Abstract
Background
We conducted a nonrandomized international study for intracranial germinoma that compared chemotherapy followed by local radiotherapy with reduced-dose craniospinal irradiation (CSI) alone, to determine whether the combined treatment regimen produced equivalent outcome and avoided irradiation beyond the primary tumor site(s).
Methods
Patients with localized germinoma received either CSI or 2 courses of carboplatin and etoposide alternating with etoposide and ifosfamide, followed by local radiotherapy. Metastatic patients received CSI with focal boosts to primary tumor and metastatic sites, with the option to be preceded with chemotherapy.
Results
Patients with localized germinoma (n = 190) received either CSI alone (n = 125) or combined therapy (n = 65), demonstrating no differences in 5-year event-free or overall survival, but a difference in progression-free survival (0.97 ± 0.02 vs 0.88 ± 0.04; P = .04). Seven of 65 patients receiving combined treatment experienced relapse (6 with ventricular recurrence outside the primary radiotherapy field), and only 4 of 125 patients treated with CSI alone experienced relapse (all at the primary tumor site). Metastatic patients (n = 45) had 0.98 ± 0.023 event-free and overall survival.
Conclusions
Localized germinoma can be treated with reduced dose CSI alone or with chemotherapy and reduced-field radiotherapy. The pattern of relapse suggests inclusion of ventricles in the radiation field. Reduced-dose craniospinal radiation alone is effective in metastatic disease.
Keywords: chemotherapy, intracranial germinoma, progression-free survival, reduced radiotherapy dose, treatment-related symptoms
Germinomas contribute to about 60% of all intracranial germ cell tumors located in the pineal gland, suprasellar region, basal ganglia, and hypothalamus.1 Intracranial germinomas are highly radiosensitive, with a tendency to spread via cerebrospinal fluid (CSF), and systemic craniospinal radiation therapy has been the standard treatment for many decades.2,3 With this treatment approach, the majority of patients have been cured.4 Concerns have long been raised about the potential adverse effects of radiotherapy.4–6 Therefore, other treatment approaches were introduced to evaluate craniospinal irradiation alone, but with reduced doses, compared with previous practice, or chemotherapy either in combination with radiotherapy or alone.7,8 In the German MAKEI 89 trial, 30 Gy were applied to the craniospinal axis (CSI) with an additional tumor boost of 15 Gy.9 With this regimen, 88% of the patients remained relapse-free at 5 years. In 1990, the French Society of Paediatric Oncology initiated a trial using chemotherapy and local field radiotherapy in localized germinomas with favorable results.10 In 1998, Matsutani et al11,12 reported excellent survival for germinomas treated with surgery, followed by extended field or whole brain radiotherapy. Patients who received chemotherapy before reduced radiotherapy (30 Gy) were all alive at a median follow-up of 4.3 years. Aoyama et al13 presented promising excellent results in a second Japanese series including 16 germinomas treated with surgery, followed by chemotherapy and low-dose involved-field radiotherapy. Approaches using chemotherapy alone have not been promising.10
Because of the rarity of the disease, an international prospective study was established in 1996 in Europe to evaluate outcomes in a larger patient cohort. In this trial, the outcomes in patients with histologically proven intracranial germinomas (with or without teratoma) treated either with a reduced dose of 24 Gy CSI and additional boost to the primary and metastatic sites of 16 Gy or with combined treatment were evaluated in a nonrandomized fashion.
Materials and Methods
Patients
A total of 235 patients (176 male, 59 female) with histologically confirmed diagnosis of a germinoma and complete examination were enrolled in SIOP CNS GCT 96 from January 1, 1996 through December 31, 2005, and followed up to July 18, 2012. The median age was 13 years (range, 4–42 years). Patients were registered from Austria, Belgium, Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Poland, Sweden, Switzerland, Spain, and the United Kingdom. Tumor symptoms, neurological status, endocrine function, and hearing and visual status were reported at diagnosis. According to usual national treatment practice, patients with localized germinoma treated in Germany, Austria, Switzerland, Norway, the Netherlands, and the United Kingdom preferably received craniospinal irradiation, whereas patients in France, Italy, Spain, Greece, Belgium, and Poland received chemotherapy and local field radiotherapy.
Diagnostic and Staging Procedures
Brain and total spine MRI, with and without gadolinium contrast, were mandatory for diagnosis and staging. In Germany, a central review of MRI has been established within the HIT network since 1998. About 50% of the German patients have been reviewed. Within the study period, a central review in the other European countries was not established. For cytological detection of microdissemination, sampling of cerebrospinal fluid (CSF) was required, obtained from lumbar puncture or from ventriculostomy in cases of raised intracranial pressure. A central review of CSF cytology was not required. Before proceeding to biopsy, the tumor markers alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) had to be evaluated in serum and CSF to exclude secreting tumor elements. By consensus between the participating national groups, AFP levels had to be ≤25 ng/mL and HCG levels ≤50 IU/L to treat for germinomas. Of the included patients, 4 showed a serum AFP ≤10 ng/mL, with no CSF-AFP >8 ng/mL in 2 of them and 2 with an AFP in CSF ≤10 ng/mL. 19 patients had a ß-HCG ≤38 IU/L in serum and 29 a ß-HCG ≤49 IU/L in CSF, including the 19 previously mentioned patients. For HCG, the values were ≤20 IU/L in serum in 6 and ≤48 IU/L in CSF in 10 patients, including the 6 previously mentioned cases.
In all other patients AFP, ß-HCG, and HCG values in serum and CSF were below the normal values (for AFP < 8 ng/mL and for ß-HCG/HCG <5 IU/L).
Local disease was defined as germinoma without evidence of dissemination (negative CSF cytology, negative imaging). Definition of bifocal disease required radiological detection of tumor in both the pineal and the suprasellar regions. Metastatic germinoma was defined as the presence of >1 intracranial focus (except bifocal disease), spinal metastases, metastases outside CNS, or tumor cells in CSF.
Of 235 total patients, 190 had localized tumors on imaging (94 were pineal, 53 supra-/intrasellar, 32 bifocal, and 11 at other sites). The remaining 45 patients had metastatic tumors (17 pineal, 13 supra- or intrasellar, 15 bifocal). Of these, 30 showed macroscopic metastases seen on MRI scan, 6 of whom also had positive CSF cytology, and 15 received a diagnosis on the basis of positive CSF cytology alone.
Pathological Analysis
The diagnosis of intracranial germinoma was histologically confirmed by the local pathologists in 235 patients. Central review was performed according to national standards. Tumors were classified according to the World Health Organization as pure germinoma (n = 224). In addition, 11 patients with components of mature (n = 9) or immature (n = 2) teratoma were included.
Treatment
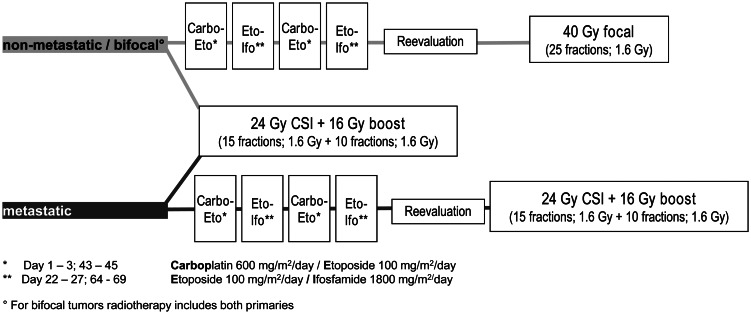
Twenty-three patients had a complete resection (22 of the 23 patients before chemotherapy or radiotherapy), 107 patients had a subtotal resection or open biopsy before chemotherapy or irradiation, and 103 patients had a stereotactic biopsy only. In 2 patients, no information concerning biopsy and resection was available. For localized germinoma, either reduced craniospinal radiotherapy alone or a combination treatment with 2 courses of carboplatin/etoposide alternating with etoposide/ifosfamide, followed by focal radiotherapy (40 Gy), were selected according to national preference (Fig. 1). The focal irradiation field was defined as the initial anatomically involved part of the brain and any postchemotherapy (or postsurgery) residue. Patients with bifocal disease in the combined treatment group received chemotherapy and focal radiotherapy to both primaries. Metastatic patients received 24 Gy craniospinal radiotherapy with 16 Gy boost to the primary site and metastases, with optional additional chemotherapy (Fig. 1).
Fig. 1.
Treatment regimen of intracranial germinoma (SIOP CNS GCT 96 protocol). Abbreviations: Carbo, carboplatin; CSI, craniospinal radiotherapy; Eto, etoposide; Ifo, ifosfamide.
Follow-Up
Evaluation was performed regularly after therapy and during follow-up, combined with a full clinical examination (including neurologic status, vision, hearing, and endocrine assessment), laboratory studies, measurements of markers, and imaging. MRI with contrast of brain and spine (if metastatic) were performed at least every 6 months for 2 years and annually thereafter. Median follow-up of all surviving patients was 6 years (range, 32 months to 14 years) from diagnosis to the last follow-up for all patients. Residual lesions at the end of treatment were defined as any persistent enhancing visible lesions at the site of the tumor, regardless of treatment modality. Time points for evaluation of response to treatment were after radiotherapy or chemotherapy. Long-term assessment via follow-up questionnaires included patient's condition, relapse, second malignancy, and persistent adverse effects of therapy. Patients were followed up until July 18, 2012.
Statistical Analysis
The probability of survival was estimated using the Kaplan–Meier method. Five-year overall survival was calculated from the date of diagnosis to the date of last follow-up or death. Progression-free survival measures the proportion of patients among those treated for GCT whose disease will remain stable (without signs of progression) at least 5 years after treatment. Event-free survival was calculated to measure the proportion of patients who remain free of an event (relapse or death of any cause). To compare the survival distributions of 2 samples, the log-rank test was used. For multivariate analysis, the variables age (<10 years, ≤16 years, and >16 years), sex, primary tumor site (pineal, supra-/intrasellar, bifocal, others), and metastatic status within 5 years were examined. Data were recorded and monitored at the database at the University Hospital of Muenster. SAS (version 9.2 for Windows; SAS Institute, Cary, NC) was used for statistical analysis.
Results
Relapses
During the first 60 months after diagnosis, 11 patients with localized germinoma experienced relapse at a median time of 10 months (Table 1). Of these, 4 had received primary treatment with craniospinal radiotherapy alone, and all experienced relapse at the original tumor site. In 2 of these recurrences, histopathological analysis was performed, demonstrating an immature teratoma (n = 1) and a yolk sac tumor (n = 1).
Table 1.
Chacteristics of 11 patients with relapses, by age, site, dissemination, tumor markers, radiological response, time to relapse, tumor markers at relapse, site of relapse, and pathology at relapse
| Variable | Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Female | Male | Male | Male | Male | Male | |
| primary diagnosis | age (years/months) | 11;2 | 10;6 | 15;9 | 4;10 | 7;3 | 15;7 | 9;0 | 16;4 | 12;10 | 14;11 | 10;4 |
| tumour site | Pineal | suprasellar | basal ganglia | bifocal | pineal | corpus callosum/epiphysis/hypothal | pineal | pineal | pineal | pineal + suprasellar | suprasellar | |
| metastases | no | no | no | no | no | no | no | no | no | no | no | |
| AFP serum | 3 ng/mL | 5 ng/mL | n.d. | 3 kU/L | 2,3 ng/mL | 1 ng/mL | 2,9 ng/mL | 3,3 ng/mL | 2 kU/L | 2 ng/mL | 1 ng/mL | |
| AFP CSF | 1 ng/mL | n.d | n.d. | <1 kU/L | n.d. | 1 ng/mL | <4 ng/mL | 1,3 ng/mL | <2 kU/L | n.d. | n.d. | |
| HCG serum | 4 U/L | 38 U/L | n.d. | 6 IU/L | 29,3 mIU/mL | 2 U/L | 2 U/L | <2 U/L | <2 kU/L | 2 U/L | 2 U/L | |
| HCG CSF | 1 U/L | n.d. | n.d. | 8 IU/L | n.d. | 5 U/L | 10 U/L | 3,7 U/L | 2 kU/L | n.d. | n.d. | |
| therapy group | chemo + focal irradiation | chemo + focal irradiation | chemo + focal irradiation | chemo + focal irradiation | chemo + focal irradiation | chemo + focal irradiation | chemo + focal irradiation | CSI | CSI | CSI | CSI | |
| radiological response | CR | CR | PR | PR | CR | CR | CR | CR | no information available | CR | CR | |
| At relapse | Time to relapse from original diagnosis | 10 months | 19 months | 9 months | 34 months | 13 months | 21 months | 9 months | 8 months | 4 months | 10 months | 8 months |
| AFP serum | 2,5 U/mL | normal | 2 kU/L | 2 kU/ | 0,99 ng/mL | 2 kU/l | <4 ng/mL | 1272 ng/mL | n.d. | 1483 kU/L | 2 ng/mL | |
| AFP CSF | n.d. | n.d. | 1 kU/L | 0.2 kU/L | 0,38 ng/mL | n.d. | n.d. | 109 ng/mL | n.d. | n.d. | n.d. | |
| HCG serum | 7,7 mU/mL | 123 U/L | <2 U/L | 22 IU/L | 14,8 U/L | <2 kU/L | <1 U/L | negativ | n.d. | not done | <1 U/L | |
| HCG CSF | n.d. | 734 U/L | HCG 3 U/L | 27 IU/L | 231 U/L | n.d. | n.d. | negativ | n.d. | n.d. | n.d. | |
| pathology | nekrotic tissue | not done | teratoma (after chemo) | no information available | not done | not done | not done | YST | TD immature | necrotic and reactive tissue (after chemo) | not done | |
| Site of relapse | right frontal horn | leptomeningeal at bottom of 4th ventricle | Local ventricles | no information available | lateral ventricles, CSF- cytology positive | frontal horn/corpus callosum | spinal C1–5 3. Ventricle | pineal | right parietal lobe incl. Pineal area | pineal | local |
Seven cases experienced relapse after combined treatment with primary tumors located in the pineal (n = 3), bifocal (n = 1), suprasellar (n = 1), and basal ganglia (n = 1) and one as a large midline tumor (patient 6; see Table 1). Six of these patients demonstrated ventricular relapse either alone or combined with tumor recurrence at the primary site. One patient had a spinal relapse with a cervical tumor mass at C1–C5 and a ventricular relapse. There was no reported case of relapse among metastatic patients.
Progression-Free and Event-Free Survival at 5 Years after Diagnosis
In the group of patients with localized tumor, disease was the cause of death in 2 patients receiving radiotherapy only and 3 receiving combined treatment.
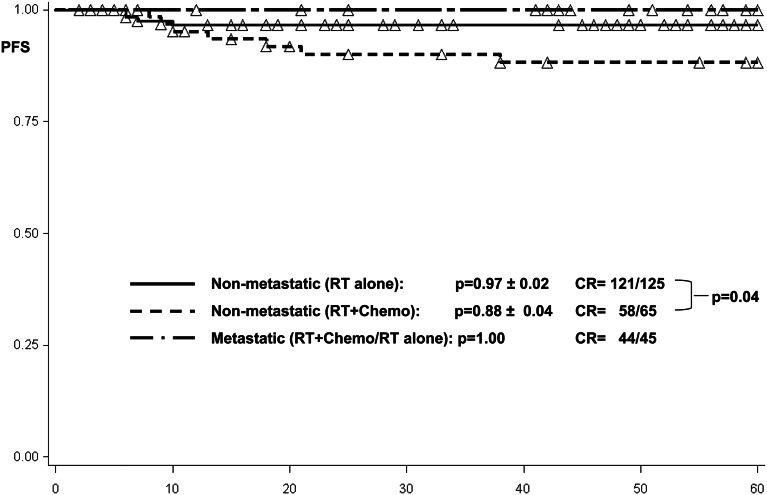
Patients with localized tumors treated with craniospinal radiotherapy alone (n = 125) demonstrated a 5-year progression-free survival of 0.97 ± 0.02, compared with 0.88 ± 0.04, among those treated with chemotherapy and focal radiotherapy (n = 65; P = .04 by log-rank test) (Fig. 2).
Fig. 2.
Progression-free survival among 65 patients with local disease and combined treatment, 125 patients with local disease and craniospinal radiotherapy, and 45 metastatic patients treated with craniospinal radiotherapy. Abbreviations: Chemo, chemotherapy; CR, complete remission; PFS, progression-free survival; RT, radiotherapy.
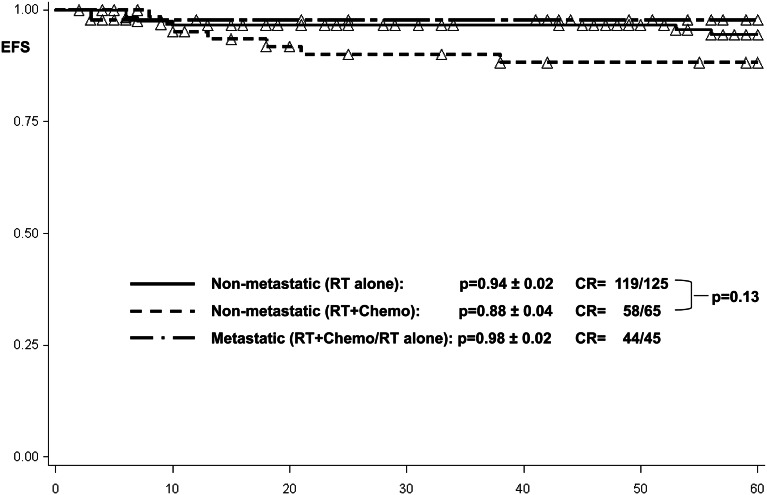
Two patients with localized disease after radiotherapy alone died of other causes (sickle-cell crisis and multiorgan failure). The event-free survival among patients with localized germinoma and craniospinal radiotherapy was estimated to be 0.94 ± 0.02 and, with combined treatment, was 0.88 ± 0.04 (P = .13 by log-rank test) (Fig. 3).
Fig. 3.
Event-free survival among 65 patients with local disease and combined treatment, 125 patients with local disease and craniospinal radiotherapy, and 45 metastatic patients treated with craniospinal radiotherapy. Abbreviations: Chemo, chemotherapy; CR, complete remission; EFS, event-free survival; RT, radiotherapy.
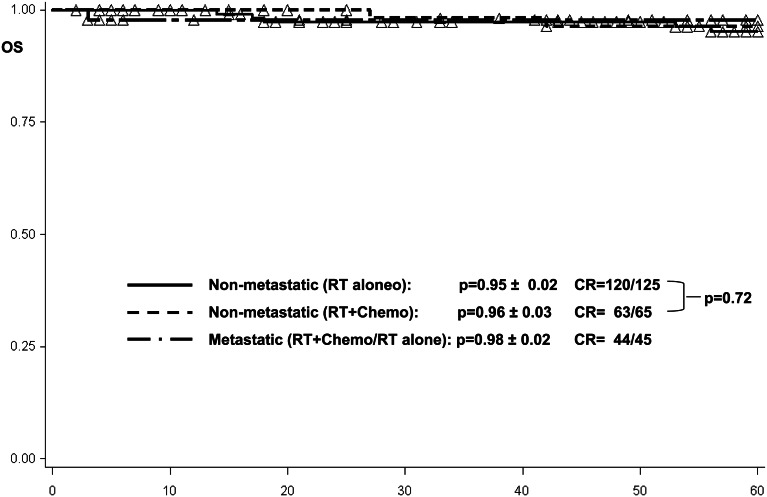
The overall survival among patients with localized germinoma and craniospinal radiotherapy was estimated to be 0.95 ± 0.02 and, with combined treatment, was 0.96 ± 0.03 (P = .72 by log-rank test) (Fig. 4).
Fig. 4.
Overall survival among 65 patients with local disease and combined treatment, 125 patients with local disease and craniospinal radiotherapy, and 45 metastatic patients treated with craniospinal radiotherapy ± chemotherapy. Abbreviations: Chemo, chemotherapy; CR, complete remission; OS, overall survival; RT, radiotherapy.
One metastatic patient receiving craniospinal radiotherapy as single treatment modality died 1 month after the end of therapy in remission due to severe diabetes insipidus followed by sepsis. For metastatic tumors (n = 45), progression-free survival among the metastatic patients was estimated to be 1.00 (Fig. 2). Event-free survival at 5 years was 0.98 ± 0.02 among all patients, of whom 28 received craniospinal radiotherapy alone and 17 had received additional chemotherapy before craniospinal radiotherapy (Fig. 3). Overall survival at 5 years was 0.98 ± 0.02 among all patients (Fig. 4).
Risk Factors
Multivariate analysis of age, sex, primary tumor site, and stage identified treatment modality as the single prognostic indicator in these groups. Frequency of relapse differed significantly (P = .0126) between combined treatment and CSI in localized disease (8.5% vs 2.6%).
We also evaluated the impact of the initial tumor marker positivity at diagnosis after first relapse. Neither AFP elevation nor HCG elevation after normal values (as described in the section diagnostic and staging procedures) had an impact on the probability of the appearance of a relapse (χ2 0.1378 for AFP, and 0.3374 for HCG;Fisher's exact test 0.2427 for AFP and 0.3457 for HCG).
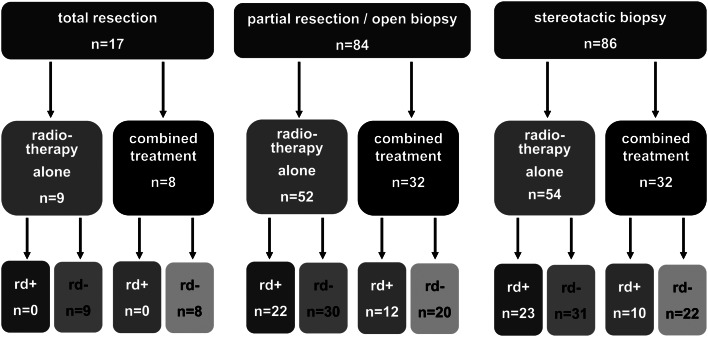
The impact of residual lesions was evaluated by radiological review at the end of treatment in 187 of 235 patients either with total resection (n = 17), partial resection (n = 84), or stereotactic biopsy (n = 86; Fig. 5). Patients with residual lesions (n = 67) did not demonstrate a difference from patients without residual lesions (n = 120) in terms of event-free (0.95 ± 0.03 vs 0.93 ± 0.03; P = 0.54) or progression-free survival at 5 years (0.97 ± 0.02 vs 0.94 ± 0.02; P = .41 by log-rank test).
Fig. 5.
Radiologically reviewed residual disease after stereotactic biopsy or partial resection. Abbreviations: rd+, residual disease; rd-, no evidence of residual disease.
Toxicity
Toxicities were classified according to National Cancer Institute criteria. Treatment was well tolerated in the majority of patients. Acute grade III or IV toxicities among patients receiving combined treatment (n = 83) occurred in 27 patients with either localized or metastatic disease. These toxicities consisted mainly of myelosuppression (grade IV; n = 9) and vomiting or diarrhea (grade III; n = 8). The only reported patient with hemorrhage showed tumor bleeding at stereotactic and open biopsy. Four patients with grade III or IV infections were reported, including one episode of sepsis followed by local infection related to a peripheral venous catheter. Six patients had electrolyte disturbances, 5 patients experienced renal impairment, and elevation of liver enzymes was seen in 7 patients. Two patients developed seizures during chemotherapy because of electrolyte disturbances. Other reported grade III or IV toxicities, included single patients with headaches, symptoms of raised intracranial pressure, and elevated serum creatinine or bilirubin level. All observed acute toxicities related to treatment resolved completely.
Grade III and IV toxicities related to radiotherapy (n = 154) included nausea and vomiting in 26 patients. One patient was admitted because of major infection. Myelosuppression (n = 2), transient cerebral edema (n = 1), oral ulcers (n = 1), and encephalopathy (n = 1) were rarely observed.
The evaluation of late effects in terms of development of permanent endocrine disturbances, neuropsychological deficits, and neurological deficits with respect to disease and treatment will be the subject of a follow-up article.
Discussion
We describe the largest prospective series of intracranial germinoma treated in a multinational European protocol. Our study shows that patients with localized germinoma can be treated effectively with 2 treatment options: radiotherapy alone at reduced doses of 24 Gy CSI and additional boost to the primary and metastatic sites of 16 Gy or a combined treatment approach using carboplatin-based chemotherapy followed by local radiotherapy (40 Gy). The primary rationale for limiting the extent of radiation in young patients is to minimize potential adverse effects.7–9,14
Chemotherapy is central to the treatment of testicular and ovarian germ cell tumors.15,16 However, chemotherapy alone should not be regarded as adequate for primary treatment of intracranial germinoma because of high relapse rates in reported series.14,17 Pre-irradiation phase II trials have confirmed the chemosensitivity of CNS germinoma.12 The rationale for the use of chemotherapy for localized intracranial germinomas is to reduce sequelae by limiting the radiation burden to the brain and spine.18–21 In our study, chemotherapy was well tolerated, and there was no major unexpected toxicity or death, with myelotoxicity and vomiting as the most frequent reported adverse effects. Chemotherapy followed by local field radiotherapy has shown a good initial response, but this treatment was insufficient to control subependymal growth in the ventricular area.3 At relapse, 6 patients demonstrated involvement of the ventricular system. This finding is consistent with previous reports of increased risk of ventricular relapse, suggesting the need to enlarge the radiation field to include the ventricles.22,23 Ventricular radiotherapy following chemotherapy is likely to be a safe treatment to control subclinical disease.24 In metastatic disease, our study results did not demonstrate additional benefit of chemotherapy. Craniospinal radiotherapy followed by a boost to the tumor site remains the mainstay of treatment for metastatic patients.9,25
Reducing the dose of radiation in patients receiving radiotherapy only (24/16 Gy) demonstrated excellent 5-year event-free and 5-year progression-free survival rates of 0.94 ± 0.02 and 0.97 ± 0.02. This treatment was well tolerated, with vomiting as the main adverse effect. The 4 relapses were all located at the primary tumor site. Further reduction of doses delivered to the neuroaxis should be investigated. Dose-reduced radiotherapy combined with chemotherapy is a promising approach to treat localized germinomas but requires further study.26
The presence of residual lesions at the end of treatment is considered to be a risk factor for most malignant pediatric brain tumors. Albright et al concluded that extent of residual lesions correlates with prognosis.27,28 However, this has not been demonstrated for germinomas, and our study did not identify the presence of residual lesions at the end of treatment as an adverse prognostic factor. There was also no evidence of an impact of the elevation of AFP (≤10 ng/mL) or HCG (≤50 IU/L) on the probability to develop a relapse.
These findings of the largest multicenter trial suggest that both chemotherapy followed by radiotherapy and dose-reduced radiotherapy as a single treatment are treatment options for localized germinomas. When combined treatment is used, the ventricular area should be included in the radiation field.29 Implementation of an international protocol demonstrated good feasibility in a multicenter setting and should be the mainstay in patients with such rare diseases to evaluate treatment and late effects and to improve outcome.
Limitations
The trial was not conducted as a randomized study, and the choice of treatment strategy was made in accordance with a national decision; thus, the comparison made is not totally independent from external factors. Therefore, the choice of a combined treatment or radiotherapy alone in localized germinomas might have been affected by local decisions on patients' status at diagnosis. This was not evaluated specifically for this manuscript but will be a focus of a further analysis in a subset of patients, for whom we are able to centrally review their radiological pictures and compare them with the clinical records. Such an investigation will not be feasible for the whole cohort, overlooking a time span of 9 years and comparing 14 different national systems.
Funding
This work was supported by the Deutsche Krebshilfe e-V., Bonn, Germany. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the supporting association.
Conflict of interest statement. None declared.
Acknowledgments
We thank all the medical centers that contributed to the SIOP '96 study and Jillian Mann, Marie Christine Baranzelli, Ulrich Göbel, and Barbara Krefeld for their valuable contributions.
References
- 1.Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991;74:545–551. doi: 10.3171/jns.1991.74.4.0545. [DOI] [PubMed] [Google Scholar]
- 2.Maity A, Shu HK, Janss A, et al. Craniospinal radiation in the treatment of biopsy-proven intracranial germinomas: twenty-five years’ experience in a single center. Int J Radiat Oncol Biol Phys. 2004;58:1165–1170. doi: 10.1016/j.ijrobp.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Brada M, Rajan B. Spinal seeding in cranial germinoma. Br J Cancer. 1990;61:339–340. doi: 10.1038/bjc.1990.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappaport R, Brauner R. Growth and endocrine disorders secondary to cranial irradiation. Pediatr Res. 1989;25:561–577. doi: 10.1203/00006450-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Mechanick JI, Hochberg FH, LaRocque A. Hypothalamic dysfunction following whole-brain irradiation. J Neurosurg. 1986;65:490–494. doi: 10.3171/jns.1986.65.4.0490. [DOI] [PubMed] [Google Scholar]
- 6.Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10:1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 7.Bouffet E, Baranzelli MC, Patte C, et al. Combined treatment modality for intracranial germinomas: results of a multicentre SFOP experience. Societe Francaise d'Oncologie Pediatrique. Br J Cancer. 1999;79:1199–1204. doi: 10.1038/sj.bjc.6690192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner JC, Peethambaram PP, Smithson WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17:933–940. doi: 10.1200/JCO.1999.17.3.933. [DOI] [PubMed] [Google Scholar]
- 9.Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585–2592. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 10.Baranzelli MC, Patte C, Bouffet E, et al. Nonmetastatic intracranial germinoma: the experience of the French Society of Pediatric Oncology. Cancer. 1997;80:1792–1797. [PubMed] [Google Scholar]
- 11.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O. Combined treatment with chemotherapy and radiation therapy for intracranial germ cell tumors. Childs Nerv Syst. 1998;14:59–62. doi: 10.1007/s003810050176. [DOI] [PubMed] [Google Scholar]
- 12.Allen JC, DaRosso RC, Donahue B, Nirenberg A. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74:940–944. doi: 10.1002/1097-0142(19940801)74:3<940::aid-cncr2820740323>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama H, Shirato H, Ikeda J, Fujieda K, Miyasaka K, Sawamura Y. Induction chemotherapy followed by low-dose involved-field radiotherapy for intracranial germ cell tumors. J Clin Oncol. 2002;20:857–865. doi: 10.1200/JCO.2002.20.3.857. [DOI] [PubMed] [Google Scholar]
- 14.Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation - a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996;14:2908–2915. doi: 10.1200/JCO.1996.14.11.2908. [DOI] [PubMed] [Google Scholar]
- 15.Adams M, Calvert AH, Carmichael J, et al. Chemotherapy for ovarian cancer - a consensus statement on standard practice. Br J Cancer. 1998;78:1404–1406. doi: 10.1038/bjc.1998.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shelley MD, Burgon K, Mason MD. Treatment of testicular germ-cell cancer: a cochrane evidence-based systematic review. Cancer Treat Rev. 2002;28:237–253. doi: 10.1016/s0305-7372(02)00059-2. [DOI] [PubMed] [Google Scholar]
- 17.da Silva NS, Cappellano AM, Diez B, et al. Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer. 2010;54:377–383. doi: 10.1002/pbc.22381. [DOI] [PubMed] [Google Scholar]
- 18.Merchant TE, Pollack IF, Loeffler JS. Brain tumors across the age spectrum: biology, therapy, and late effects. Semin Radiat Oncol. 2010;20:58–66. doi: 10.1016/j.semradonc.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sklar CA, Constine LS. Chronic neuroendocrinological sequelae of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:1113–1121. doi: 10.1016/0360-3016(94)00427-M. [DOI] [PubMed] [Google Scholar]
- 20.Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 21.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas JG, Rockhill JK, Olson JM, Ellenbogen RG, Geyer JR. Cisplatin-based chemotherapy followed by focal, reduced-dose irradiation for pediatric primary central nervous system germinomas. J Pediatr Hematol Oncol. 2006;28:36–39. [PubMed] [Google Scholar]
- 23.Alapetite C, Brisse H, Patte C, et al. Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol. 2010;12:1318–1325. doi: 10.1093/neuonc/noq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatua S, Dhall G, O'Neil S, et al. Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr Blood Cancer. 2010;55:42–46. doi: 10.1002/pbc.22468. [DOI] [PubMed] [Google Scholar]
- 25.Jensen AW, Issa Laack NN, Buckner JC, Schomberg PJ, Wetmore CJ, Brown PD. Long-Term Follow-Up of Dose-Adapted and Reduced-Field Radiotherapy With or Without Chemotherapy for Central Nervous System Germinoma. Int J Radiat Oncol Biol Phys. 2010;77:1449–1456. doi: 10.1016/j.ijrobp.2009.06.077. [DOI] [PubMed] [Google Scholar]
- 26.Haas-Kogan DA, Missett BT, Wara WM, et al. Radiation therapy for intracranial germ cell tumors. Int J Radiat Oncol Biol Phys. 2003;56:511–518. doi: 10.1016/s0360-3016(02)04611-4. [DOI] [PubMed] [Google Scholar]
- 27.Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;33:4961–4968. doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 28.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children's Cancer Group. Neurosurgery. 1996;38:265–271. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 29.O'Neil S, Ji L, Buranahirun C, et al. Neurocognitive outcomes in pediatric and adolescent patients with central nervous system germinoma treated with a strategy of chemotherapy followed by reduced-dose and volume irradiation. Pediatr Blood Cancer. 2011;57(4):669–673. doi: 10.1002/pbc.23146. [DOI] [PubMed] [Google Scholar]