow-up. The median OS for the whole series was 11.8 months (95% CI, 10.7–12.8 months) (Fig. 1). Analysis of outcome with regard to treatment modalities is shown in Table 2. Excluding patients who died of surgical complications, the Cox regression analysis revealed that a postoperative KPS ≥70 (hazard ratio [HR], 0.77; 95% CI, 0.63–0.93; P = .007); surgical resection instead of biopsy (HR, 0.50; 95% CI, 0.41–0.60; P < .001); and treatment modalities, including RT alone (HR, 0.30; 95% CI, 0.22–0.42; P < .001) and RT plus chemotherapy (HR, 0.14; 95% CI, 0.11–0.19; P < .001), instead of palliative support were independently associated with longer OS. Younger age showed a marked tendency for better OS (HR, 0.99; 95% CI, 0.98–1.00; P = .055).
Fig. 1.
Kaplan-Meier estimates of survival in the whole series and patients who received or not postoperative oncological treatment. MS, median survival.
Impact of RT Delay on Outcome
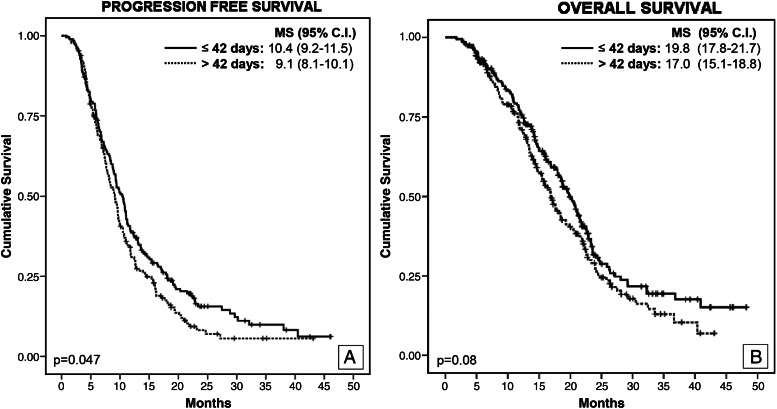
The median time to start RT after surgery for the whole series was 42 days (range, 9–161 days). To explore the potential impact on survival, we first analyzed the relationship between the RT delay and well-recognized prognostic survival predictors to avoid multicollinearity in the multivariate analysis. This showed that patients with biopsy had a shorter waiting time, compared with those who underwent surgery (median, 35 vs 42 days; P = .001). For this reason, we decided to analyze the impact of RT delay in the group of patients with surgical resection. However, in this group, the delay to start RT was shorter among patients treated with chemotherapy and RT than among those treated only with RT (median, 42 vs 46 days; P = .023). To prevent this potential bias, we restricted the multivariate analysis to the group of patients who underwent surgery and received RT and chemotherapy (n = 396). The comparison between pretreatment characteristics and first- and second-line treatments in this group of patients subdivided according to the RT delay did not show differences (Table 3). The multivariate analysis demonstrated that initiation of RT ≤42 days was independently associated with longer PFS (HR, 0.8; 95% CI, 0.64–0.99; P = .042) but not with OS (HR, 0.79; 95% CI, 0.62–1.00; P = .055) (Fig. 2).
Table 3.
Clinical characteristics of 396 patients with GBM with surgical resection classified according the RT delay
| Characteristics | ≤42 days, n (%) 206 (52.0) | >42 days, n (%) 190 (48.0) | P |
|---|---|---|---|
| Age (years)a | 59 (25–78) | 60 (20–80) | .92 |
| Sex | |||
| Male | 133 (64.6) | 120 (63.2) | .92 |
| Female | 73 (35.4) | 70 (36.8) | |
| Symptoms | |||
| Focal | 143 (69.1) | 116 (60.7) | .09 |
| Cognitive | 63 (30.6) | 63 (33.5) | .59 |
| Epilepsy | 48 (23.2) | 56 (29.3) | .17 |
| MRI location | |||
| Single lesion | 190 (92.2) | 174 (91.6) | .81 |
| Lobar | 166 (87.3) | 160 (91.9) | .19 |
| Surgery | |||
| Gross total resection | 122 (59.2) | 112 (59.0) | 1.00 |
| Partial resection | 84 (40.8) | 78 (41.0) | |
| Postoperative KPSa | 80 (100–50) | 80 (100–50) | .7 |
| 1st line chemotherapies | |||
| C-A TMZb | 180 (87.4) | 159 (83.7) | .23 |
| C-A TMZ + BCNU wafers | 18 (8.7) | 16 (8.4) | |
| BCNU wafersc | 8 (3.9) | 15 (7.9) | |
| 2nd line treatments | n = 160 | n = 155 | |
| Bevacizumab-based | 64 (40.0) | 62 (40.0) | .95 |
| Nitrosurea-based | 37 (23.1) | 35 (22.6) | |
| Radiosurgery | 7 (4.4) | 5 (3.2) | |
| Clinical Trials | 5 (3.1) | 6 (3.9) | |
| Palliative | 47 (29.4) | 47 (30.3) | |
aData expressed as median (range).
b22 patients also received bevacizumab or were included in clinical trials that tested bevacizumab or cilengitide.
c9 patients also received adjuvant TMZ. C-A TMZ: Concomitant and adjuvant temozolomide.
Fig. 2.
Progression-free survival (A) and overall survival (B), according to delay to initiation of RT. MS, median survival.
Older Patients
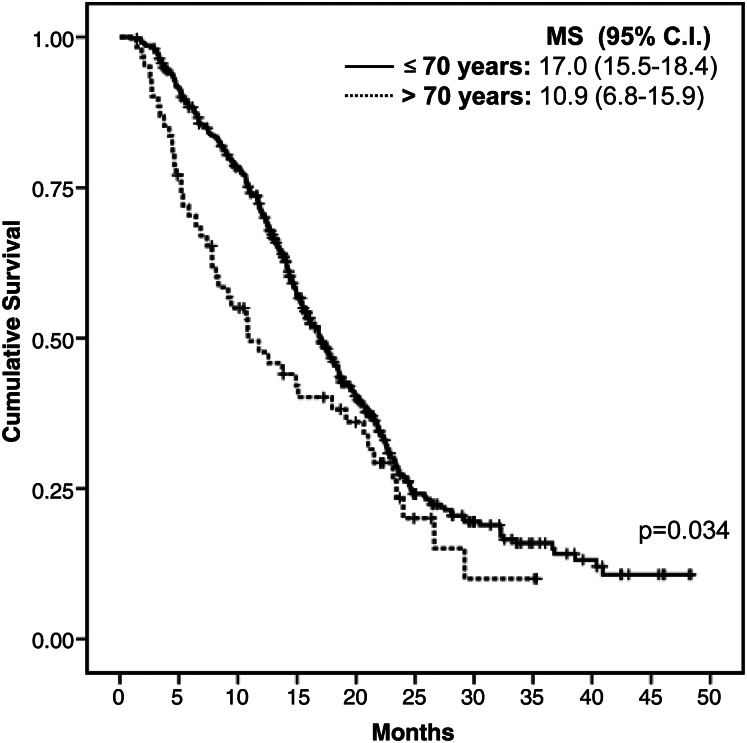
The main clinical, treatment, and outcome variables in patients with GBM who were >70 years of age are shown in Table 4. Compared with younger patients, older patients were more likely to present with cognitive impairment (47.9% vs 35.0%), only have a biopsy (45.8% vs 30.4%), have more major surgical complications (18.8% vs 12.6%), have a lower median postoperative KPS (60 vs 80), and to receive only palliative treatment (41.1% vs 16.7%; P < .001) or hypofractionated RT instead of standard RT (23.8% vs 5.4%; P < .001) (Table 4). Median OS was 5.2 months (95% CI, 4.3–6.1 months), and the survival probability at 2 years was 10.1%. The main prognostic factors of OS identified in the multivariate analysis were gross total resection (HR, 0.49; 95% CI, 0.32–0.75; P = .002) and treatment with RT and TMZ (HR, 0.22; 95% CI, 0.14–0.33; P < .001) or RT alone (HR, 0.37; 95% CI, 0.23–0.59; P < .001). Only 61 (31.8%) older patients were treated with RT and concomitant adjuvant TMZ, with a median OS of 10.9 months (95% CI, 6.8–14.9 months). In contrast, the group of younger patients who received the same treatment had an OS of 17.0 months (95% CI, 15.5–18.4 months; P = .034) (Fig. 3).
Table 4.
Clinical, management and outcome characteristics of patients with GBM older and younger than 70 years
| Characteristics | ≤70 years, n (%) 642 (77.0) | >70 years, n (%) 192 (23.0) | P |
|---|---|---|---|
| Age (years)* | 59 (20–70) | 75 (71–85) | <.001 |
| Sex | |||
| Male | 407 63.4) | 104 (54.2) | .023 |
| Female | 235 (36.6) | 88 (45.8) | |
| Symptoms | |||
| Focal | 433 (67.6) | 138 (71.9) | .290 |
| Cognitive | 222 (35.0) | 91 (47.9) | .002 |
| Epilepsy | 161 (25.1) | 44 (22.9) | .570 |
| MRI location | |||
| Single lesion | 534 (83.2) | 166 (86.5) | .430 |
| Lobara | 417 (78.1) | 138 (83.1) | .089 |
| Right side | 258 (48.3) | 93 (56.0) | .051 |
| Delay 1stimage-surgery (days)* | 15 (0–177) | 20 (0–152) | .002 |
| Surgery | |||
| Gross total resection | 257 (40.2) | 56 (29.2) | <.001 |
| Partial resection | 188 (29.4) | 48 (25) | |
| Biopsy | 194 (30.4) | 88 (45.8) | |
| Post-surgical complicationsb | 78 (12.1) | 37 (19.3) | .017 |
| 30- days mortality | 44 (6.9) | 22 (11.5) | .047 |
| Postoperative KPS* | 80 (100–10) | 60 (100–10) | <.001 |
| ≥70 | 239 (69.2) | 45 (44.2) | |
| 60 | 51 (14.8) | 31 (30.4) | |
| <60 | 55 (15.9) | 26 (25.5) | |
| 1st line treatments | |||
| RT+ C plus A TMZ** | 417 (65.0) | 61 (31.8) | <.001 |
| RT+ A TMZ*** | 26 (4.0) | 5 (2.6) | |
| RT+ BCNU wafers | 16 (2.5) | 7 (3.6) | |
| RT alone | 34 (5.3) | 36 (18.8) | |
| Clinical Trials | 42 (6.5) | 4 (2.1) | |
| Palliative | 107 (16.7) | 79 (41.1) | |
| No salvage treatment | 175 (40.1) | 59 (77.7) | <.001 |
| OS in months (95%CI) | 13.7 (12.6–14.8) | 5.2 (4.3–6.1) | <.001 |
| 1 year SR | 55.90% | 24.50% | |
| 2 years SR | 21.50% | 10.10% | |
Abbreviations: C, concomitant; A, adjuvant; TMZ, temozolomide; OS, overall survival; SR, survival rate.
aIsolate lobar lesion without corpus callosum and basal ganglia involvement.
bOnly included major complication that significantly delayed or prevented oncological treatment.
*value expressed as median (range).
**43 patients also received BCNU wafers.
***10 patients also received BCNU wafers.
Fig. 3.
Survival among patients stratified by age and treated with the standard protocol of RT with concomitant and adjuvant TMZ. Abbreviation: MS, median survival.
Discussion
Community-based studies are important to ascertain the degree of implementation of new standards of care defined by pivotal clinical trials and to identify relevant variables that prevent or have a negative impact in outcome. Our study showed that the standard protocol with RT and concurrent and adjuvant TMZ was initiated in 79% of the 602 patients treated outside clinical trials. This data indicate a good diffusion of the standard protocol in Spain. However, we found that 22% of patients did not receive any oncological treatment after surgery, a similar percentage reported in previous studies.5,6,8,12,13 In addition, we also investigated important issues usually not described in similar community-based series, such as the degree of implementation of postoperative MRI, to define the extent of tumor resection, frequency of major postoperative complications, reasons for withholding treatment after surgery, and specific treatment of patients with GBM who were >70 years of age.
Similar to previous series, 66% of our patients were treated with surgical resection,5,12 38% of which were considered to be gross total. However, only 41% of patients had a postoperative MRI to confirm the extent of resection. Similar community-based studies describe a frequency of gross total resection of 28%–80.5%, but the percentage of postoperative imaging is not registered or the extent of resection was not known in a substantial number of patients.5,7,12–15 Correct evaluation by postoperative MRI is important, because gross total resection has a prognostic impact on survival16 and provides practical clinical information, such as the identification of perioperative infarcts, which may present as new contrast-enhancing lesions in follow-up MRI's and may be mistaken with tumor relapse.17
We observed major postoperative complications in almost 14% patients, and age was the only predictor in our study. The predicted risk of major complications after craniotomies for gliomas, not only GBM, was 5%–26%, and it depended on age, KPS, and functional location of the tumor.10 Postoperative mortality and frequency of perioperative complications are not reported in most population-based studies.6,7,12,13,15,18,19 The Glioma Outcome Project described the perioperative complications of initial craniotomies in 408 patients with anaplastic gliomas and GBM from 52 hospitals. The study found that 8% patients had a worse postoperative neurological status, but the authors did not indicate whether these patients were less likely to receive RT and chemotherapy.20 In a single institution study of 144 patients with GBM, the rate of major complications after surgery was 9%, and these patients were less likely to receive postoperative treatment.21 We did not observe a correlation between the hospital caseload and frequency of complications, although the number of patients with GBM who underwent surgery at each hospital was uniformly low. Previous studies suggested a lower frequency of postoperative complications in hospitals with a higher number of craniotomies, but the minimal volume of surgeries for acceptable frequency of complications has not been established.22–24
The optimal time from surgery to RT is a matter of debate in GBM.25 On the basis of data from 16 randomized studies, the Radiation Therapy Oncology Group concluded that there was no decrement in survival with increasing time to initiation of RT, provided that RT was started within the first 42 days after surgery.11 However, in the series of 952 patients with GBM diagnosed in 2004 in 43 French hospitals, the median time from surgical resection to RT was 44 days and, in the present series, was 42 days.12 These data emphasize that the number of patients with GBM who start RT beyond 6 weeks is not small, at least in some countries, and the need to study the impact on survival when RT is delayed beyond this time point. In the French study, the delay beyond 41 days did not affect OS in those patients treated with the standard protocol of RT and TMZ.26 However, the study included patients who underwent biopsy and who had longer waiting times than patients who underwent surgery. In our series, we analyzed the impact of RT delay in the subgroup of patients who had surgical resection and were treated with RT and chemotherapy to avoid multicollinearity with other predictors of survival. We observed a longer PFS and a trend toward a better OS among patients who started RT within 42 days. Although our findings on PFS are limited by the retrospective nature of the study, GBM progression was radiologically confirmed in 94% of the patients. In addition, we observed an almost significant impact on OS, suggesting that the advantage in survival obtained with optimal surgery may be reduced if RT is delayed beyond 42 days.27
The best treatment for older patients with GBM is unsettled.9,28 Older patients treated with RT have a median survival advantage of 3 months, compared with those who receive supportive care alone.29 Two recent multicentric studies randomized patients to receive TMZ or RT. Both studies showed that treatment with TMZ was not inferior to RT, with a median OS of around 8 months. MGMT promoter methylation was associated with a better outcome in patients treated with TMZ but not in those who received RT.30,31 Taken together, the data from both studies indicate that treatment with TMZ alone should be considered as an option in older patients, particularly when the genetic analysis of the tumor is feasible and shows methylation of the MGMT promoter.32 The role of the treatment with RT and concomitant TMZ in patients >70 years of age will be clarified in ongoing randomized clinical trials. In retrospective single institution studies, patients treated with this regimen have median OS of 10.6–13.7 months.33–37 In a population-based study, median OS was 13.4 months.8 However, only 8% of older patients received chemoradiation, suggesting that the improved outcome was attributable to selection bias.8
In summary, our results show that the postsurgical treatment regimen with RT and TMZ for newly diagnosed GBM has been widely accepted in the community setting, with similar outcomes to that reported in the pivotal trial.4 However, only 57% of the patients could receive this treatment. In the group of patients treated with surgical resection, RT, and chemotherapy, a delay of ≥42 days to initiate RT was associated with a shorter PFS. Lastly, a significant proportion of patients, particularly those >70 years of age, do not receive any treatment after surgery, in part because of major perioperative complications.
Funding
This work was supported in part by funds from the Neuro-Oncology Section of the Spanish Society of Neurology.
Acknowledgments
We thank all members of the brain tumor teams at the participating hospitals that provided the clinical information of their patients; our research nurse Ms. Verónica Mato, for her excellent work in data management; and Dr. Myrna Rosenfeld, for critical review of the manuscript.
Conflict of interest statement. None declared.
References
- 1.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro-Oncol. 2012;14:351–359. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darefsky AS, King JT, Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118:2163–2172. doi: 10.1002/cncr.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107:207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rønning PA, Helseth E, Meling TR, Johannesen TB. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro-Oncol. 2012;14:1178–1184. doi: 10.1093/neuonc/nos153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2012 doi: 10.1016/j.ctrv.2012.05.008. http//dx.doi.org/10.1016/xtrv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal DT, Won M, Mehta MP, et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27:733–739. doi: 10.1200/JCO.2008.18.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauchet L, Mathieu-Daudé H, Fabbro-Peray P, et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro-Oncol. 2010;12:725–735. doi: 10.1093/neuonc/noq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557–564. doi: 10.1001/jama.293.5.557. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal MA, Drummond KJ, Dally M, et al. Management of glioma in Victoria (1998–2000): retrospective cohort study. MJA. 2006;184:270–273. doi: 10.5694/j.1326-5377.2006.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 15.Salmaggi A, Silvani A, Merli R, et al. Multicentre prospective collection of newly diagnosed glioblastoma patients: update on the Lombardia experience. Neurol Sci. 2008;29:77–83. doi: 10.1007/s10072-008-0865-x. [DOI] [PubMed] [Google Scholar]
- 16.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 17.Ulmer S, Braga TA, Barker FG, et al. Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology. 2006;67:1668–1670. doi: 10.1212/01.wnl.0000242894.21705.3c. [DOI] [PubMed] [Google Scholar]
- 18.Arrigo RT, Boakye M, Skirboll SL. Patterns of care and survival of glioblastoma patients in the Veterans population. J Neurooncol. 2012;106:627–635. doi: 10.1007/s11060-011-0702-6. [DOI] [PubMed] [Google Scholar]
- 19.Filippini G, Falcone Ch, Boiardi A, et al. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro-Oncol. 2008;10:79–87. doi: 10.1215/15228517-2007-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang S, Parney IF, MCDermott M, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 21.Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: Surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76:572–579. doi: 10.1016/j.wneu.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Braker FG, II, Curry WT, Jr, Carter BS. Surgery for primary supratentorial brain tumors in the United States, 1988 to 2000: The effect of provider caseload and centralization of care. Neuro-Oncol. 2005;6:49–63. doi: 10.1215/S1152851704000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowan JA, Jr, Dimick JB, Leveque JCH, et al. The impact of provider volume on mortality and intracranial tumor resection. Neurosurgery. 2003;52:48–54. doi: 10.1097/00006123-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Long DM, Gordon T, Bowman H, et al. Outcome and cost of craniotomy performed to treat tumors in regional academic referral centers. Neurosurgery. 2003;52:1056–1065. [PubMed] [Google Scholar]
- 25.Lawrence YR, Blumenthal DT, Matceyevsky D, et al. Delayed initiation of radiotherapy for glioblastoma: how important is it to push to the front (or the back) of the line? J Neurooncol. 2011;105:1–7. doi: 10.1007/s11060-011-0589-2. [DOI] [PubMed] [Google Scholar]
- 26.Noel G, Huchet A, Feuvret L, et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. 2012;109:167–175. doi: 10.1007/s11060-012-0883-7. [DOI] [PubMed] [Google Scholar]
- 27.Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007;85:339–343. doi: 10.1007/s11060-007-9426-z. [DOI] [PubMed] [Google Scholar]
- 28.Chargari C, Feuvret L, Bauduceau O, et al. Treatment of elderly patients with glioblastoma: From clinical evidence to molecular highlights. Cancer Treat Rev. 2012;38:988–995. doi: 10.1016/j.ctrv.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 30.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 31.Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 32.Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131:1342–1350. doi: 10.1002/ijc.27385. [DOI] [PubMed] [Google Scholar]
- 33.Floyd SR, Kasper EM, Uhlmann EJ, et al. Hypofractionated radiotherapy and stereotactic boost with concurrent and adjuvant temozolamide for glioblastoma in good performance status elderly patients - Early results of a phase II trial. Front Oncol. 2012;2:122. doi: 10.3389/fonc.2012.00122. doi:10.3389/fonc.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker CA, Chang M, Chou JF, et al. Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J Neurooncol. 2012;109:391–397. doi: 10.1007/s11060-012-0906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 36.Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 37.Combs SE, Wagner J, Bischof M, et al. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys. 2008;70:987–992. doi: 10.1016/j.ijrobp.2007.07.2368. [DOI] [PubMed] [Google Scholar]