Abstract
Vascular endothelial growth factor A (VEGF-A) is classically viewed as a key factor in angiogenesis and tissue remodeling. However, recent evidence suggests a potential role of this growth factor in the control of energy metabolism and adipose tissue function. In this regard, we and others have described the effects of the up and downregulation of VEGF-A in adipose tissue on the control of energy homeostasis. VEGF-A overexpression protects against diet-induced obesity and insulin resistance. The observation that VEGF-A overexpression leads to an increase in brown adipose tissue (BAT) thermogenesis and also promotes a “BAT-like” phenotype in white adipose tissue depots is of particular relevance for the understanding of the mechanisms underlying obesity development. In addition, VEGF-A may not only have pro-inflammatory but also anti-inflammatory properties, with a chemotactic activity specific for M2 anti-inflammatory macrophages. This new scientific evidence highlights the importance that VEGF-A actions on metabolism could have on the design of new treatments for obesity, insulin resistance and obesity-related disorders.
Keywords: VEGF-A, adipose tissue, insulin resistance, inflammation, obesity
VEGF is Involved in Energy Homeostasis Control
Vascular endothelial growth factor A (VEGF-A) is acknowledged as a key molecule in processes such as vasculogenesis, angiogenesis, control of vascular permeability or tissue remodeling.1 However, VEGF-A has also been recognized to exert metabolic effects, and both its up and downregulation have been described to control energy metabolism.2,3 In this regard, we have recently reported that VEGF-A overexpression in adipose tissue protects transgenic mice against diet-induced obesity and insulin resistance.2 In agreement with our data, Sun et al. demonstrated that a mouse model that allows inducible overexpression of VEGF-A in adipose tissue is also protected against high fat diet-induced insulin resistance.4 In both models, VEGF-A overproduction led to a higher rate of energy expenditure, which was probably due to an increase in thermogenesis. In our transgenic model,2 thermogenesis was enhanced mainly in brown adipose tissue (BAT), as supported by increased protein levels of UCP1 and PGC-1α in BAT. However, in the inducible model,4 VEGF-A overexpression exerted its effects mostly on white adipose tissue (WAT) and, increased thermogenesis was the result of a “browning” of WAT without changes in BAT depots. All these results clearly suggest a potential role of VEGF-A in BAT differentiation and function. This is of particular importance in light of recent evidence demonstrating that BAT has physiological relevance in adult humans and that obese patients have decreased BAT activity.5-7 Thus, increasing thermogenic activity may be of interest for the treatment of obesity but requires a better understanding of the mechanisms underlying the activation of this process in BAT. Cold-exposure is one of the inducers of non-shivering thermogenesis and this process involves a hypoxia-independent increase in the vascularization of BAT and WAT.8 This is accompanied by an increase in VEGF-A gene expression that has been postulated to be secondary to adrenergic stimulation that induces PGC-1α expression, which in turn can control VEGF-A gene promoter.8 However, in our study, VEGF-A overexpression per se leads to an increase in PGC-1α and UCP-1 levels in BAT, suggesting that VEGF-A, either directly or indirectly, may trigger thermogenesis activation.2 Similarly, inducible VEGF-A overexpression leads to upregulation of PGC-1α and UCP-1 in WAT, but without effect in BAT.4 Indeed, in the inducible VEGF-A overexpressing-model, VEGF-A overexpression and increased vascularization were exclusive of white adipose depots, whereas in our model, both effects were present in white and brown adipose tissue. This apparent discrepancy between models may be attributed to the fact that mutant mice obtained by classical transgenesis express the transgene during adipose tissue development, whereas inducible transgenic mice begin to overexpress VEGF-A at 5 weeks of age. This also suggests that the timeframe of VEGF-A expression is crucial to determinate its action. This idea is consistent with the observation that the blockage of VEGF-A signaling has opposite effects on adipose tissue metabolism depending on the pre-existence of obesity in the animal model used.4 Indeed, inhibition of VEGF-A-induced activation of VEGF receptor 2 (VEGFR2) at the beginning of a high fat diet causes an aggravation of metabolic alterations in mice. In contrast, the same VEGF-A-VEGFR2 blockage in ob/ob obese mice leads to reduced body weight gain, improved insulin sensitivity and a decrease in inflammatory factors.4 These results illustrate the complexity of the VEGF system, which may have either beneficial or deleterious effects depending on the context. For example, VEGF-A overexpression in β-cell leads to disorganized, hypervascularized and fibrotic islets, progressive macrophage infiltration and proinflammatory cytokine production, resulting in impaired insulin secretion, decreased β-cell mass and hyperglycemia with age.9 Thus, VEGF-A may have opposite actions on inflammation and metabolism with a tissue-specific pattern. These different actions of VEGF-A may rely on different signaling mechanisms, which remain to be fully elucidated. In addition to VEGFR2, VEGF-A exerts its effects through another tyrosine-kinase receptor, VEGF receptor 1 (VEGFR1). However, VEGF-A has also been recently described to regulate adipocyte differentiation independently of VEGFR1 and VEGFR2.10 Furthermore, not only VEGF-A but also VEGF-B may have metabolic effects, adding to the complexity of the VEGF system.11 VEGF-B controls endothelial uptake of fatty acids through activation of VEGFR1 and neuropilin-1.11 Moreover, both genetic and pharmacological inhibition of VEGF-B signaling in mice, have been shown to reduce ectopic lipid accumulation, improve peripheral insulin sensitivity and muscle glucose uptake and preserve islet functionality.12 In addition, compensatory VEGF-B upregulation associated to VEGF-A downregulation leads to brown-like WAT differentiation.3 Altogether, these results demonstrate that the VEGF system is crucial in the regulation of energy homeostasis but further studies are necessary to understand the underlying mechanisms.
VEGF Acts as an Anti-Inflammatory M2 Macrophages Attractant
VEGF is also well-known for being involved in inflammatory processes13 but little is known about its role in adipose tissue inflammation during obesity. It is well-accepted that low-grade inflammation in the adipose tissue can promote obesity-associated insulin resistance.14,15 This process occurs when the recruitment of M1 macrophages promotes the change of the anti-inflammatory milieu, maintained by M2 macrophages, toward an inflammatory enviroment.16 Our work points out that VEGF-A has a protective effect against adipose tissue inflammation, by recruiting M2 macrophages to adipose depots, thus maintaining an anti-inflammatory milieu.2
Although VEGF has been widely described to be chemotactic for macrophages,17 in our animal model, the chemotactic activity of VEGF-A in adipose tissue seemed to be specific for M2 macrophages. In line with our findings, a spatiotemporal link between VEGF and M2 macrophages has been described in several processes, in which a parallel increase in VEGF and M2 macrophage recruitment has been observed. One example is wound healing. Early in wound healing, M1 macrophages likely direct the inflammatory response that helps clear microbes, cellular debris and damaged matrix from the wound. When the infection recedes, the composition of the local environment changes, facilitating either the differentiation of monocytes or the polarization of macrophages toward anti-inflammatory M2 macrophages. These M2 macrophages produce considerable amounts of anti-inflammatory cytokines, and also VEGF, in order to direct the angiogenic response that results in the repair of the wound.18,19 Similarly, both VEGF and M2 macrophages are involved in tumor development. Tumor-associated macrophages (TAMs) have been reported to be M2 macrophages.20 Indeed, TAMs produce a host of growth factors that affect tumor-cell proliferation, angiogenesis and the deposition and dissolution of connective tissues. These growth factors include VEGF and transforming growth factor-β (TGF-β).21,22 M2 macrophages and VEGF also play a key role in anastomosis, by which endothelial cells from the tip of adjacent growing vessels fuse leading to the connection of main vessels. This process involves tissue-resident macrophages located in the vicinity of the vessel branches.23 These macrophages are polarized toward the M2 phenotype and release pro-angiogenic factors such as VEGF.24
Nevertheless, a causative link between expression of VEGF and the presence of M2 macrophages has only recently been established. We have demonstrated that, in adipose tissue, VEGF-A enhances the presence of M2 anti-inflammatory macrophages. In order to discern whether VEGF-A was acting as a chemoattractant for M2 macrophages or if it was promoting the polarization switch from M1 to M2 macrophages, bone marrow derived macrophages (BMDM) were treated with VEGF-A. After treatment with the growth factor, we did not find an increase in M2 markers in comparison with non-treated BMDM.2 These observations therefore suggested that VEGF per se was not able to induce the switch toward an M2 phenotype. In line with our results, it has recently been reported that subcutaneous grafts of engineered VEGF-A-overexpressing keratinocytes have increased amounts of macrophages polarized toward an M2 phenotype.25 The authors also demonstrate that VEGF-A per se is not able to induce a polarization switch in BMDM. While VEGF-A is needed initially for macrophage recruitment, a subsequent activation by IL-4 and IL-10 is essential for M2 polarization.25,26 One possible mechanism through which VEGF-A could attract M2 macrophages is through the chemokine CXCL12. CXCL12 expression has been reported to be linked to VEGF-A expression27 and recent findings support that CXCL12 functions as an anti-inflammatory chemokine.28-30 Moreover, CXCL12 expression is very high in some pathologic conditions associated with hypoxia and in proangiogenic environments, such as in tumors.28 Indeed, in our transgenic mice, the expression of CXCL12 in adipose tissue was upregulated, suggesting that the increase in M2 macrophages induced by VEGF-A could be mediated through CXCL12.2 Thus, altogether these results suggest that VEGF-A could, under certain conditions, act as anti-inflammatory molecule.
VEGF May Protect against Obesity-Induced Insulin Resistance in Humans
The anti-inflammatory properties of VEGF-A together with its role in thermogenesis activation may be of particular relevance during obesity. Although VEGF-A expression has been extensively measured in obese humans and animal models, results are unclear, and there are conflicting reports regarding the local and systemic levels of VEGF during obesity. Several reports argue that VEGF-A concentration in serum correlates significantly with BMI,31,32 with higher VEGF-A levels observed in overweight and obese subjects and animal models. However, other authors have failed to reproduce these results33 and a few studies have reported decreased VEGF-A expression in adipose tissue of obese mice34 and obese humans.35 These latter results, and the fact that VEGF-A overexpression protects mice from diet-induced insulin resistance, are in line with a recent report indicating that morbidly obese subjects with low insulin resistance had higher adipose tissue VEGF-A levels than obese subjects with high insulin resistance.36 Although there are few reports regarding changes of VEGF-A expression during diabetes, VEGF-A production seems to be decreased in cells of Type 2 diabetic patients.37,38 This clearly suggests a protective role of VEGF-A against obesity-induced insulin resistance.
Concluding Remarks
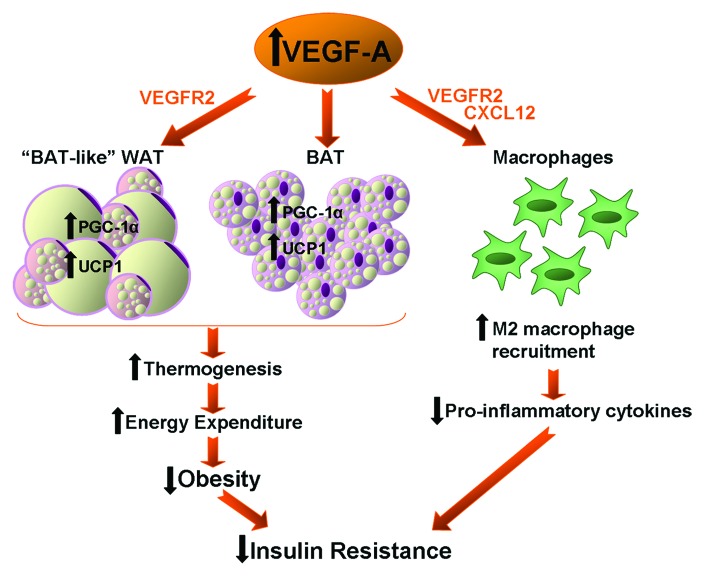
Therefore, all these data suggest that VEGF-A overexpression in adipose tissue promotes a “BAT-like” phenotype in WAT depots and enhances BAT PGC-1α and UCP-1 expression, thus increasing thermogenesis and energy expenditure and decreasing obesity. Moreover, VEGF-A exerts an anti-inflammatory role in adipose tissue, increasing the recruitment of M2 alternatively activated macrophages. Both the reduction of obesity and the decrease in pro-inflammatory cytokine levels induced by VEGF-A overexpression would be responsible for the amelioration of insulin sensitivity (Fig. 1). The elucidation of the precise mechanisms by which VEGF-A exerts these actions on adipose tissue warrant further studies. A better understanding of VEGF-A actions on metabolism would be of crucial importance to the development of treatments for obesity, insulin resistance and obesity-related disorders.
Figure 1. Schematic representation of the impact of VEGF-A overexpression in adipose tissue on insulin resistance. VEGF-A overexpression promotes a “BAT-like” phenotype in WAT depots and enhances PGC-1α and UCP-1 expression in BAT, leading to increased thermogenesis and energy expenditure and reduced obesity. VEGF-A overexpression also exerts its action on macrophages by increasing the recruitment of M2 anti-inflammatory macrophages to fat depots. The decreased obesity and the anti-inflammatory milieu induced by VEGF-A in adipose tissue may be responsible for the reduction of insulin resistance in transgenic mice.
Acknowledgments
The work in our lab relevant to this commentary was supported by grants from the Ministerio de Ciencia e Innovación, Plan Nacional I+D+I (SAF2008-00962 and SAF2011-24698) and Generalitat de Catalunya (2009 SGR-224), Spain and EUMODIC (LSHG-CT-2006-037188). The authors declare no conflicts of interest.
Glossary
Abbreviations:
- BAT
brown adipose tissue
- SDF-1
stromal cell-derived factor (CXCL12)
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- TAMs
tumor-associated macrophages
- TGF-β
transforming growth factor-beta
- UCP1
uncoupling protein 1
- VEGF-A
vascular endothelial growth factor A
- VEGF-B
vascular endothelial growth factor B
- VEGFR1
vascular endothelial growth factor receptor 1
- VEGFR2
vascular endothelial growth factor receptor 2
- WAT
white adipose tissue
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/22880
References
- 1.Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–87. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 2.Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X, Ji Y, Zhang L, Zhang Y, Zhang S, An Y, et al. Resistance to obesity by repression of VEGF gene expression through induction of brown-like adipocyte differentiation. Endocrinology. 2012;153:3123–32. doi: 10.1210/en.2012-1151. [DOI] [PubMed] [Google Scholar]
- 4.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–9. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248–52. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Agudo J, Ayuso E, Jimenez V, Casellas A, Mallol C, Salavert A, et al. Vascular Endothelial Growth Factor-Mediated Islet Hypervascularization and Inflammation Contribute to Progressive Reduction of β-Cell Mass. Diabetes. 2012;61:2851–61. doi: 10.2337/db12-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–13. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–21. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–30. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 18.Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Plas MJ, van Dissel JT, Nibbering PH. Maggot secretions skew monocyte-macrophage differentiation away from a pro-inflammatory to a pro-angiogenic type. PLoS One. 2009;4:e8071. doi: 10.1371/journal.pone.0008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, et al. Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J Leukoc Biol. 2007;81:557–66. doi: 10.1189/jlb.0806517. [DOI] [PubMed] [Google Scholar]
- 22.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–25. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt T, Carmeliet P. Blood-vessel formation: Bridges that guide and unite. Nature. 2010;465:697–9. doi: 10.1038/465697a. [DOI] [PubMed] [Google Scholar]
- 24.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 26.De Palma M. Partners in crime: VEGF and IL-4 conscript tumour-promoting macrophages. J Pathol. 2012;227:4–7. doi: 10.1002/path.4008. [DOI] [PubMed] [Google Scholar]
- 27.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88:463–73. doi: 10.1189/jlb.0909602. [DOI] [PubMed] [Google Scholar]
- 29.Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–55. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega MA, Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- 31.Loebig M, Klement J, Schmoller A, Betz S, Heuck N, Schweiger U, et al. Evidence for a relationship between VEGF and BMI independent of insulin sensitivity by glucose clamp procedure in a homogenous group healthy young men. PLoS One. 2010;5:e12610. doi: 10.1371/journal.pone.0012610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–14. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 33.Rehman J, Considine RV, Bovenkerk JE, Li J, Slavens CA, Jones RM, et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–13. doi: 10.1016/S0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 34.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinahones FJ, Coín-Aragüez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J, et al. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12:4. doi: 10.1186/1472-6793-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106:13505–10. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–9. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]