Abstract
Background
Bevacizumab, an anti–vascular endothelial growth factor antibody, has been used for the treatment of radiation necrosis. Thus far, however, there has been no definitive report on its use for the treatment of symptomatic pseudoprogression. Here we report 2 cases of successful treatment with bevacizumab for symptomatic pseudoprogression after boron neutron capture therapy (BNCT) was applied for recurrent malignant gliomas.
Methods
Two recurrent malignant gliomas received BNCT. Both cases were treated with intravenous administration of bevacizumab at the deterioration that seemed to be symptomatic pseudoprogression.
Results
The first case was recurrent glioblastoma multiforme and the second was recurrent anaplastic oligoastrocytoma. Both cases recurred after standard chemoradiotherapy and were referred to our institute for BNCT, which is tumor-selective particle radiation. Just prior to neutron irradiation, PET with an amino acid tracer was applied in each case to confirm tumor recurrence. Both cases showed deterioration in symptoms, as well as on MRI, at intervals of 4 months and 2 months, respectively, after BNCT. For the first case, a second PET was applied in order to confirm no increase in tracer uptake. We diagnosed both cases as symptomatic pseudoprogression and started the intravenous administration of 5 mg/kg bevacizumab biweekly with 6 cycles. Both cases responded well to this, showing rapid and dramatic improvement in neuroimaging and clinical symptoms. No tumor progression was observed 8 months after BNCT.
Conclusions
Bevacizumab showed marked effects on symptomatic pseudoprogression after BNCT. BNCT combined with bevacizumab may prolong the survival of patients with recurrent malignant gliomas.
Keywords: bevacizumab, boron neutron capture therapy, malignant gliomas, pseudoprogression
With the advent of temozolomide (TMZ), concomitant chemoradiation and maintenance chemotherapy with TMZ have become the worldwide standard treatment for malignant gliomas (MGs), especially glioblastoma multiforme (GBM).1 In GBM treatments, pseudoprogression (psPD) can be encountered with a relatively high frequency, especially in O6-DNA methylguanine-methyltransferase (MGMT) promoter methylated cases,2 and intensive treatment might be the primary factor in psPD, as Brandsma et al reported.3 Boron neutron capture therapy (BNCT) is biochemically targeted radiation based on the nuclear capture and fission reactions that occur when nonradioactive boron-10, which is a constituent of natural elemental boron, is irradiated with low-energy thermal neutrons to yield high linear energy transfer alpha particles and recoiling lithium-7 nuclei. Because these particles are released within a very short range, such as 9 μm, the cytotoxic effects are confined within boron-10–containing cells.4 Boron-10–containing compounds can be accumulated selectively into tumor cells by several mechanisms. For example, boronophenylalanine (BPA) is selectively and preferentially accumulated into tumor cells via the augmented metabolism of amino acids in comparison with normal cells. We applied BNCT aggressively to newly diagnosed and recurrent MGs.5–7 We previously reported a high incidence of psPD after BNCT, not only in MGs but also in malignant meningiomas.8 However, it is difficult for us to estimate precisely the psPD occurrence rate after BNCT, because many cases were followed up after BNCT by physicians in charge in many towns in Japan. Nevertheless, we have the impression that psPD might occur more frequently by BNCT than by X-ray treatment and that the rate of psPD after BNCT might be higher in recurrent cases than in newly diagnosed cases.
Bevacizumab, an anti–vascular endothelial growth factor (VEGF) antibody, has been used for the treatment of symptomatic radiation necrosis (RN).9,10 It is difficult to definitively distinguish RN from psPD. We therefore applied intravenous administration of bevacizumab to cases we highly suspected to be symptomatic psPD encountered after BNCT for recurrent MGs. Here we report 2 successfully treated cases of symptomatic psPD after BNCT with bevacizumab.
Case Presentation
Case 1
A 56-year-old male experienced speech disturbance and consequently retired from his job. First he received a craniotomy in April 2008 with a diagnosis of gemistocytic astrocytoma followed by fractionated X-ray treatment (total 50 Gy) and repetitive chemotherapy with nitrosourea. In April 2011, a recurrent lesion appeared with gadolinium (Gd) enhancement on MRI. Re-craniotomy revealed GBM histologically. After surgery, the enhanced lesion gradually grew, and sensory aphasia was aggravated despite the repeated administration of TMZ. Also, carbon 11–labeled methionine PET (C-Met-PET) showed high uptake of the tracer beyond the Gd-enhanced lesion. The patient was then referred to our institute for BNCT. Upon referral, MRI showed a small ringlike enhanced lesion having satellite-enhanced dots in the left temporal lobe, with a relatively large volume of fluid-attenuated inversion recovery (FLAIR) at high intensity, as shown in Fig. 1A and D. A simultaneous fluorine 18–labeled (F)-BPA-PET image showed marked tracer uptake in the left temporo-parietal region, as shown in Fig. 2A, with a 5.5 lesion/normal (L/N) brain ratio of the tracer, indicating that the lesion was a highly malignant tumor.
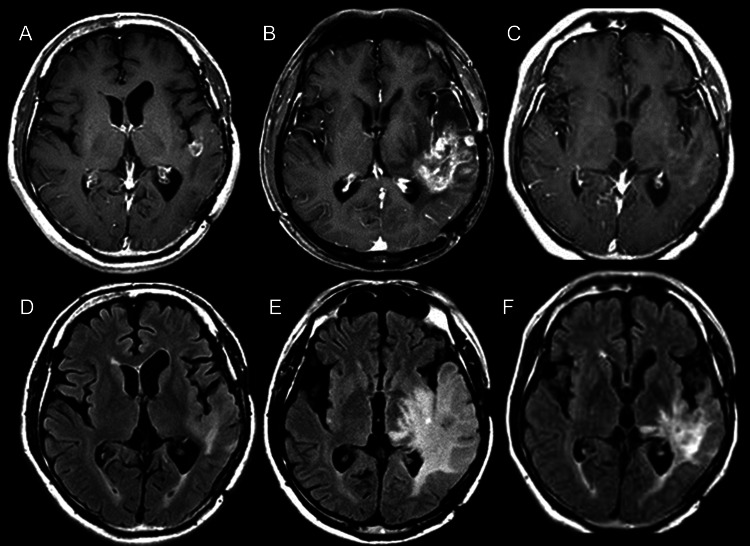
Fig. 1.
Periodic MRI changes in case 1. (A–C) Gd-enhanced T1-weighted MRI. (D–F) FLAIR MRI. (A and D) Just prior to BNCT; (B and E) 4 months after BNCT; (C and F) 7 months after BNCT (3 cycles after initial bevacizumab treatment).
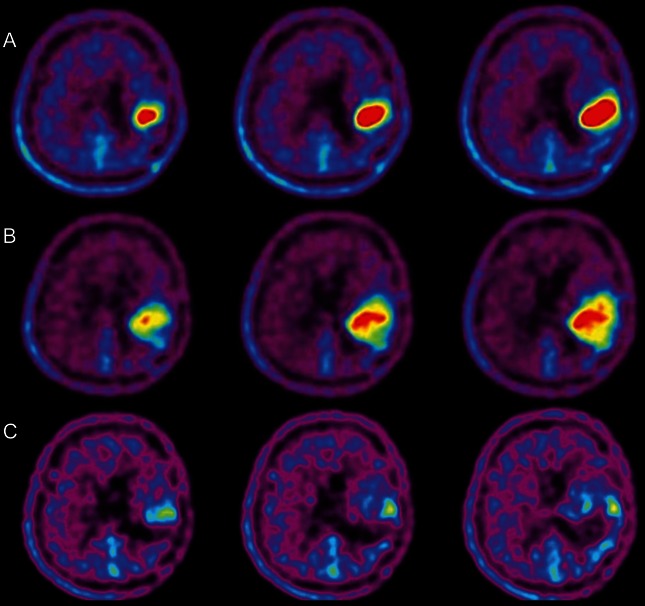
Fig. 2.
F-BPA-PET in case 1, prior to BNCT and at aggravation as well as in follow-up with the patient in good condition. (A) Prior to BNCT; (B) 4 months after BNCT (at aggravation); (C) 8 months after BNCT.
We administered BNCT to our patient according to our recent protocol for recurrent MGs and malignant meningiomas. Briefly, only BPA was administered in the 2 h (200 mg/kg/h) just prior to neutron irradiation and then during neutron irradiation (100 mg/kg/h). The irradiation time was decided by simulation not to exceed 12.0 Gy-Eq (Gray-equivalent) for the peak brain dose. Using BNCT, we estimated maximum brain dose, maximum tumor dose, and minimum tumor dose as 10.8, 110, and 82.3 Gy-Eq, respectively. Here, Gy-Eq corresponds to the biologically equivalent X-ray dose that would have equivalent effects on tumors and on the normal brain. The dose estimation method was described previously.8
One week after BNCT, anticoagulant and vitamin E were administered. This was for the prevention of RN, as we reported previously.9 Right hemiparesis and aphasia occurred and became aggravated gradually after BNCT, even with an escalated dose of corticosteroids. Then, 4 months after BNCT, follow-up MRI and F-BPA-PET were applied simultaneously. In MRI, the Gd-enhanced lesion and the high-intensity area in FLAIR increased markedly (Fig. 1B and E). The second F-BPA-PET, taken 4 months after BNCT, showed decreased uptake of the tracer, as shown in Fig. 2B (L/N ratio, 4.7). Thereafter, the aggravation of clinical symptoms and MRIs was attributed not to tumor progression but to psPD.
We proposed bevacizumab treatment to the patient, his family, and the physician in charge. Thereafter, he was administered 5 mg/kg bevacizumab biweekly with 6 cycles. MRI taken after 3 cycles showed marked improvement in both Gd-enhanced and FLAIR images, as shown in Fig. 1C and F. The patient's speech disturbance and hemiparesis improved markedly by the treatment. The third F-BPA-PET, undertaken 8 months after BNCT with the patient in a stable state, showed a further decrease of tracer uptake, with an L/N ratio of 1.8, as shown in Fig. 2C. This finding suggests no tumor progression and good control of the tumor so far. The follow-up MRI showed no tumor progression (data not shown).
Case 2
A 27-year-old female developed left hemiparesis. A right frontal enhanced mass was removed gross totally in May 2005. The histological diagnosis was anaplastic oligoastrocytoma. She received fractionated X-ray treatment (total 72 Gy) and repetitive chemotherapy with nitrosourea. The lesion recurred and re-craniotomy was applied in November 2009 with the same pathological diagnosis. This was followed by successive TMZ chemotherapy. Unfortunately, the recurrence was confirmed by MRI and C-Met-PET, and the patient retired from her job as a nurse due to progression of left hemiparesis and seizures. She was referred to us for BNCT. Upon referral, MRI showed a Gd-enhanced lesion in the right frontal lobe with moderate perifocal edema, as shown in Fig. 3A and D.
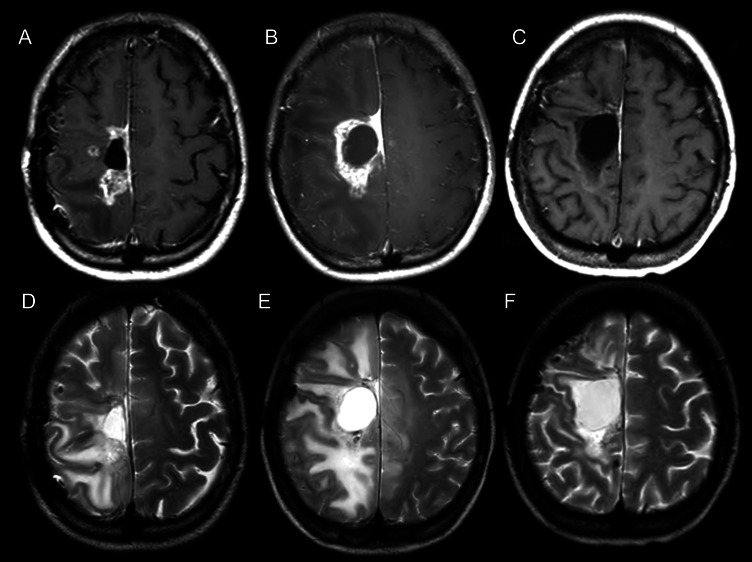
Fig. 3.
Periodic MRI changes in case 2. (A–C) Gd-enhanced T1-weighted MRI. (D–F) T2-weighted MRI. (A and D) Just prior to BNCT; (B and E) 2 months after BNCT; (C and F) 6 months after BNCT (4 cycles after initial bevacizumab treatment).
For this case, BNCT was applied using the same protocol described in case 1. In BNCT, the maximum brain dose, maximum tumor dose, and minimum tumor dose were 11.5, 71.6, and 30.1 Gy-Eq, respectively. In this case, anticoagulant and vitamin E were also administered 1 week after BNCT to prevent RN. After BNCT, her hemiparesis became aggravated gradually even with an increasing dose of corticosteroids. MRI taken 2 months after BNCT showed an enlarged enhanced lesion with increased perilesional edema (Fig. 3B and E). The patient had no chance to receive further amino acid PET, but we considered this aggravation as symptomatic of psPD based on the duration of aggravation after BNCT. This patient and her physician in charge also accepted our proposal of bevacizumab treatment on the same schedule and dosage described in case 1. The patient was bed-ridden just prior to bevacizumab treatment, but her hemiparesis improved markedly and she could walk after 2 cycles of the treatment. MRI taken after 4 cycles, at 6 months after BNCT, showed marked improvement not only in Gd enhancement but also in the perilesional edema in FLAIR images, as shown in Fig. 3C and F. Her clinical condition has remained stable and good since the treatment ended.
Discussion
In our limited experience, there is no obvious histological difference between RN and psPD.8,11 Necrosis is the central histopathological feature of each, and prominent angiogenesis is common at the boundary of central necrosis and normal brain tissue in each clinicopathological entity. Clinically, psPD usually occurs at a relatively early stage after some intensive treatments and is self-limiting. In most cases it improves over time without intensive treatments. On the other hand, RN often shows severe symptoms and occurs at least 6 months after radiotherapy. It is often long-lasting and improves only with intensive treatment, such as lesionectomy or bevacizumab administration. In human surgical specimens of RN, we previously demonstrated that overproduction of VEGF in reactive astrocytes in the perinecrotic area caused leaky angiogenesis, and this is the cause of perifocal edema in RN.10 So we speculated that bevacizumab might neutralize this overproduced VEGF in the perinecrotic area and subsequently reduce the edema.10 This is why we used bevacizumab for symptomatic psPD.
Originally, F-BPA-PET was developed for the simulation of absorbed dose in BNCT.6,12,13 On the other hand, the background uptake of the tracer F-BPA is very low compared with that of fluorodeoxyglucose and even with that of methionine as a tracer. Thereafter, RN and psPD have been differentially diagnosed from tumor progression by F-BPA-PET.8,14 On the basis of our experience, an L/N ratio of <2.0 in F-BPA-PET indicates a high possibility of RN and does not indicate tumor progression. We are now performing a nationwide multicenter clinical trial of bevacizumab treatment for symptomatic RN in the brain with diagnosis made by amino acid tracer PET. F-BPA-PET and C-Met-PET are equally useful for the differential diagnosis between RN and tumor progression. Both PETs show the same tendencies of tracer uptake and distribution, as Nariai et al reported.15
Both cases presented here were recurrent MGs and had received fractionated X-ray treatment previously. They showed aggravated clinical symptoms and MRI results a couple of months after BNCT. Therefore, we considered both cases to be symptomatic psPD. Especially in case 1, repetitive F-BPA-PETs were applied before BNCT and upon aggravation after BNCT, as well as in a stable state during follow-up. The second F-BPA-PET showed a lower L/N ratio than the first, but it was still higher than our criterion for RN at the aggravation. This may suggest that the pathology of case 1 was psPD and not RN. Although the essential difference between them is still unclear, we speculated that they may have similar pathophysiology.
Usually we can treat asymptomatic psPD only with corticosteroids, or we can only observe the patient in asymptomatic psPD without treatments. Unfortunately, both cases presented here continued their clinical deterioration despite the escalating doses of corticosteroids. Fortunately, however, we used bevacizumab thereafter, to which both cases responded well. The physicians in charge decreased the corticosteroid dose for each patient after bevacizumab treatment.
To improve the effectiveness of radiotherapy, one study used bevacizumab with hypofractionated stereotactic irradiation for the treatment of recurrent MGs.16 However, the literature contains no obvious reports about bevacizumab's effects on symptomatic psPD. We applied bevacizumab treatment to symptomatic RN in some cases, and all the patients responded well.9 Based on these findings, as noted, we are performing a nationwide multicenter clinical trial of bevacizumab treatment for symptomatic RN in the brain. We therefore treated the present 2 cases with bevacizumab and confirmed marked effects. Some of the literature supports this concept.17
We applied BNCT, a tumor-selective particle radiation, aggressively even for recurrent MGs with satisfactory results, as reported elsewhere.7 In that previous report, we used Carson et al18 as our reference regarding BNCT's effectiveness for recurrent MGs; those authors advocated, and we adopted, recursive portioning analysis (RPA) classification for recurrent MGs. In our previous report,7 we showed good effectiveness, especially in poor prognosis groups (RPA classes 3 and 718) in BNCT in comparison with Carson's original data sets. Those authors reported that RPA classes 3 and 7 showed the poorest prognosis, with median survival times (MSTs) of 3.8 months and 4.9 months, respectively, after recurrence that followed some treatments. Both of the cases presented here should be considered RPA class 3 because they showed poor performance status at recurrence and because the initial histological diagnosis was not GBM. Carson's data sets revealed an MST of 3.8 months in RPA class 3 after recurrence. Both cases presented here survived more than 8 months after BNCT without tumor progression, continuing up to the writing of this manuscript. Although the 2 cases reported here are the only 2 that we have experienced with symptomatic psPD treated by bevacizumab after BNCT, BNCT plus bevacizumab at psPD improves a patient's condition and may prolong survival more effectively for recurrent MGs than we suggested in our previous report.
Bevacizumab treatment had no adverse effect in either of the present cases. As we described for each case, we routinely used anticoagulant after BNCT for recurrent MGs. This was to prevent anticipated RN. This anticoagulant administration probably decreases the possible adverse effects of thromboembolitic complications of bevacizumab, as we and Levin et al have reported.9,10
As noted at the beginning of this paper, it is widely accepted that MGMT promoter methylation status plays a significant role in the incidence of psPD in newly diagnosed GBM cases treated by concomitant chemo therapy and radiation.2 So let us add finally some information regarding MGMT in both cases presented here. In case 1, MGMT protein expression was positive in immunohistochemistry, and in case 2, the MGMT promoter was methylated. These observations might suggest that MGMT status is not so important for the incidence of symptomatic psPD for recurrent MGs receiving BNCT.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (B) (19390385) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology to S.-I. M.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 3.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 4.Barth RF, Vicente MG, Harling OK, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabata S, Miyatake S, Kuroiwa T, et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res (Tokyo) 2009;50:51–60. doi: 10.1269/jrr.08043. [DOI] [PubMed] [Google Scholar]
- 6.Miyatake S, Kawabata S, Kajimoto Y, et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg. 2005;103:1000–1009. doi: 10.3171/jns.2005.103.6.1000. [DOI] [PubMed] [Google Scholar]
- 7.Miyatake S, Kawabata S, Yokoyama K, et al. Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol. 2009;91:199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 8.Miyatake S, Kawabata S, Nonoguchi N, et al. Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro Oncol. 2009;11:430–436. doi: 10.1215/15228517-2008-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuse M, Kawabata S, Kuroiwa T, Miyatake S. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol. 2011;102:471–475. doi: 10.1007/s11060-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 10.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor–producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105:423–431. doi: 10.1007/s11060-011-0610-9. [DOI] [PubMed] [Google Scholar]
- 12.Imahori Y, Ueda S, Ohmori Y, et al. Positron emission tomography–based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part I. Clin Cancer Res. 1998;4:1825–1832. [PubMed] [Google Scholar]
- 13.Imahori Y, Ueda S, Ohmori Y, et al. Positron emission tomography–based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part II. Clin Cancer Res. 1998;4:1833–1841. [PubMed] [Google Scholar]
- 14.Miyashita M, Miyatake S, Imahori Y, et al. Evaluation of fluoride-labeled boronophenylalanine-PET imaging for the study of radiation effects in patients with glioblastomas. J Neurooncol. 2008;89:239–246. doi: 10.1007/s11060-008-9621-6. [DOI] [PubMed] [Google Scholar]
- 15.Nariai T, Ishiwata K, Kimura Y, et al. PET pharmacokinetic analysis to estimate boron concentration in tumor and brain as a guide to plan BNCT for malignant cerebral glioma. Appl Radiat Isot. 2009;67:S348–S350. doi: 10.1016/j.apradiso.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khasraw M, Simeonovic M, Grommes C. Bevacizumab for the treatment of high-grade glioma. Expert Opin Biol Ther. 2012;12:1101–1111. doi: 10.1517/14712598.2012.694422. [DOI] [PubMed] [Google Scholar]
- 18.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]